Abstract
The extent and position of the single-stranded gap in DNA molecules from Dane particles isolated from two donors of the adw serotype were determined by molecular hybridization and electron microscopic methods. The results showed that in each preparation more than 99% of the circular molecules are of uniform length and contain both single- and double-stranded regions. They confirmed that one end of the short strand is fixed with respect to the single EcoRI site within the molecule and to the nick in the long strand, but they also showed that although the position of the other end is variable, there is a preferred minimum length of about 650 to 700 nucleotides for the single-stranded region.
Full text
PDF

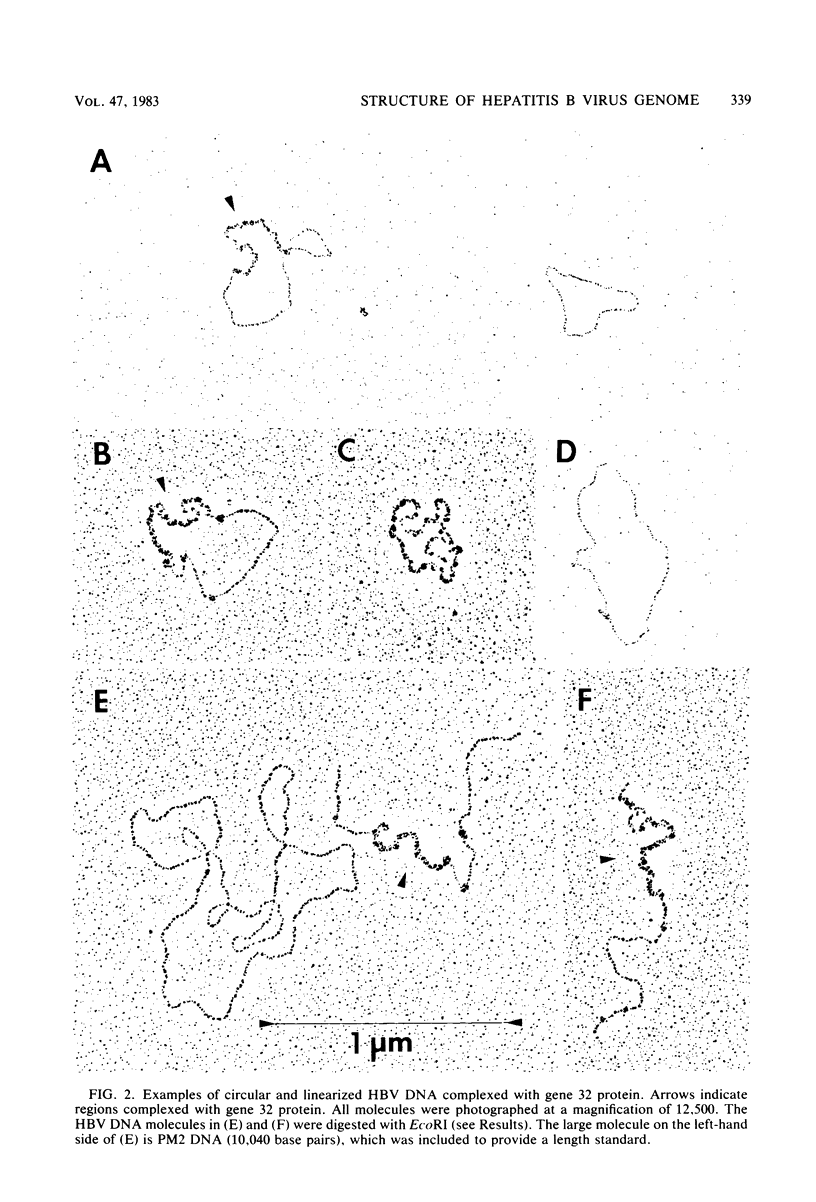
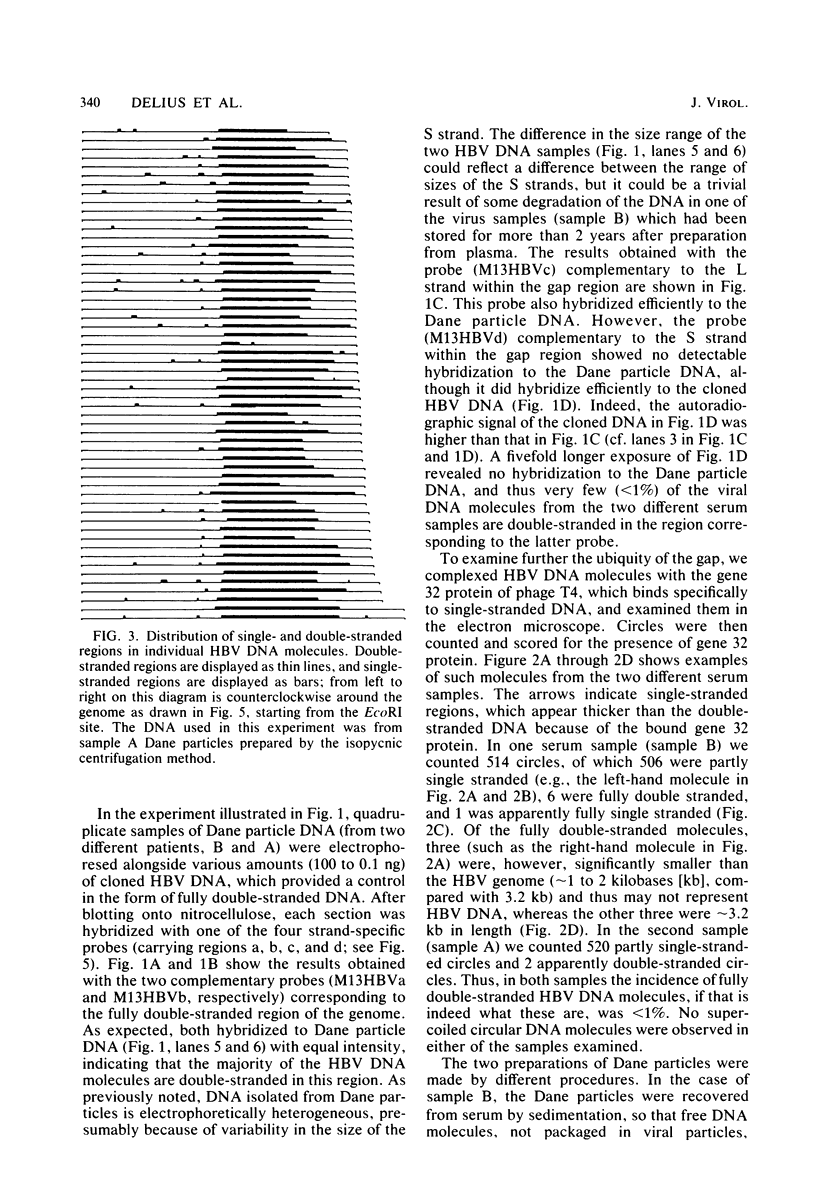
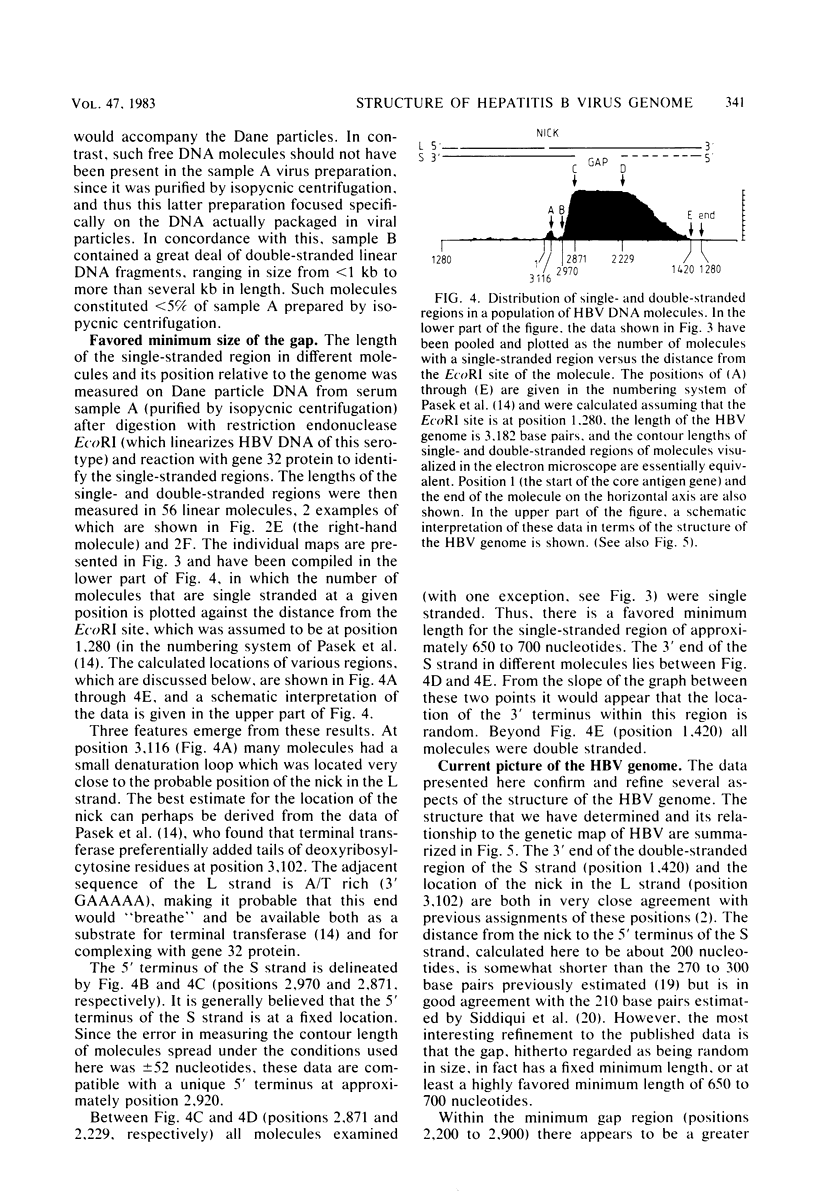
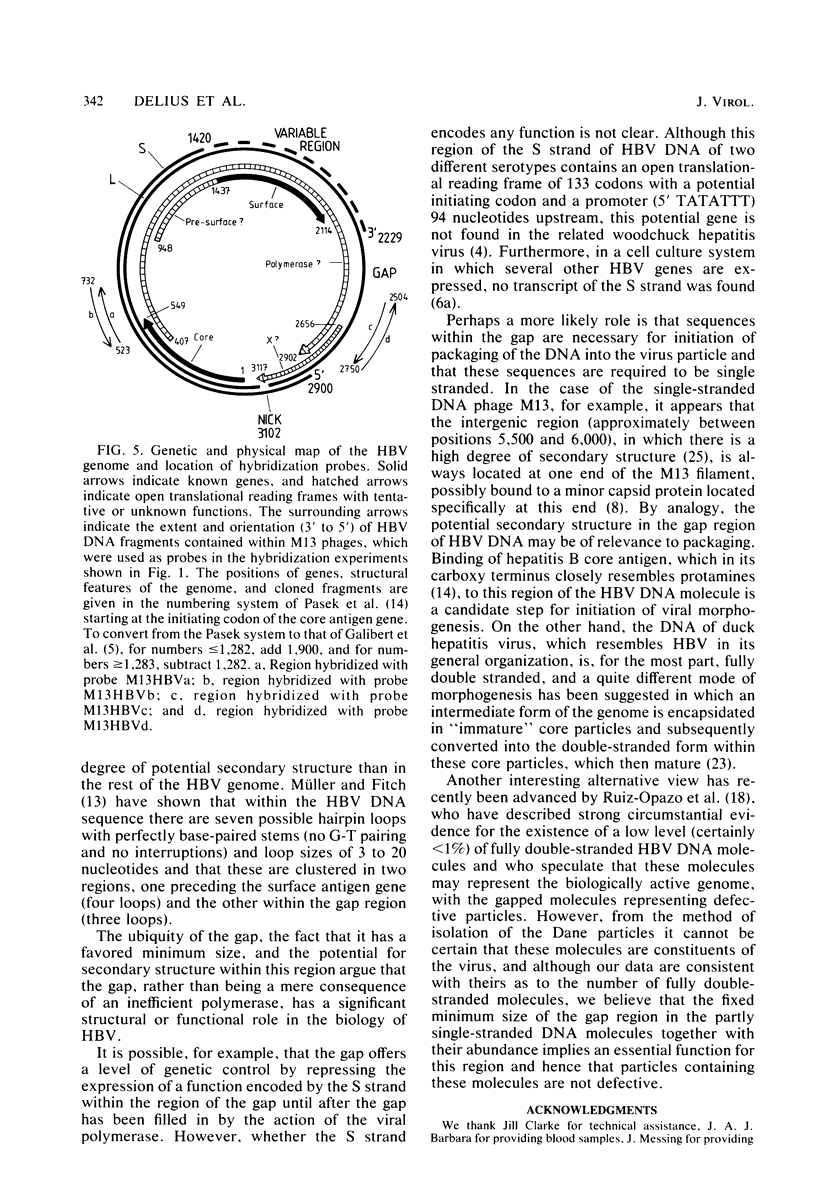

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron C. H., Combridge B. S., Howell D. R., Barbara J. A. A sensitive immunoradiometric assay for the detection of hepatitis B surface antigen. J Virol Methods. 1980;1(6):311–323. doi: 10.1016/0166-0934(80)90048-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Core and E antigen synthesis in rodent cells transformed with hepatitis B virus DNA is associated with greater than genome length viral messenger RNAs. J Mol Biol. 1983 Apr 25;165(4):683–699. doi: 10.1016/s0022-2836(83)80274-5. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Murray K. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J Mol Biol. 1982 Nov 25;162(1):43–67. doi: 10.1016/0022-2836(82)90161-9. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Lin T. C., Webster R. E., Konigsberg W. Structure of filamentous bacteriophage: isolation, characterization, and localization of the minor coat proteins and orientation of the DNA. Prog Clin Biol Res. 1981;64:413–428. [PubMed] [Google Scholar]
- Hruska J. F., Clayton D. A., Rubenstein J. L., Robinson W. S. Structure of hepatitis B Dane particle DNA before and after the Dane particle DNA polymerase reaction. J Virol. 1977 Feb;21(2):666–672. doi: 10.1128/jvi.21.2.666-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers T. A., Greenberg H. B., Robinson W. S. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol. 1977 Aug;23(2):368–376. doi: 10.1128/jvi.23.2.368-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. R., Fitch W. M. Evolutionary selection for perfect hairpin structures in viral DNAs. Nature. 1982 Aug 5;298(5874):582–585. doi: 10.1038/298582a0. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Priess H., Koller B., Hess B., Delius H. Electron microscopic mapping and sequence analysis of the terminator for the early message of E. coli phage T7. Mol Gen Genet. 1980 Apr;178(1):27–34. doi: 10.1007/BF00267209. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Greenman R. L. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974 Jun;13(6):1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Opazo N., Chakraborty P. R., Shafritz D. A. Evidence for supercoiled hepatitis B virus DNA in chimpanzee liver and serum Dane particles: possible implications in persistent HBV infection. Cell. 1982 May;29(1):129–136. doi: 10.1016/0092-8674(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Sattler F., Robinson W. S. Restriction endonuclease cleavage map and location of unique features of the DNA of hepatitis B virus, subtype adw2. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4664–4668. doi: 10.1073/pnas.76.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981 Mar-Apr;1(2):179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]