Abstract
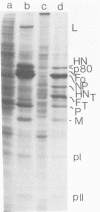
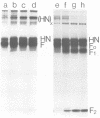
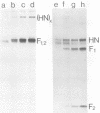
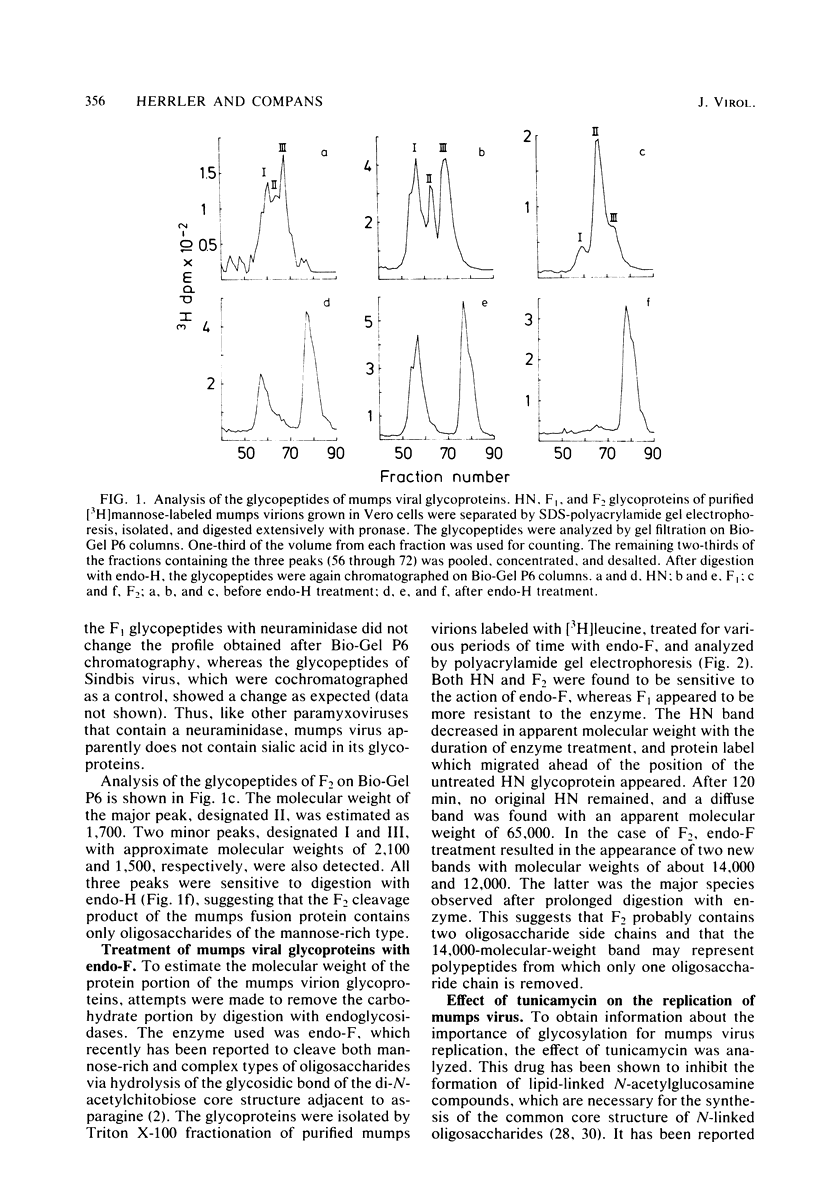
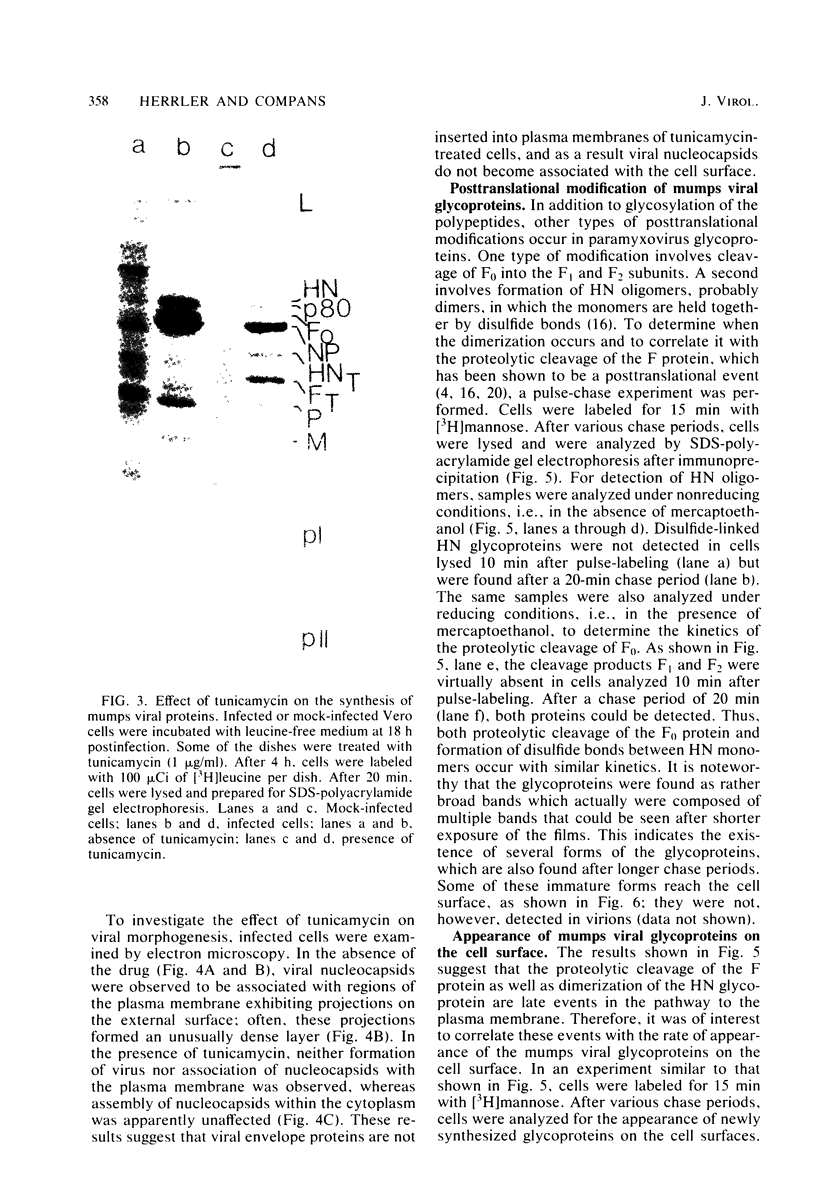
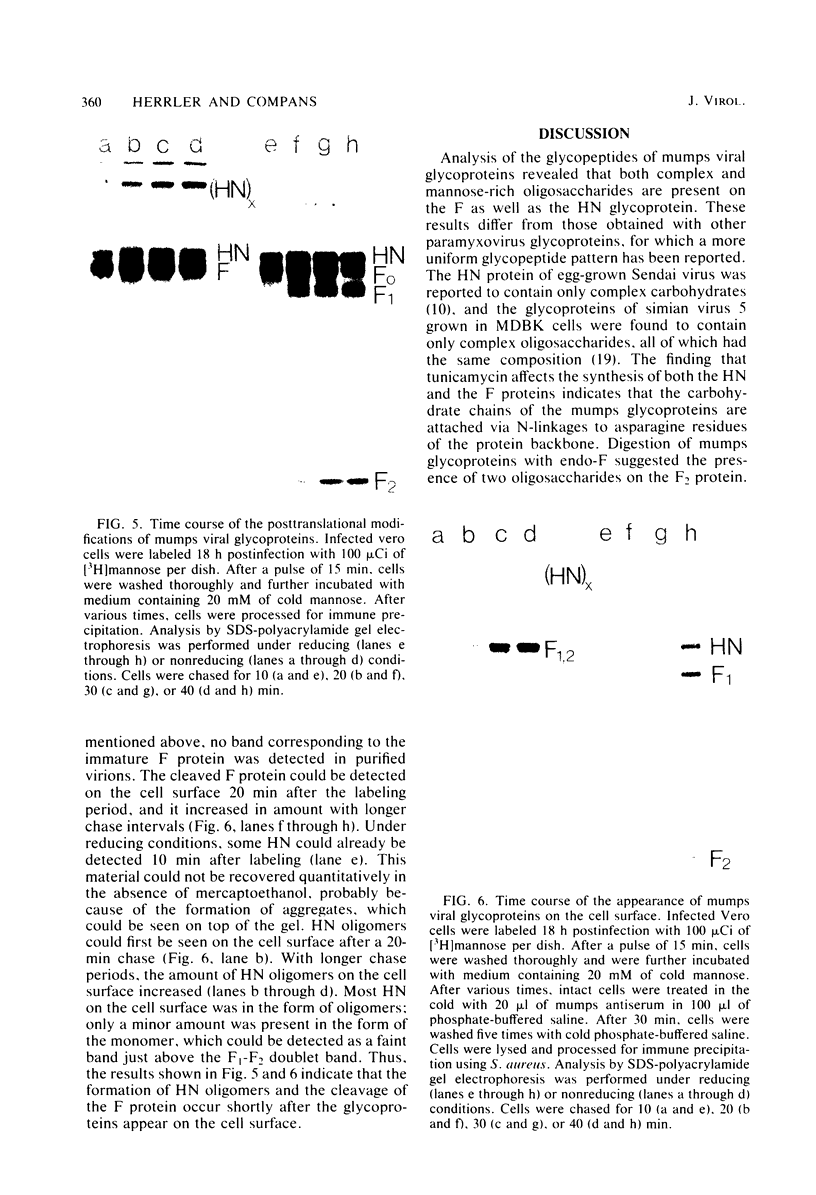
Analysis of the pronase-derived glycopeptides of isolated mumps virus glycoproteins revealed the presence of both complex and high-mannose-type oligosaccharides on the HN and F1 glycoproteins, whereas only high-mannose-type glycopeptides were detected on F2. Endoglycosidase F, a newly described glycosidase that cleaves N-linked high mannose as well as complex oligosaccharides, appeared to completely cleave the oligosaccharides linked to HN and F2, whereas F1 was resistant to the enzyme. Two distinct cleavage products of F2 were observed, suggesting the presence of two oligosaccharide side chains. Tunicamycin was found to reduce the infectious virus yield and inhibit mumps virus particle formation. The two glycoproteins, HN and F, were not found in the presence of the glycosylation inhibitor. However, two new polypeptides were detected, with molecular weights of 63,000 (HNT) and 53,000 (FT), respectively, which may represent nonglycosylated forms of the glycoproteins. Synthesis of the nonglycosylated virus-coded proteins (L, NP, P, M, pI, and pII) was not affected by tunicamycin. The formation of HN oligomers and the proteolytic cleavage of the F protein were found to occur with the same kinetics. Analysis of the time course of appearance of mumps virus glycoproteins on the cell surface suggested that dimerization of HN and cleavage of F occur immediately after their exposure on the plasma membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M., Muramatsu T. Endo-beta-N-acetylglucosaminidases acting on the carbohydrate moieties of glycoproteins. The differential specificities of the enzymes from Streptomyces griseus and Diplococcus pneumoniae. J Biochem. 1974 Aug;76(2):307–317. doi: 10.1093/oxfordjournals.jbchem.a130572. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Herrler G., Compans R. W., Meier-Ewert H. A precursor glycoprotein in influenza C virus. Virology. 1979 Nov;99(1):49–56. doi: 10.1016/0042-6822(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Herrler G., Compans R. W. Synthesis of mumps virus polypeptides in infected Vero cells. Virology. 1982 Jun;119(2):430–438. doi: 10.1016/0042-6822(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Herrler G., Nagele A., Meier-Ewert H., Bhown A. S., Compans R. W. Isolation and structural analysis of influenza C virion glycoproteins. Virology. 1981 Sep;113(2):439–451. doi: 10.1016/0042-6822(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Homma M., Compans R. W. Effect of tunicamycin on the replication of Sendai virus. Virology. 1982 Jun;119(2):474–487. doi: 10.1016/0042-6822(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Naruse H., Nagai Y., Yoshida T., Hamaguchi M., Matsumoto T., Isomura S., Suzuki S. The polypeptides of mumps virus and their synthesis in infected chick embryo cells. Virology. 1981 Jul 15;112(1):119–130. doi: 10.1016/0042-6822(81)90618-8. [DOI] [PubMed] [Google Scholar]
- Orvell C. Immunological properties of purified mumps virus glycoproteins. J Gen Virol. 1978 Dec;41(3):517–526. doi: 10.1099/0022-1317-41-3-517. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Rima B. K., Roberts M. W., McAdam W. D., Martin S. J. Polypeptide synthesis i mumps virus-infected cells. J Gen Virol. 1980 Feb;46(2):501–505. doi: 10.1099/0022-1317-46-2-501. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schwalbe J. C., Hightower L. E. Maturation of the envelope glycoproteins of Newcastle disease virus on cellular membranes. J Virol. 1982 Mar;41(3):947–957. doi: 10.1128/jvi.41.3.947-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. Virus-dependent glycosylation. J Virol. 1975 Jan;17(1):85–93. doi: 10.1128/jvi.17.1.85-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup K. C., Fields B. N. The replication of measles virus in the presence of tunicamycin. Virology. 1981 Jan 30;108(2):391–404. doi: 10.1016/0042-6822(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]