Abstract
The Rho subfamily of the Rho small G protein family (Rho) regulates formation of stress fibers and focal adhesions in many types of cultured cells. In moving cells, dynamic and coordinate disassembly and reassembly of stress fibers and focal adhesions are observed, but the precise mechanisms in the regulation of these processes are poorly understood. We previously showed that 12-O-tetradecanoylphorbol-13-acetate (TPA) first induced disassembly of stress fibers and focal adhesions followed by their reassembly in MDCK cells. The reassembled stress fibers showed radial-like morphology that was apparently different from the original. We analyzed here the mechanisms of these TPA-induced processes. Rho inactivation and activation were necessary for the TPA-induced disassembly and reassembly, respectively, of stress fibers and focal adhesions. Both inactivation and activation of the Rac subfamily of the Rho family (Rac) inhibited the TPA-induced reassembly of stress fibers and focal adhesions but not their TPA-induced disassembly. Moreover, microinjection or transient expression of Rab GDI, a regulator of all the Rab small G protein family members, inhibited the TPA-induced reassembly of stress fibers and focal adhesions but not their TPA-induced disassembly, indicating that, furthermore, activation of some Rab family members is necessary for their TPA-induced reassembly. Of the Rab family members, at least Rab5 activation was necessary for the TPA-induced reassembly of stress fibers and focal adhesions. The TPA-induced, small G protein-mediated reorganization of stress fibers and focal adhesions was closely related to the TPA-induced cell motility. These results indicate that the Rho and Rab family members coordinately regulate the TPA-induced reorganization of stress fibers and focal adhesions that may cause cell motility.
INTRODUCTION
Cell migration plays a central role in a wide variety of biological phenomena, such as inflammatory response, wound healing, organogenesis, and metastasis of malignant cancer (reviewed by Stoker and Gherardi, 1990; Roy and Mareel, 1992). In moving cells, membrane protrusions, such as lamellipodia and filopodia, at the cell front, and retraction at the cell rear are externally observed, whereas reorganization of the actin cytoskeleton, such as disassembly and reassembly of stress fibers and focal adhesions, is internally observed. These processes are dynamic, well coordinated, and essential for cell migration, but the mechanisms of these processes are not fully understood. In the case of epithelial and endothelial cells, they adhere to each other to form cell–cell junctions, including adherens junction, tight junction, desmosome, and gap junction, in addition to cell–matrix junction, and these cell–cell junctions are furthermore disrupted for migration. Some growth factors, such as hepatocyte growth factor/scatter factor (HGF/SF),1 disrupt cell–cell junctions and induce cell migration, but their modes of action in these processes have not been clarified.
The Rho small G protein family regulates various cell functions, including cell migration, through reorganization of the actin cytoskeleton (reviewed by Hall, 1994, 1998; Takai et al., 1995). The Rho family consists of the Rho, Rac, and Cdc42 subfamilies. The Rho subfamily (Rho), consisting of three members, RhoA, -B, and -C, regulates formation of stress fibers and focal adhesions in many types of cells; the Rac subfamily (Rac), consisting of two members, Rac1 and -2, regulates formation of lamellipodia and membrane ruffling; and the Cdc42 subfamily (Cdc42), consisting of G25K and Cdc42Hs, regulates formation of filopodia (reviewed by Hall, 1994, 1998; Takai et al., 1995). Three laboratories, including our own, have shown that, furthermore, Rac regulates formation of cadherin-dependent cell–cell adhesion in MDCK cells and human keratinocytes (Braga et al., 1997; Hordijk et al., 1997; Takaishi et al., 1997). In addition, Braga et al. (1997) have shown that Rho is also involved in the formation of cell–cell adhesion in human keratinocytes, but we have shown that Rho is indirectly involved in formation of cell–cell adhesion in MDCK cells (Takaishi et al., 1997).
The Rab small G protein family (Rab) consists of more than 30 members and regulates intracellular vesicle trafficking (reviewed by Simons and Zerial, 1993; Nuoffer and Balch, 1994; Pfeffer, 1994; Novick and Zerial, 1997). Of the Rab family members, Rab5 regulates early endocytosis (Bucci et al., 1992; Stenmark et al., 1994), Rab11 regulates recycling through the pericentriolar recycling endosome (Ullrich et al., 1996), and Rab8 regulates vesicular traffic from trans-Golgi network to the basolateral plasma membrane (Huber et al., 1993). Because recycling of the plasma membrane components, such as integrins, by vesicle trafficking is important for cell migration (Martenson et al., 1993; Lawson and Maxfield, 1995; Bretcher, 1996; reviewed by Lauffenburger and Horwitz, 1996), it is supposed that Rab also plays an important role in cell migration. However, the role of Rab in cell migration has not yet been investigated.
The Rho and Rab families cycle between the GDP-bound inactive and GTP-bound active forms, which are regulated by three types of regulators, GDP/GTP exchange protein (GEP), GTPase activating protein (GAP), and GDP dissociation inhibitor (GDI) (reviewed by Hall, 1994, 1998; Takai et al., 1995, 1996; Novick and Zerial, 1997). Of these regulators, Rho and Rab GDIs are general regulators of all the Rho and Rab family members, respectively, whereas GEP and GAP are specific for each Rho or Rab subfamily. GEP stimulates the conversion from the GDP-bound form to the GTP-bound form, and GAP stimulates the reverse conversion (reviewed by Takai et al., 1995, 1996; Novick and Zerial, 1997; Hall, 1998). GDI has three activities (reviewed by Takai et al., 1995, 1996; Novick and Zerial, 1997): 1) GDI keeps the GDP-bound form in the cytosol; 2) GDI transports its complexed small G protein to its respective target membrane where the GDP-bound form is converted to the GTP-bound form; and 3) Once the GDP-bound form is converted from the GTP-bound form after its function has been accomplished, GDI forms a complex with it and translocates it to the cytosol. We have shown that microinjection of Rho GDI inhibits the functions of the Rho family members, such as cytokinesis (Kishi et al., 1993), cell motility (Takaishi et al., 1993, 1994), membrane ruffling (Nishiyama et al., 1994), and formation of stress fibers and focal adhesions (Kotani et al., 1997). It is predicted that microinjection or overexpression of Rab GDI inhibits the functions of the Rab family members, but this type of experiments has not been performed thus far.
HGF/SF induces cell migration in many cultured epithelial cells including MDCK cells (reviewed by Gherardi and Stoker, 1991). We have previously shown that 12-O-tetradecanoylphorbol-13-acetate (TPA), an activator of protein kinase C, also induces the scattering of MDCK cells and that TPA first induces disassembly of stress fibers and focal adhesions followed by their reassembly in MDCK cells (Takaishi et al., 1995). The reassembled stress fibers show radial-like morphology, which apparently differs from the original. In the present study, we have examined whether the Rho and Rab family members are involved in these TPA-induced processes. We have found here that the Rho and Rab family members coordinately regulate the TPA-induced reorganization of stress fibers and focal adhesions.
MATERIALS AND METHODS
Materials and Chemicals
MDCK cells were kindly supplied by Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany). MDCK cell lines stably expressing a dominant active mutant of RhoA (V14RhoA) or Rac1 (V12Rac1) and a dominant negative mutant of Rac1 (N17Rac1) were established as described previously (Takaishi et al., 1997). Human recombinant HGF/SF was provided by Dr. T. Nakamura (Osaka University, Suita, Japan). TPA was obtained from Sigma Chemical (St. Louis, MO). The cDNAs of a dominant active mutant of Rab5 (Rab5DA) with a mutation of amino acid 79 from Gln to Leu (L79Rab5), a dominant negative mutant of Rab5 (Rab5DN) with a mutation of amino acid 34 from Ser to Asn (N34Rab5), and Rab8 were provided by Dr. M. Zerial (European Molecular Biology Laboratory, Heidelberg, Germany). The fragments containing dominant active mutants of Rab8 and -11 (Rab8DA and -11DA, respectively) were obtained by PCR mutagenesis of Gln to Leu at codon 67 of Rab8 (L67Rab8) and Gln to Leu at codon 70 of Rab11 (L70Rab11), respectively. The fragments containing the dominant negative mutants of Rab8 and -11 (Rab8DN and -11DN, respectively) were obtained by PCR mutagenesis of Thr to Asn at codon 22 of Rab8 (N22Rab8) and Ser to Asn at codon 25 of Rab11 (N25Rab11), respectively. The pSRαneo and pEF-BOS expression plasmids were donated by Dr. A. Miyajima (Tokyo University, Tokyo, Japan) and Dr. S. Nagata (Osaka University, Osaka, Japan), respectively. The guanosine 5′-(3-O-thio)-triphosphate (GTPγS)-bound form of RhoA was made as described previously (Takaishi et al., 1993). C3 was supplied by Dr. S. Narumiya (Kyoto University, Kyoto, Japan). Rab GDI was purified as a His6 fusion protein from Escherichia coli, which overexpressed His6-tagged Rab GDI (His6-Rab GDI) according to the manufacturer’s protocol. Hybridoma cells expressing the anti-myc mouse mAb (9E10) were purchased from American Type Culture Collection (Rockville, MD). An anti-vinculin mouse mAb (V115) was obtained from Sigma Chemical. Second antibodies for immunofluorescence microscopy were obtained from Chemicon International (Temecula, CA).
Construction of Expression Plasmids of Rab Mutants and Rab GDI
Expression vectors were constructed in pSRαneo or pEF-BOS using standard molecular biology methods. The pSRαneo-myc-tagged (pSRα-myc-) and pEF-BOS-myc-tagged (pEF-BOS-myc-) L79Rab5, N34Rab5, L67Rab8, N22Rab8, L70Rab11, and N25Rab11, and pEF-BOS-myc-Rab GDI were constructed as described previously (Komuro et al., 1996; Takaishi et al., 1997). The fragment containing L79Rab5-, N34Rab5-, L67Rab8-, N22Rab8-, L70Rab11-, N25Rab11-, or Rab GDI-coding sequences with the BamHI site upstream of the initiation methionine codon and downstream of the termination codon was synthesized by PCR. These fragments were digested by BamHI and ligated into the BamHI site of the pSRα-myc or pEF-BOS-myc plasmid.
Cell Culture, Transfection, and Microinjection
MDCK cells were maintained at 37°C in a humidified atmosphere of 10% CO2 and 90% air in DMEM containing 10% FCS (Life Technologies, Grand Island, NY), 100 U/ml penicillin, and 100 μg/ml streptomycin. Transient transfection of pEF-BOS-myc-L79Rab5, -N34Rab5, -L67Rab8, -N22Rab8, -L70Rab11, -N25Rab11, or -Rab GDI was carried out using a lipofectAMINE reagent as described previously (Komuro et al., 1996). At 24 h after the transfection, the cells were detached using an EDTA/trypsin solution, seeded onto 35-mm grid dishes, and further incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with 100 nM TPA. Stable transfection of pSRα-myc-N34Rab5, -L70Rab11, or -N25Rab11 was carried out using a lipofectAMINE reagent, and cell clones were isolated by resistance to G418 as described previously (Takaishi et al., 1997). MDCK cells for the microinjection experiments were seeded at a density of 1 × 105 cells per dish onto 35-mm grid dishes. At 24 h after seeding, C3, His6-Rab GDI, or GTPγS-RhoA was comicroinjected along with a marker protein (rat IgG) into the cytoplasm of the cells and then returned to the incubator for 30 min before TPA stimulation or fixation. Thirty to 50 cells were microinjected in each experiment, and more than 90% of the cells showed the same response.
Immunofluorescence Microscopy
Cells were fixed in 3.7% paraformaldehyde in PBS for 20 min. The fixed cells were incubated for 10 min with 50 mM ammonium chloride in PBS and permeabilized with PBS containing 0.2% Triton X-100 for 10 min. After the cells were soaked in 10% FCS/PBS for 30 min, they were treated with the first antibodies in 10% FCS/PBS for 1 h. The cells were then washed with PBS three times, followed by incubation with the second antibodies in 10% FCS/PBS for 1 h. For the detection of actin filaments, rhodamine-phalloidin was mixed with the second antibody solution. For the double staining, the second antibodies that did not cross-react with each other were chosen. After the cells were washed with PBS three times, they were examined using a LSM 410 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
RESULTS
TPA and HGF/SF Induce Reorganization of the Actin Cytoskeleton in MDCK Cells
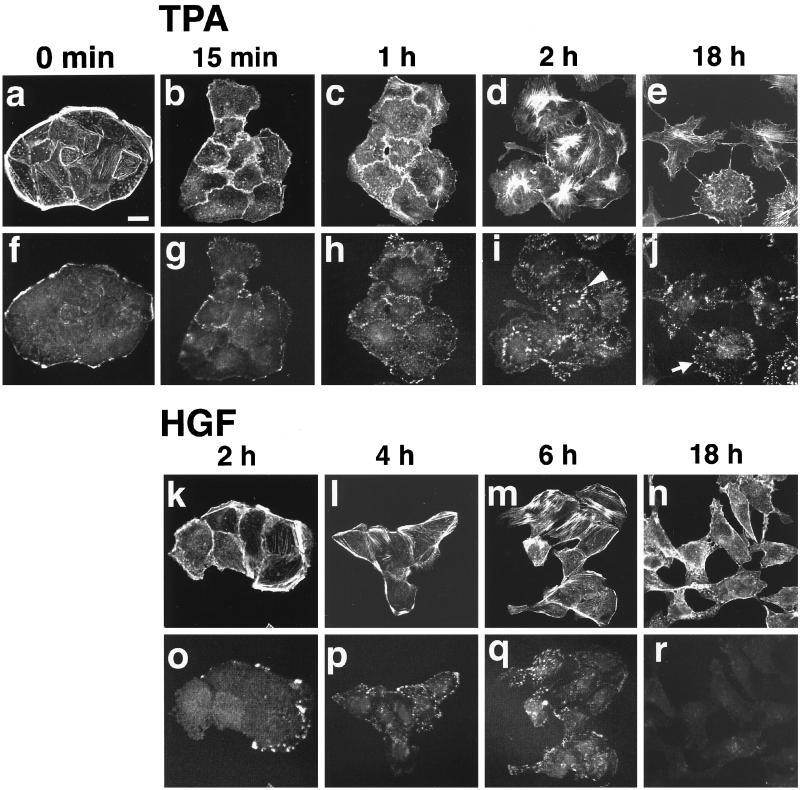
Confocal microscopic analysis of cultured MDCK cells at the basal levels showed that the cells contacted with each other, forming colonies of the cells (Figure 1a). In these cells, actin filaments showed three different structures localized at three different areas: weak stress fibers that ran parallel throughout the cells, heavy peripheral bundles that were localized at the edges of the colonies, and weak cortical bundles that were localized at the cell–cell adhesion sites (Figure 1a). Vinculin was stained both at the basal edges of the colonies and at focal adhesions, which were localized at the end of the stress fibers (Kotani et al., 1997) (Figure 1f).
Figure 1.
TPA- and HGF/SF-induced reorganization of the actin cytoskeleton in wt MDCK cells. wt MDCK cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with none (a and f), 100 nM TPA (b–e and g–j), or 20 ng/ml HGF/SF (k–r). The cells were fixed at 15 min (b and g), 1 h (c and h), 2 h (d and i), or 18 h (e and j) after TPA stimulation, and at 2 h (k and o), 4 h (l and p), 6 h (m and q), or 18 h (n and r) after HGF/SF stimulation, double stained with rhodamine-phalloidin (a–e and k–n) or the V115 anti-vinculin mAb (f–j and o–r), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm. An arrowhead in panel i indicates the staining of vinculin at the newly formed focal adhesions. An arrow in panel j indicates the dot-like staining of vinculin that is located at the sites different from the focal adhesions.
Stimulation of MDCK cells with TPA caused cell spreading within 15 min followed by dissociation and scattering of the cells at 2 h (Figure 1, b–d). At 18 h, the cells completely dissociated from and did not contact with each other (Figure 1e). The membranes of most cells started to ruffle within 15 min (Figure 1b), and the membrane ruffling was always observed, at least in a part of the cells, during the stimulation (Figure 1, b–e). The peripheral bundles decreased within 15 min and mostly disappeared at 1 h. The stress fibers mostly disappeared within 15 min, but reappeared in a part of the cells at 1 h and in most cells at 2 h (Figure 1, b–d). Even at 18 h, the newly formed stress fibers were still observed in a part of the cells (Figure 1e). The newly formed stress fibers showed radial-like morphology that apparently differed from the original (Figure 1, a and d). Stimulation of the cells with TPA, furthermore, caused decrease of vinculin at the basal edges of the colonies within 15 min. Dot-like staining of vinculin at the basal edges of the cells, which was located at the sites different from the focal adhesions, was observed between 1 h and 18 h, and it became prominent at 18 h (Figure 1, g–j). Most vinculin at the focal adhesions disappeared at 15 min, and it began to increase at 1 h, and the strong staining of vinculin at the focal adhesions was observed at 2 h (Figure 1, g–i). These newly formed focal adhesions were located at the edges of the newly formed radial stress fibers.
Stimulation of MDCK cells with HGF/SF showed similar reorganization of stress fibers and focal adhesions, but their time courses were different from those of the TPA-induced stimulation. Stimulation of the cells with HGF/SF caused spreading of the cells without dissociation of the cells during the first 4 h (Figure 1, k and l). Between 6 h and 18 h, the cell–cell contacts were disrupted, and the cells scattered (Figure 1, m and n). These results are consistent with previous observations (Ridley et al., 1995). Reorganization of the actin cytoskeleton was also observed (Figure 1, k–n). The formation of membrane ruffling was always observed in a part of the cells during the HGF/SF stimulation. Peripheral bundles decreased within 2 h and completely disappeared between 6 h and 18 h. Stress fibers decreased within 2 h, increased in a part of the cells between 4 h and 6 h, and mostly disappeared at 18 h. The morphology of the newly formed stress fibers was not identical but similar to that induced by TPA. The time course of the localization of vinculin was similar to that of the disassembly and reassembly of the stress fibers (Figure 1, o–r). The localization of vinculin at the basal edges of the colonies of the cells decreased within 2 h and disappeared between 6 h and 18 h. The localization of vinculin at the focal adhesions decreased within 2 h, increased between 4 h and 6 h, and decreased again at 18 h.
Cycloheximide, an inhibitor of protein synthesis, has previously been reported to inhibit the HGF/SF-induced cell scattering (Rosen et al., 1990). We next examined the effect of cycloheximide on the TPA- and HGF/SF-induced cell scattering and reorganization of the actin cytoskeleton. As reported previously (Rosen et al., 1990), the HGF/SF-induced cell scattering was completely inhibited by cycloheximide (Figure 2, a and b). However, the TPA-induced cell scattering, membrane ruffling, and disassembly or reassembly of focal adhesions and stress fibers were not inhibited by cycloheximide (Figure 2, c–f), indicating that the TPA-induced motile response and reorganization of the actin cytoskeleton do not require protein synthesis.
Figure 2.
Effect of cycloheximide on the TPA- and HGF/SF-induced reorganization of actin filaments in wt MDCK cells. wt MDCK cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with 20 ng/ml HGF/SF (a and b) or 100 nM TPA (c–f) in the absence (a) or presence (b–f) of 10 μg/ml cycloheximide. Cycloheximide was added 30 min before HGF/SF or TPA stimulation. At 18 h after HGF/SF stimulation, the cells were fixed and stained with rhodamine-phalloidin (a and b). At 15 min (c and d) or 2 h (e and f) after TPA stimulation, the cells were fixed, double stained with rhodamine-phalloidin (c and e) or the V115 anti-vinculin mAb (d and f), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm.
To further clarify the mechanisms of the disassembly and reassembly of stress fibers and focal adhesions in motile cells, we analyzed the TPA-induced reorganization of the actin cytoskeleton for the following reasons: 1) The TPA-induced motile response was apparently faster than the HGF/SF-induced one; 2) The TPA-induced disassembly and reassembly of stress fibers and focal adhesions were synchronized in most MDCK cells, whereas the HGF/SF-induced ones were not synchronized; and 3) The TPA-induced motile response and reorganization of the actin cytoskeleton did not require protein synthesis, whereas the HGF/SF-induced ones did, suggesting that the mechanisms of the HGF/SF-induced processes are more complicated than those of the TPA-induced processes. The TPA-induced disassembly of stress fibers and focal adhesions was analyzed at 15 min after the stimulation and the TPA-induced reassembly of stress fibers and focal adhesions was analyzed at 2 h.
Rho Inactivation Is Necessary for the TPA-induced Disassembly of Stress Fibers and Focal Adhesions
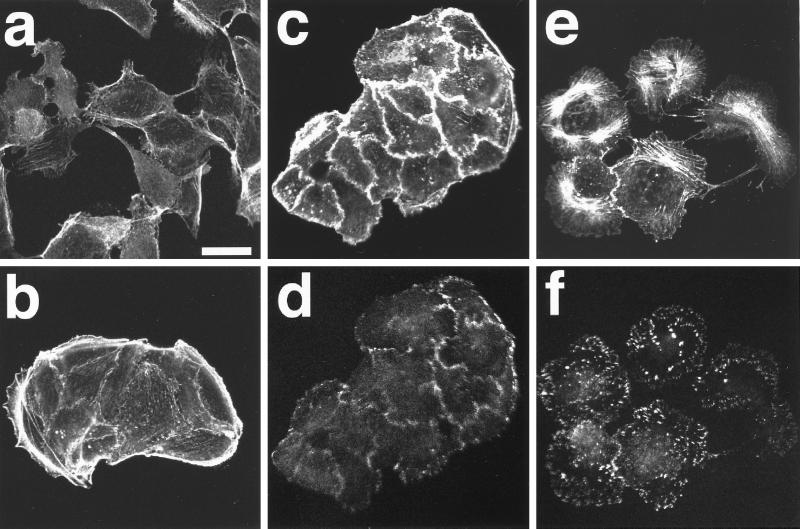
To study the effect of Rho inactivation on the TPA-induced effects, we took advantage of the MDCK cell lines stably expressing the dominant active mutant of RhoA (sMDCK-RhoDA) that were established in our preceding paper (Takaishi et al., 1997). In sMDCK-RhoDA cells, strong formation of stress fibers was observed as described (Takaishi et al., 1997) (Figure 3a). Stimulation of the cells with TPA for 15 min did not reduce the stress fibers (Figure 3b). The cells slightly spread and the colonies became larger, but the strong formation of stress fibers did not change even at 2 h (Figure 3c). The morphology of stress fibers after the stimulation in sMDCK-RhoDA cells was not apparently different from that before the stimulation, but was different from the TPA-induced radial-type one in wild-type (wt) MDCK cells (see Figure 1d). Stimulation of the cells with TPA for both 15 min and 2 h slightly reduced the peripheral bundles of actin filaments (Figure 3, b and c) and induced weak membrane ruffling (our unpublished results) in a part of the cells. Accumulation of vinculin at the focal adhesions in sMDCK-RhoDA cells was much more than that in the wt cells as described (Takaishi et al., 1997), and the stimulation of sMDCK-RhoDA cells with TPA for 2 h did not affect the distribution pattern or the accumulation level of vinculin (Figure 3, d–f). The TPA-induced cell scattering was also inhibited in sMDCK-RhoDA cells at 2 h (Figure 3c). sMDCK-RhoDA cells slightly scattered at 6 h, but TPA was apparently less effective on the scattering response in sMDCK-RhoDA cells than in wt MDCK cells (our unpublished results). These results are consistent with the earlier observations that Rho activation enhances the formation of stress fibers and focal adhesions (Ridley and Hall, 1992; Self et al., 1993), and indicate that Rho inactivation is necessary for the TPA-induced disassembly of stress fibers and focal adhesions and cell scattering.
Figure 3.
Inhibition of the TPA-induced disassembly of stress fibers and focal adhesions in sMDCK-RhoDA cells. sMDCK-RhoDA cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with none (a and d) or 100 nM TPA (b, c, e, and f). The cells were fixed at 15 min (b and e) or 2 h (c and f) after TPA stimulation, double stained with rhodamine-phalloidin (a–c) or the V115 anti-vinculin mAb (d–f), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm. Arrowheads in panels b and c indicate the reduced peripheral bundles.
In this paper we examined the effect of the stable expression of the dominant active mutant of RhoA, but not that of RhoB or -C. However, because the expression of a dominant active mutant of either RhoA, -B, or -C induced the formation of stress fibers in MDCK cells (Adamson et al., 1992), the effect of the stable expression of a dominant active mutant of either RhoB or -C may be similar to that of RhoA in MDCK cells.
Rho Activation Is Necessary for the TPA-induced Reassembly of Stress Fibers and Focal Adhesions
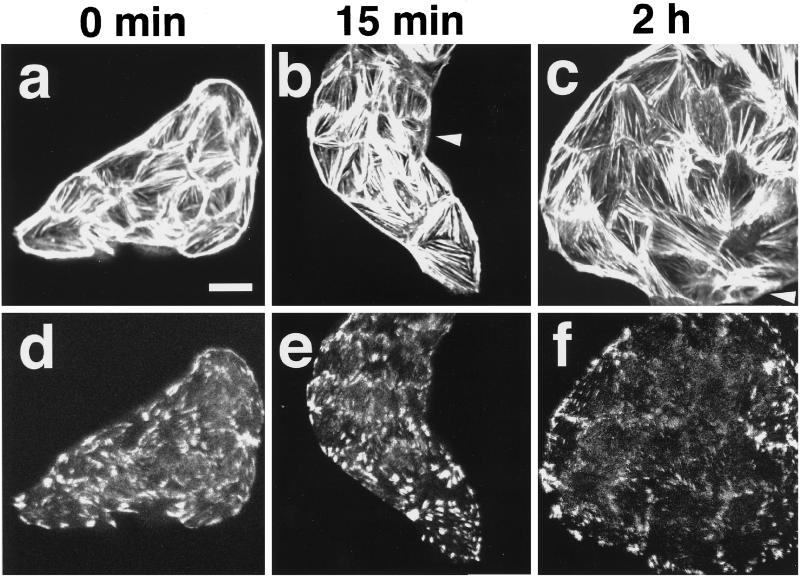
To study the effect of Rho inactivation on the TPA-induced effects, we used C3, a Clostridium botulinum exoenzyme known to ADP ribosylate Rho (Aktories et al., 1988; Kikuchi et al., 1988; Narumiya et al., 1988; Braun et al., 1989), to inhibit its functions, because we could not obtain the MDCK cell lines stably expressing a dominant negative mutant of RhoA. Microinjection of C3 into wt MDCK cells induced disappearance of stress fibers and peripheral bundles, which is consistent with earlier observations (Kotani et al., 1997) (Figure 4, a and e). At 15 min after TPA stimulation, the staining pattern of the actin filaments in the microinjected cells was indistinguishable from that in the unmicroinjected cells (Figure 4, b and f), but at 2 h after the stimulation, neither the TPA-induced reassembly of stress fibers nor the staining of vinculin at the focal adhesions was observed in the microinjected cells (Figure 4, c, d, g, and h). These results indicate that Rho activation is necessary for the TPA-induced reassembly of radial stress fibers and focal adhesions.
Figure 4.
Inhibition by C3 of the TPA-induced reassembly of stress fibers and focal adhesions. wt MDCK cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were microinjected with 40 μg/ml C3 plus 5 mg/ml rat IgG. At 30 min after the microinjection, the cells were stimulated with none (a and e) or 100 nM TPA (b–d and f–h). At 15 min (b and f) or 2 h (c, d, g, and h) after TPA stimulation, the cells were fixed, stained with rhodamine-phalloidin (a–c) or the V115 anti-vinculin mAb (d), and analyzed by confocal microscopy. The microinjected cells are shown by the staining of microinjected rat IgG (e–h). Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm.
Rac Activation and Inactivation Inhibit the TPA-induced Reassembly of Stress Fibers and Focal Adhesions but Not Their TPA-induced Disassembly
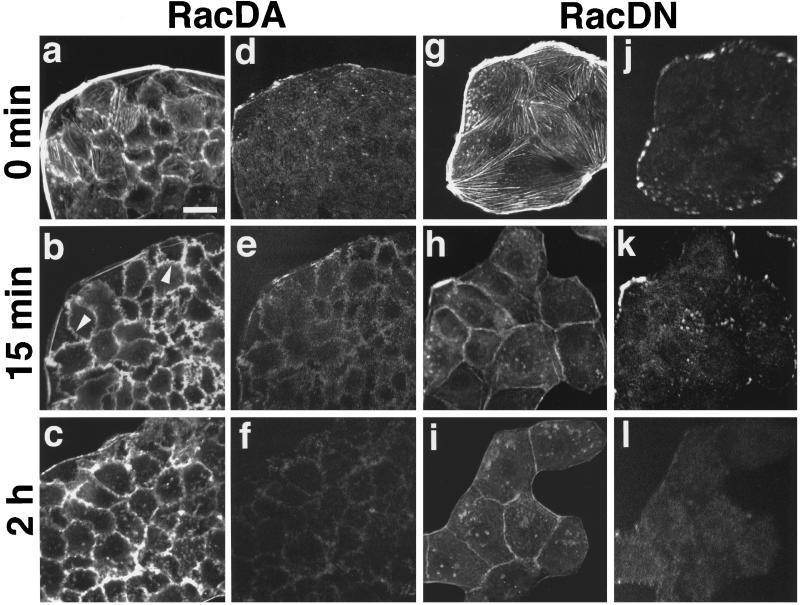
To study the effect of Rac activation and inactivation on the TPA-induced effects, we took advantage of the MDCK cell lines stably expressing the dominant active mutant of Rac1 (sMDCK-RacDA) or the dominant negative mutant of Rac1 (sMDCK-RacDN), which were established in our preceding paper (Takaishi et al., 1997). In sMDCK-RacDA and -RacDN cells, actin filaments at the cell–cell adhesion sites markedly increased and decreased, respectively, as described (Takaishi et al., 1997). The stress fibers and the peripheral bundles were observed in both sMDCK-RacDA and -RacDN cells (Figure 5, a and g). Stimulation of sMDCK-RacDA cells with TPA for 15 min caused formation of membrane ruffling, which was prominently observed at the cell–cell adhesion sites, and disappearance of the stress fibers and the peripheral bundles (Figure 5b). The TPA-induced reassembly of radial stress fibers was not observed at 2 h after the stimulation (Figure 5c). Confocal microscopic analysis at the junctional levels showed that the increased localization of actin filaments at the cell–cell adhesion sites continued even at 2 h after the stimulation (our unpublished results). The weak staining of vinculin at the focal adhesion sites disappeared at 15 min and 2 h (Figure 5, d–f). The TPA-induced cell scattering was also inhibited at 2 h (Figure 5c) and 6 h (our unpublished results).
Figure 5.
Inhibition of the TPA-induced reassembly of stress fibers and focal adhesions in sMDCK-RacDA and -RacDN cells. sMDCK-RacDA and -RacDN cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, sMDCK-RacDA (a–f) and -RacDN (g–l) cells were stimulated with none (a, d, g, and j) or 100 nM TPA (b, c, e, f, h, i, k, and l). At 15 min (b, e, h, and k) or 2 h (c, f, i, and l) after TPA stimulation, the cells were fixed, double stained with rhodamine-phalloidin (a–c and g–i) or the V115 anti-vinculin mAb (d–f and j–l), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm. Arrowheads in panel b indicate membrane ruffling at the cell–cell adhesion sites.
Stimulation of sMDCK-RacDN cells with TPA for 15 min did not induce membrane ruffling, but reduced the formation of stress fibers and the peripheral bundles (Figure 5h). The stress fibers and the peripheral bundles observed at 2 h were similar to those at 15 min, and the TPA-induced assembly of stress fibers was not observed (Figure 5i). Moreover, the TPA-induced dissociation of the cell–cell adhesion was not observed at 2 h. The staining of vinculin at the focal adhesions and the basal edges of the colonies decreased at both 15 min and 2 h (Figure 5, j–l). The TPA-induced cell scattering was also inhibited at 2 h (Figure 5i) and 6 h (our unpublished results).
These results indicate that cyclical activation and inactivation of Rac are necessary for the TPA-induced reassembly of stress fibers and focal adhesions and cell scattering and that neither activation nor inactivation of Rac is necessary for their TPA-induced disassembly.
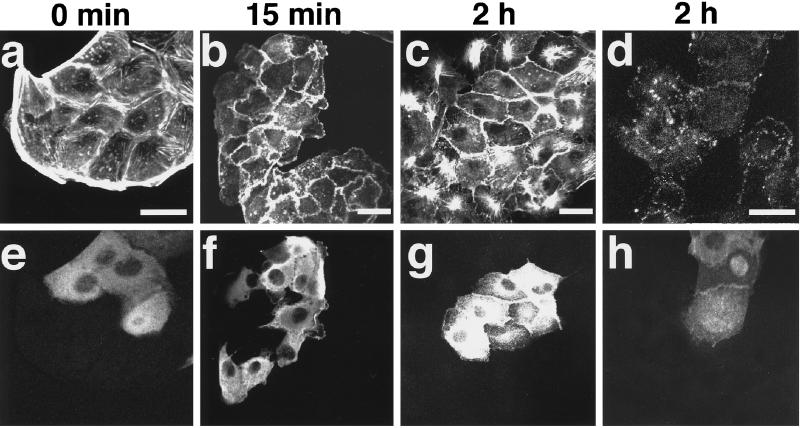
Rab GDI Inhibits the TPA-induced Assembly of Stress Fibers and Focal Adhesions but Not Their TPA-induced Disassembly
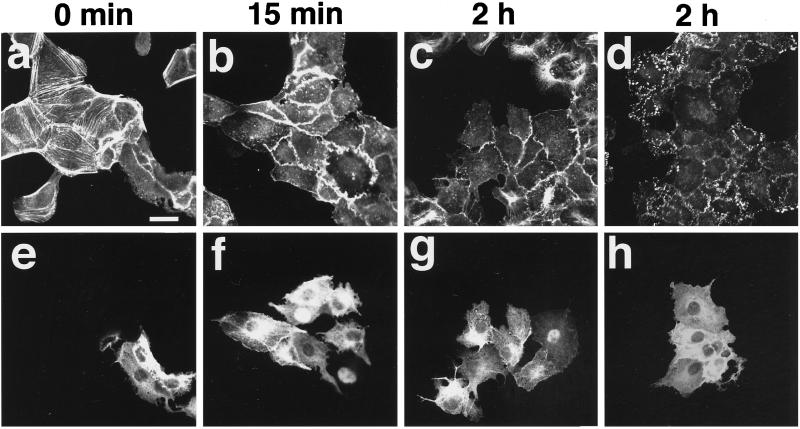
Microinjection of Rho GDI into cultured cells inhibits various functions of its substrate small G proteins (Kishi et al., 1993; Takaishi et al., 1993, 1994; Nishiyama et al., 1994; Kotani et al., 1997). By analogy with this, we microinjected Rab GDI into wt MDCK cells and examined its effect on the TPA-induced reorganization of the actin cytoskeleton. Microinjection of Rab GDI into wt MDCK cells did not apparently affect the actin cytoskeleton (Figure 6, a and e). The TPA-induced formation of membrane ruffling and disappearance of stress fibers and peripheral bundles were similarly observed at 15 min after the stimulation in the Rab GDI-microinjected cells (Figure 6, b and f). However, at 2 h after stimulation, the TPA-induced reassembly of stress fibers was not observed in the Rab GDI-microinjected cells (Figure 6, c and g). The staining of vinculin at the newly formed focal adhesions was not observed at 2 h in the Rab GDI-microinjected cells (Figure 6, d and h). Overexpression of Rab GDI in wt MDCK cells by the transient expression method also inhibited the TPA-induced reassembly of stress fibers at 2 h, but not their TPA-induced disassembly at 15 min (Figure 7). These results indicate that activation of some Rab family members is necessary for the TPA-induced reassembly of stress fibers and focal adhesions but not for their disassembly.
Figure 6.
Inhibition by Rab GDI of the TPA-induced reassembly of stress fibers and focal adhesions. wt MDCK cells were incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were microinjected with 5 mg/ml His6-Rab GDI plus 5 mg/ml rat IgG. At 30 min after the microinjection, the cells were stimulated with none (a and e) or 100 nM TPA (b–d and f–h). At 15 min (b and f) or 2 h (c, d, g, and h) after TPA stimulation, the cells were fixed, stained with rhodamine-phalloidin (a–c) or the V115 anti-vinculin mAb (d), and analyzed by confocal microscopy. The microinjected cells are shown by the staining of microinjected rat IgG (e–h). Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bars, 10 μm.
Figure 7.
Effect of transient expression of Rab GDI, Rab5DA, -5DN, -8DA, -8DN, -11DA, and -11DN on the TPA-induced disassembly and reassembly of stress fibers. wt MDCK cells were transfected with pEF-BOS-myc-Rab GDI, -L79Rab5 (Rab5DA), -N34Rab5 (Rab5DN), -L67Rab8 (Rab8DA), -N22Rab8 (Rab8DN), -L70Rab11 (Rab11DA), or -N25Rab11 (Rab11DN) using a lipofectAMINE reagent. At 24 h after the transfection, the cells were detached using an EDTA/trypsin solution, seeded onto 35-mm grid dishes, and further incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with 100 nM TPA and fixed at 15 min (A) or 2 h (B) after TPA stimulation. The cells were then double stained with rhodamine-phalloidin or the 9E10 anti-myc mAb to detect the transfected cells, and analyzed by confocal microscopy. (A) The percentage of the cells in which disassembly of stress fibers was observed at 15 min after TPA stimulation. (B) The percentage of the cells in which reassembly of stress fibers was observed at 2 h after TPA stimulation. None, untransfected cells; Rab GDI, the cells expressing myc-Rab GDI; Rab5DA, the cells expressing myc-L79Rab5; Rab5DN, the cells expressing myc-N34Rab5; Rab8DA, the cells expressing myc-L67Rab8; Rab8DN, the cells expressing myc-N22Rab8; Rab11DA, the cells expressing myc-L70Rab11; Rab11DN, the cells expressing myc-N25Rab11. The results shown are mean obtained from at least three independent experiments.
Rab5 Activation Is Necessary for the TPA-induced Reassembly of Stress Fibers and Focal Adhesions but Not for Their TPA-induced Disassembly
We next examined which member of the Rab subfamily is involved in the TPA-induced reassembly of stress fibers and focal adhesions. We chose Rab5, -8, and -11 for further analysis, because their functions thus far reported (Bucci et al., 1992; Huber et al., 1993; Stenmark et al., 1994; Ullrich et al., 1996) suggest that they regulate the transport of integrins, which is involved in the stress fiber formation (Hotchin and Hall, 1995; Lawson and Maxfield, 1995). We transfected transiently the plasmid expressing Rab5DA, -5DN, -8DA, -8DN, -11DA, or -11DN into wt MDCK cells and stimulated the cells with TPA for 15 min or 2 h (Figures 7 and 8). The expression of these mutants affected neither the actin cytoskeleton in the cells that were not stimulated with TPA (our unpublished results) nor the disassembly of stress fibers in the cells that were stimulated with TPA for 15 min (Figure 7). The expression of Rab5DN apparently inhibited the TPA-induced reassembly of stress fibers at 2 h, whereas the expression of Rab11DN inhibited their TPA-induced reassembly to a small extent (Figure 7 and Figure 8, c, d, k, and l).
Figure 8.
Inhibition of the TPA-induced reassembly of stress fibers by transient expression of Rab5DN. wt MDCK cells were transfected with pEF-BOS-myc-L79Rab5 (Rab5DA) (a and b), -N34Rab5 (Rab5DN) (c and d), -L67Rab8 (Rab8DA) (e and f), -N22Rab8 (Rab8DN) (g and h), -L70Rab11 (Rab11DA) (i and j), and -N25Rab11 (Rab11DN) (k and l) using a lipofectAMINE reagent. At 24 h after the transfection, the cells were detached using an EDTA/trypsin solution, seeded onto 35-mm grid dishes, and further incubated in DMEM containing 10% FCS for 24 h. After the incubation, the cells were stimulated with 100 nM TPA and fixed at 2 h after TPA stimulation. The cells were then double stained with rhodamine-phalloidin (a, c, e, g, i, and k) or the 9E10 anti-myc mAb (b, d, f, h, j, and l), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm. Panels k and l show the Rab11DN-expressing cell in which the TPA-induced reassembly of stress fibers is observed, whereas the expression of Rab11DN inhibits their TPA-induced reassembly to a small extent (see Figure 7).
The expression of Rab5DA, -8DA, -8DN, and -11DA did not affect the TPA-induced reassembly of stress fibers at 2 h (Figure 7 and Figure 8, a, b, and e–j). The expression of Rab5DA and -8DA formed a large vesicular structure in which both the proteins were localized (Figure 8, b and f). Expressed Rab11DA was concentrated at the perinuclear region (Figure 8j). The expression of Rab5DN, -8DN, and -11DN showed dot-like staining that was relatively concentrated at the perinuclear region (Figure 8, d, h, and l). These results indicate that Rab5 activation is necessary for the TPA-induced reassembly of stress fibers, that Rab11 activation is slightly involved in their TPA-induced reassembly, and that Rab5 or Rab11 inactivation, or Rab8 activation or inactivation, is not necessary for their TPA-induced reassembly. These results also indicate that neither inactivation nor activation of Rab5, -8, or -11 is necessary for the TPA-induced disassembly of stress fibers.
The effects of Rab5 and -11 on the TPA-induced reassembly of stress fibers and focal adhesions were further examined using the MDCK cell lines stably expressing a dominant negative mutant of Rab5 (sMDCK-Rab5DN) or a dominant active or negative mutant of Rab11 (sMDCK-Rab11DA or -Rab11DN, respectively). We obtained five sMDCK-Rab5DN cell lines, two sMDCK-Rab11DA cell lines, and seven sMDCK-Rab11DN cell lines, but could not obtain the MDCK cell lines stably expressing a dominant active mutant of Rab5. In the sMDCK-Rab5DN cell line clone 10 (sMDCK-Rab5DN-10 cells), the staining patterns of actin filaments and vinculin were indistinguishable from those in wt MDCK cells that were stimulated without or with TPA for 15 min, but the TPA-induced reassembly of stress fibers and focal adhesions was inhibited at 2 h after the stimulation (Figure 1, a, b, d, f, g, and i, and Figure 9, a–f). The TPA-induced dissociation of cell–cell adhesion was not observed at 2 h (Figure 9, c and f). The same results were obtained in all the other sMDCK-Rab5DN cell lines (our unpublished results). In both sMDCK-Rab11DA cell line clone 3 (sMDCK-Rab11DA-3 cells) and sMDCK-Rab11DN cell line clone 5 (sMDCK-Rab11DN-5 cells), the staining patterns of actin filaments and vinculin were indistinguishable from those in wt MDCK cells that were not stimulated or were stimulated with TPA for 15 min or 2 h (Figure 1, a, b, d, f, g, and i, and Figure 9, g–r). The TPA-induced dissociation of cell–cell adhesion was also observed at 2 h (Figure 9, i, l, o, and r). The same results were obtained in all the other clones of sMDCK-Rab11DA and -Rab11DN cells (our unpublished results). These results have provided additional evidence that Rab5 activation is necessary for the TPA-induced reassembly of stress fibers and focal adhesions. However, the effects of Rab11 on these TPA-induced processes are apparently inconsistent with those obtained by use of the transient transfection assay. The exact reason for this inconsistency is not known but might be due to the different expression levels of N25Rab11 by the transfection of pEF-BOS-myc-N25Rab11, which was used in the transient transfection, and of pSRα-myc-N25Rab11, which was used in the stable transfection, because the expression level of N25Rab11 by pEF-BOS-myc-N25Rab11 was at least two- to threefold higher than that by pSRα-myc-N25Rab11 as estimated by the transient transfection method (our unpublished results). Therefore, we cannot conclude the definitive role of Rab11 in the TPA-induced reassembly of stress fibers and focal adhesions, but Rab11 activation may be at least slightly involved.
Figure 9.
Inhibition of the TPA-induced reassembly of stress fibers and focal adhesions in sMDCK-Rab5DN cells. sMDCK-Rab5DN-10 (a–f), -Rab11DA-3 (g–l), or -Rab11DN-5 (m–r) cells were incubated in DMEM containing 10% FCS for 24 h. The cells were stimulated with none (a, d, g, j, m, and p) or 100 nM TPA (b, c, e, f, h, i, k, l, n, o, q, and r). At 15 min (b, e, h, k, n, and q) or 2 h (c, f, i, l, o, and r) after TPA stimulation, the cells were fixed, double stained with rhodamine-phalloidin (a–c, g–i, and m–o) or the V115 anti-vinculin mAb (d–f, j–l, and p–r), and analyzed by confocal microscopy. Confocal images are shown at the basal levels. The results shown are representative of three independent experiments. Bar, 10 μm.
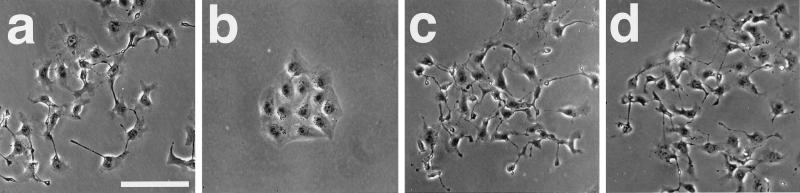
Rab5 Activation Is Necessary for the TPA-induced Cell Scattering
The TPA-induced cell scattering was inhibited in sMDCK-Rab5DN cells, but not in sMDCK-Rab11DA or -Rab11DN cells at 2 h (Figure 9, c, i, and o). These effects were more marked when the cells were analyzed at 6 h after TPA stimulation (Figure 10, a, c, and d). The TPA-induced cell scattering was inhibited in sMDCK-Rab5DN-10 cells, but these cells spread slightly (Figure 10b). These results indicate that Rab5 activation is necessary not only for the TPA-induced reassembly of stress fibers and focal adhesions but also for the TPA-induced cell scattering.
Figure 10.
Inhibition of the TPA-induced cell scattering in sMDCK-Rab5DN cells. wt MDCK (a), sMDCK-Rab5DN-10 (b), -Rab11DA-3 (c), or -Rab11DN-5 (d) cells were incubated in DMEM containing 10% FCS for 24 h. The cells were stimulated with 100 nM TPA. At 6 h after TPA stimulation, the cells were fixed, and analyzed by phase-contrast microscopy. The results shown are representative of three independent experiments. Bar, 50 μm.
The Rho-induced Formation of Stress Fibers and Focal Adhesions Is not Mediated through Rab Activation
Rho, Rab5, and -11 activation is involved in the TPA-induced assembly of stress fibers and focal adhesions as described above. We lastly examined whether Rab activation is necessary for the RhoA-induced formation of stress fibers and focal adhesions. Microinjection of GTPγS-RhoA alone into wt MDCK cells induced formation of stress fibers (Kotani et al., 1997). Comicroinjection of GTPγS-RhoA with Rab GDI did not inhibit the RhoA-induced formation of stress fibers (our unpublished results). Moreover, microinjection of Rab GDI did not inhibit the increased formation of stress fibers and focal adhesions in sMDCK-RhoDA cells (our unpublished results). These results indicate that the Rho-induced formation of stress fibers and focal adhesions is not mediated by Rab activation.
DISCUSSION
In this study we have first confirmed in wt MDCK cells that TPA and HGF/SF induce disassembly of stress fibers and focal adhesions followed by their reassembly, and that the reassembled stress fibers show radial-like morphology that is apparently different from the original. We have then shown, by use of both sMDCK-RhoDA cells and wt MDCK cells microinjected with C3, that Rho inactivation and activation are necessary for the TPA-induced disassembly and reassembly, respectively, of stress fibers and focal adhesions. These results are consistent with the earlier observations that Rho activation and inactivation stimulate and inhibit, respectively, formation of stress fibers and focal adhesions (Ridley and Hall, 1992; Self et al., 1993). We have shown here that TPA furthermore inhibits the peripheral bundle formation in wt MDCK cells, but not in sMDCK-RhoDA cells. We have previously shown that Rho activation is necessary for both the peripheral bundle formation and the localization of the ERM family, consisting of three members, ezrin, radixin, and moesin, at the peripheral bundles in MDCK cells (Kotani et al., 1997). Therefore, the inhibitory effect of TPA on peripheral bundle formation may also be mediated by Rho inactivation. TPA may transduce a negative signal to inhibit Rho and a positive signal to reactivate it.
We have shown here that TPA does not reduce the localization of actin filaments at the cell–cell adhesion sites or disrupt the cell–cell adhesion at least for 2 h stimulation in both sMDCK-RacDA and sMDCK-RacDN cells. As to membrane ruffling, TPA induces its formation in sMDCK-RacDA cells but not in sMDCK-RacDN cells. These results are consistent with earlier observations (Ridley et al., 1995; Braga et al., 1997; Hordijk et al., 1997; Takaishi et al., 1997) and have provided additional evidence that Rac has two functions: one is to strengthen cell–cell adhesion, and the other is to induce membrane ruffling. The Rac-strengthened cell–cell adhesion may make the cells resistant to the TPA-induced disruption of cell–cell adhesion, and the Rac-induced membrane ruffling may stimulate cell scattering and thereby secondarily reduce cell-cell adhesion. TPA may transduce negative and positive signals to Rac at cell–cell adhesion sites and membrane ruffling area, respectively. We have moreover shown here that the TPA-induced reassembly of stress fibers and focal adhesions is inhibited in both sMDCK-RacDN and -RacDA cells. The disruption of cell–cell adhesion may be necessary for the TPA-induced reassembly of stress fibers and focal adhesions.
We have shown here for the first time that activation of some Rab family members is furthermore necessary for the TPA-induced reassembly of stress fibers and focal adhesions but not for their TPA-induced disassembly. Because recycling of the plasma membrane components, especially integrins, by vesicle trafficking is important for the formation of focal adhesions that are dynamically controlled during cell motility (Martenson et al., 1993; Lawson and Maxfield, 1995; Bretcher, 1996; reviewed by Lauffenburger and Horwitz, 1996), we have analyzed here the effect of the dominant active or dominant negative mutant of Rab5, which regulates early endocytosis (Bucci et al., 1992; Stenmark et al., 1994). It has previously been shown that the dominant negative mutant of Rab5, N34Rab5, inhibits the internalization of transferrin in BHK cells (Stenmark et al., 1994). This earlier observation, together with the present result that the transient or stable expression of N34Rab5 inhibits the TPA-induced reassembly of stress fibers and focal adhesions in MDCK cells, indicates that early endocytosis of the plasma membrane components is related to these TPA-induced processes. It has also previously been shown that overexpression of the dominant active mutant of Rab5, L79Rab5, stimulates the internalization of transferrin but inhibits its recycling in BHK cells (Stenmark et al., 1994). Lower expression of L79Rab5, however, stimulates the internalization of transferrin but does not inhibit its recycling, although the modes of action of L79Rab5 at higher and lower expression levels have not been shown (Stenmark et al., 1994). Therefore, the present result, that the transient expression of L79Rab5 does not affect the TPA-induced reassembly of stress fibers and focal adhesions in MDCK cells, may be due to the fact that recycling of the plasma membrane components is not completely inhibited in the L79Rab5-expressing cells under our experimental conditions.
Rab11 regulates recycling through the pericentriolar recycling endosome (Ullrich et al., 1996). It has previously been shown that the dominant negative mutant of Rab11, N25Rab11, markedly inhibits the recycling of transferrin, whereas the dominant active mutant of Rab11, L70Rab11, moderately inhibits it in BHK cells (Ullrich et al., 1996). We have shown here that the transient expression of N25Rab11 slightly inhibits its TPA-induced reassembly of stress fibers and focal adhesions, although its stable expression does not affect them, and that the transient or stable expression of L70Rab11 does not affect their TPA-induced disassembly. Because the inability of the stable expression of N25Rab11 to inhibit the TPA-induced processes appears to be simply due to its insufficient expression level as described above, the Rab11-regulated recycling pathway through the pericentriolar recycling endosome may be at least slightly involved in the TPA-induced reassembly of stress fibers and focal adhesions. The direct recycling pathway from early endosome may also be responsible for the TPA-induced processes.
In Swiss 3T3 cells, it has been shown that Cdc42 induces filopodia formation followed by membrane ruffling formation through Rac activation, and that Rac induces membrane ruffling formation followed by assembly of stress fibers through Rho activation, indicating that there is cross-talk among the Rho family members (Ridley et al., 1992; Kozma et al., 1995; Nobes and Hall, 1995). In BHK cells, transient or stable expression of L67Rab8, but not N22Rab8, induces processes extending outward through reorganization of actin filaments and microtubules (Peränen et al., 1996), suggesting that there is cross-talk between the Rho family members and Rab8. There may also be cross-talk between the Rho and Rab family members in the TPA-induced reorganization of the actin cytoskeleton in MDCK cells. Because the Rho-induced formation of stress fibers and focal adhesions is not inhibited by Rab GDI, it is likely that the Rab family members do not act downstream of Rho in the TPA-induced reassembly of stress fibers and focal adhesions. We have shown here that both the transient and stable expressions of the dominant negative mutant of Rab5, but not the dominant active mutant, inhibit the TPA-induced reassembly of stress fibers and focal adhesions. Some Rab family members, at least Rab5, may act upstream of Rho in these TPA-induced processes. The mechanism of this cross-talk between Rab5 and Rho is not known, but Rho inactivation may first induce dissociation of stress fibers from integrins, Rab5 may then regulate recycling of the integrins, and Rho activation may finally stimulate formation of the new focal adhesions by use of these recycled integrins. It is also possible that the TPA-induced reactivation of Rho is mediated by the activation of Rac as shown in Swiss3T3 cells (Ridley et al., 1992). However, this possibility seems unlikely, because the TPA-induced reassembly of stress fibers and focal adhesions was not observed in sMDCK-RacDA cells. We have shown here that the TPA-induced disassembly of peripheral bundles, stress fibers, or focal adhesions is not inhibited in sMDCK-RacDA, -RacDN, -Rab5DN, -Rab11DA, or -Rab11DN cells. These results suggest that the TPA-induced Rho inactivation is not mediated by Rac or the Rab family members. Further study is necessary for our understanding of the cross-talk between the Rho and Rab family members in the dynamic and coordinate reorganization of the actin cytoskeleton.
We have shown here that the TPA-induced cell scattering as well as the TPA-induced reassembly of stress fibers and focal adhesion is inhibited in sMDCK-RhoDA, -RacDA, -RacDN, and Rab5DN cells. These results suggest that dissassembly and reassembly of stress fibers and focal adhesions are necessary for cell motility. Moreover, the result, that the TPA-induced disruption of the cell–cell adhesion is inhibited in sMDCK-Rab5DN cells, suggests that Rab5 activation is necessary for the TPA-induced disruption of the cell–cell adhesion. Further investigation is necessary to clarify the role and mode of action of the TPA-induced reassembly of stress fibers and focal adhesions in cell motility.
ACKNOWLEDGMENTS
We thank Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany) for providing MDCK cells, Dr. A. Hall (University College London, London, England) for the cDNAs of V12Rac1 and N17Rac1, Dr. P. Madaule (Kyoto University, Kyoto, Japan) for the cDNA of RhoA, Dr. A. Miyajima (Tokyo University, Tokyo, Japan) for the pSRαneo expression plasmid, Dr. S. Nagata (Osaka University, Osaka, Japan) for the pEF-BOS expression plasmid, Dr. T. Nakamura (Osaka University, Osaka, Japan) for HGF/SF, Dr. S. Narumiya (Kyoto University, Kyoto, Japan) for C3, and Dr. M. Zerial (European Molecular Biology Laboratory, Heidelberg, Germany) for the cDNAs of L79Rab5, N34Rab5, and Rab8. This investigation was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, Sports, and Culture, Japan (1997), by grants-in-aid for Abnormalities in Hormone Receptor Mechanisms and for Aging and Health from the Ministry of Health and Welfare, Japan (1997), and by a grant from the Human Frontier Science Program (1997).
Abbreviations used:
- GAP
GTPase activating protein
- GDI
GDP dissociation inhibitor
- GEP
GDP/GTP exchange protein
- GTPγS
guanosine 5′-(3-O-thio)-triphosphate
- HGF/SF
hepatocyte growth factor/scatter factor
- His6-Rab GDI
His6-tagged Rab GDI
- pEF-BOS-myc-
pEF-BOS-myc tagged
- pSRα-myc-
pSRαneo-myc tagged
- TPA
12-O-tetradecanoylphorbol-13-acetate
REFERENCES
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K, Rösener S, Blaschke U, Chhatwal GS. Botulinum ADP-ribosyltransferase C3: purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988;172:445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Habermann B, Just I, Aktories K, Vandekerckhove J. Purification of the 22kDa protein substrate of botulinum ADP-ribosyltransferase C3 from porcine brain cytosol and its characterization as a GTP-binding protein highly homologous to the rho gene product. FEBS Lett. 1989;243:70–76. doi: 10.1016/0014-5793(89)81220-7. [DOI] [PubMed] [Google Scholar]
- Bretcher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Stoker M. Hepatocyte growth factor-scatter factor: mitogen, motogen, and Met. Cancer Cells A Mon Rev. 1991;3:227–232. [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, Van der Kammen RA, Michiels F, Oomen LCJM, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto K, Fujita T, Takai Y. ADP-ribosylation of the bovine brain rho protein by botulinum toxin type C1. J Biol Chem. 1988;263:16303–16308. [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro R, Sasaki T, Takaishi K, Orita S, Takai Y. Involvement of Rho and Rac Small G proteins and Rho GDI in Ca2+-dependent exocytosis from PC12 cells. Genes Cells. 1996;1:943–951. doi: 10.1046/j.1365-2443.1996.760276.x. [DOI] [PubMed] [Google Scholar]
- Kotani H, Takaishi K, Sasaki T, Takai Y. Rho regulates association of both the ERM family and vinculin with the plasma membrane in MDCK cells. Oncogene. 1997;14:1705–1713. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The ras-related protein cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Martenson C, Stone K, Reedy M, Sheetz M. Fast axonal transport is required for growth cone advance. Nature. 1993;366:66–69. doi: 10.1038/366066a0. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sekine A, Fujiwara M. Substrate for botulinum ADP-ribosyltransferase, Gb, has an amino acid sequence homologous to a putative rho gene product. J Biol Chem. 1988;263:17255–17257. [PubMed] [Google Scholar]
- Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Peränen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rosen EM, Meromsky L, Goldberg I, Bhargava M, Setter E. Studies on the mechanism of scatter factor. Effect of agents that modulate intracellular signal transduction, macromolecule synthesis and cytoskeleton assembly. J Cell Sci. 1990;96:639–649. doi: 10.1242/jcs.96.4.639. [DOI] [PubMed] [Google Scholar]
- Roy FV, Mareel M. Tumor invasion: effects of cell adhesion and motility. Trends Cell Biol. 1992;2:163–169. doi: 10.1016/0962-8924(92)90035-l. [DOI] [PubMed] [Google Scholar]
- Self AJ, Paterson HF, Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993;8:655–661. [PubMed] [Google Scholar]
- Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gherardi E. Regulation of cell movement: the mitogenic cytokines. Biochim Biophys Acta. 1990;1072:81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in Ca2+-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita Sa, Tsukita Sh, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]