Abstract
Antigen presentation to CD4+ T lymphocytes requires transport of newly synthesized major histocompatibility complex (MHC) class II molecules to the endocytic pathway, where peptide loading occurs. This step is mediated by a signal located in the cytoplasmic tail of the MHC class II-associated Ii chain, which directs the MHC class II-Ii complexes from the trans-Golgi network (TGN) to endosomes. The subcellular machinery responsible for the specific targeting of MHC class II molecules to the endocytic pathway, as well as the first compartments these molecules enter after exit from the TGN, remain unclear. We have designed an original experimental approach to selectively analyze this step of MHC class II transport. Newly synthesized MHC class II molecules were caused to accumulate in the Golgi apparatus and TGN by incubating the cells at 19°C, and early endosomes were functionally inactivated by in vivo cross-linking of transferrin (Tf) receptor–containing endosomes using Tf-HRP complexes and the HRP-insoluble substrate diaminobenzidine. Inactivation of Tf-containing endosomes caused a marked delay in Ii chain degradation, peptide loading, and MHC class II transport to the cell surface. Thus, early endosomes appear to be required for delivery of MHC class II molecules to the endocytic pathway. Under cross-linking conditions, most αβIi complexes accumulated in tubules and vesicles devoid of γ-adaptin and/or mannose-6-phosphate receptor, suggesting an AP1-independent pathway for the delivery of newly synthesized MHC class II molecules from the TGN to endosomes.
INTRODUCTION
Most immune responses are generated after activation of helper T lymphocytes by antigenic peptides loaded on major histocompatibility complex (MHC) class II molecules and displayed at the plasma membrane of antigen-presenting cells (Cresswell, 1994; Germain, 1994; Watts, 1997). The generation of antigenic peptides and their association to MHC class II molecules occur in the endocytic pathway. This process requires antigen internalization, as well as delivery of newly synthesized MHC class II molecules to endocytic compartments (Cresswell, 1994; Germain, 1994; Watts, 1997). Transport of class II molecules to and from endosomes is mediated and regulated by the class II associated invariant chain (Ii) (Cresswell, 1996). In the endoplasmic reticulum (ER), homotrimers of Ii chain associate with three αβ heterodimers of class II molecules (Cresswell, 1996). A targeting signal, located in the NH2-terminal cytoplasmic domain of Ii chain, directs the transport of αβ-Ii complexes to the endocytic pathway (Cresswell, 1996). Immediately upon delivery to endosomes, the Ii chain is degraded in a stepwise manner, with several Ii chain fragments remaining transiently associated with class II molecules (Cresswell, 1996). The exchange of these Ii fragments by antigenic peptides is catalyzed by the nonpolymorphic MHC class II molecules HLA-DM and HLA-DO before transport to the cell surface (Roche, 1995) (Kropshofer et al., 1997).
In different antigen-presenting cells, specific peptide-loading compartments, called MHC class II compartments (MIICs) (Peters et al., 1991; Tulp et al., 1994; West et al., 1994) or class II vesicles (CIIVs) (Amigorena et al., 1994; Pierre et al., 1997), have been described. MIICs are found in Epstein–Barr virus (EBV)-transformed human B-lymphocytes, melanoma cells, and immature dendritic cells, whereas CIIVs were described in murine B lymphoma cells and developing dendritic cells. CIIVs and MIICs represent heterogeneous sets of multivesicular and multilaminar compartments, related to endosomes and lysosomes, respectively, and which may represent consecutive compartments along the endocytic pathway (Kleijmeer et al., 1997). In contrast to MIICs, which bear lysosomal markers (such as Lamp1 and 2) (Peters et al., 1991, 1995), CIIVs contain low levels of transferrin receptor (TfR) and mannose 6-phosphate receptor (M6PR) but are devoid of Lamp1 and 2 (Amigorena et al., 1994; Pierre et al., 1996). The distribution of MHC class II molecules between the plasma membrane and endosomal and lysosomal compartments is correlated with the rate of Ii chain degradation (Pieters et al., 1991; Brachet et al., 1997; Pierre et al., 1997).
The intracellular routes of MHC class II molecule transport to peptide-loading compartments are still unclear. In B-lymphocytes, MHC class II molecules enter endocytic compartments either directly from the trans-Golgi network (TGN) or through the cell surface (Neefjes et al., 1990; Peters et al., 1991; Pieters et al., 1991; Roche et al., 1993; Bénaroch et al., 1995; Saudrais et al., 1998). In the case of direct TGN to endosome transport, the compartments to which MHC class II molecules are first delivered are not well defined. It was proposed that αβIi complexes are directly targeted to multivesicular MIICs, based on the presence of an intact Ii chain in these organelles (Neefjes et al., 1990; Peters et al., 1995; Glickman et al., 1996; Kleijmeer et al., 1997). Yet, in other studies, αβIi complexes were transiently detected in TfR-containing early endosomes before reaching later endocytic compartments (Cresswell, 1985; Romagnoli et al., 1993; Castellino and Germain, 1995; Warmerdam et al., 1996).
Another related controversial issue concerns the cytosolic machinery involved in sorting of MHC class II molecules in the TGN (Robinson, 1997). At least two cytosolic complexes are involved in TGN–endosomal sorting: AP1 and clathrin determine the transport of M6PR to endosomes, whereas AP3 was more recently proposed to mediate direct transport of lysosomal resident proteins to lysosomes (or to the vacuole in yeast) (Odorizzi et al., 1998). Whether MHC class II molecules and the M6PR use the same transport pathway is still a matter of debate. On one hand, Glickman et al. (1996) showed that class II molecules can be transported to MIICs via an M6PR-independent pathway that does not appear to involve AP-1. In addition, Liu et al. (1998) showed that blocking clathrin function with a dominant negative mutant did not affect MHC class II-Ii chain complexes direct delivery to the endocytic pathway. On the other hand, overexpression of Ii chain recruits AP-1 in vitro (Salamero et al., 1996; Rodionov and Bakke, 1998), indicating that MHC class II molecules could potentially be transported in AP1-clathrin–coated vesicles. However, no evidence for colocalization between AP1 γ-adaptin or M6PR and MHC class II molecules was reported.
In the present study, we analyzed MHC class II transport from the TGN to the endocytic pathway in human and murine B cells. We selectively inactivated Tf-positive early endosomes in living cells and found that functional Tf-containing compartments were required for MHC class II transport to the peptide-loading compartments. These results indicate that MHC class II molecules are not directly delivered to late endocytic compartments. In addition, inactivation of early endosomes caused the accumulation of αβIi complexes in transport vesicles lacking both M6PR and γ-adaptin, indicating that MHC class II molecules and the M6PR use independent transport routes from the TGN to the endocytic pathway.
MATERIALS AND METHODS
Cells
Experiments on mouse cells were performed using an A20 B lymphoma cell line transfected with a cDNA encoding class II molecules I-Ab and called B4–14 (kindly provided by Avlin Barlow and Charles Janeway, Yale University, New Haven, CT) and an FcγR-negative mutant cell line derived from A20 cells and transfected with a cDNA encoding a chimera composed of the extracytoplasmic and transmembrane regions of mouse type II Fcγ receptors bound to the cytoplasmic region of mouse Igβ chain of the B cell receptor. This cell line was called A6B9 (Amigorena et al., 1992). Experiments on human cells were performed using EBV-transformed B-lymphocyte cell line Laz-B29 and hybridoma cell line T2bb provided by N.S. Braunstein (Columbia University, New York, NY) (Eastman et al., 1996). The Laz-B29 cell line was transfected with the chimeric cDNA encoding FcγRII-Igβ and called Laz-B29 B29. Stable clones were selected in hygromycin-containing medium and tested as described (Choquet et al., 1994).
Antibodies
The antibodies used in this work are anti-mouse I-Ab MHC class II molecule mAb Y3P (Janeway et al., 1984), which binds to mature MHC class II molecules; anti-mouse CD107b (lamp2) mAb (clone ABL-93; PharMingen, San Diego, CA); anti-mouse TfR (CD71) mAb (clone C2, PharMingen); anti-mouse Fc receptor mAb 2.4G2 (Unkeless, 1979); anti-human MHC class II molecule mAb L243, which binds to mature class II molecules (Shackelford et al., 1981); anti-human MHC class II molecule mAb DA6.147 (Guy et al., 1982); anti-human CD107a (lamp1) mAb (clone H4A3, PharMingen); anti-human invariant chain mAb PIN1 (Denzin and Cresswell, 1995); and anti-γ-adaptin mAb (Sigma, St. Louis, MO). We also used several rabbit polyclonal sera directed against HRP molecules (Sigma), human class II molecules (anti-αβDR) (Peters et al., 1991), the lumenal domain of human invariant chain Ab ICC5 (Morton et al., 1995), the cytoplasmic tail of mouse I-Ab β chain (Théry et al., 1998), and dinitrophenol (DNP) molecule (kindly provided by Dr. Ira Mellman, Yale University). The antibody directed against the CI-M6PR (46 kDa MPR) was kindly provided by Dr. Kurt von Figura (University of Gottingen, Gottingen, Germany).
Cross-Linking of Tf Receptor Compartments
Cells were starved for 1.5 h in RPMI 1640 medium supplemented with 0.1% BSA and then incubated for 2 h at 19°C at 107 cells/ml in the same medium containing 20 μg/ml human or mouse HRP-Tf (Pierce, Rockford, IL) and 20 mM HEPES, pH 7.4. To remove the HRP-Tf bound to the cell surface, the cells were washed in cold PBS and incubated for 10 min at 0°C with PBS containing 20% FCS. Inactivation of the TfR-positive compartments was performed by incubating the cells at 0°C, for 1 h at 107 cells/ml, in the dark, with diaminobenzidine (fast DAB, Sigma) diluted in 15 ml of Tris-buffered saline (and not 5 ml as suggested by the manufacturer’s instructions) and supplemented with 0.01 μl/ml H2O2 30% (a final concentration of 0.003% H2O2). Under these conditions cell viability (as measured by trypan blue staining) was not modified (<5%), and protein synthesis (as assessed by [35S]methionine incorporation) was not affected (our unpublished results). Control cells were submitted to the same treatment but were incubated with uncoupled Tf.
Metabolic Labeling and Immunoprecipitation
The experiments were performed as described (Amigorena et al., 1994). Briefly, the cells were metabolically labeled for 20 min with [35S]methionine/cysteine labeling mix at 500 μCi/ml, (Amersham, Arlington Heights, IL) and chased for various periods. When necessary, the cell surface biotinylation and immunoprecipitation were performed as described (Amigorena et al., 1994) and analyzed by electrophoresis on SDS-acrylamide gels (12 or 10–15% gradient acrylamide gels). Immunoprecipitated class II molecules were loaded with or without previous boiling to detect SDS-resistant compact dimers that migrate at a molecular mass of 60 kDa and correspond to mature peptide-loaded class II molecules. When boiled, the SDS-resistant class II molecules dissociate in α and β chains. The quantifications were performed using a video camera (Bio-print system; Vuilbert-Lourmat, Marne la Vallée, France). Optical densitometry was performed using Bio1D software (Vilbert-Lourmat).
Endoglycosidase H Digestion
The cells were metabolically labeled for 10 min, processed for early endosome inactivation, and chased for various periods. After immunoprecipitation, molecules were eluted at 95°C for 10 min with 50 μl of 1% SDS and 1 mM DTT in 50 mM Tris, pH 6.8, and then digested or not at 30°C overnight with 0.2 U/ml endoglysosidase H (Oxford GlycoSystem, Abingdon, United Kingdom). Samples were then analyzed on 12% SDS-PAGE, and the proteins were revealed by autoradiography.
125I Protein Labeling
The mAb rat anti-mouse Fc receptors (2.4G2) and DNP-BSA protein were labeled with 125I-Na (Amersham) using iodobeads (Pierce), according to the manufacturer’s instructions. Labeled proteins were separated from free 125I-Na on a PD 10 column (Amersham). The percentage of free 125I was always <5%.
Endocytosis of 2.4G2 mAb
Endocytosis was measured as described (Mellman and Plutner, 1984). Briefly, human Laz-B29 and mouse A6B9 cells were processed for inactivation of the TfR-containing compartments, washed, and incubated with iodinated 2.4G2 for 30 min at 0°C. After two additional washes, the cells were incubated at 37°C for various periods. The mAb bound to the cell surface was removed by a 3-min acid wash in 0.5 M acetic acid and 50 mM NaCl, pH 2.9, on ice. Cell-associated radioactivity was measured after neutralization with 100 mM Tris, pH 7.4.
Degradation of 125I-DNP-BSA
Degradation of DNP-BSA complexed with anti-DNP antibodies and internalized via FcR was measured as described (Mellman and Plutner, 1984). Briefly, mouse A6B9 cells were processed for the inactivation of TfR compartments and incubated with immune complexes (ICs; 10 μg/ml 125I-DNP-BSA and 20 μg/ml rabbit anti-DNP antibodies) at 4°C for 1.5 h. IC internalization was achieved by incubating the cells for 15 min at 37°C. After two washes in cold culture medium, cells were incubated at 37°C for various periods. Degradation of DNP-BSA was estimated by measuring the soluble radioactivity in 20% trichloroacetic acid (TCA).
Electron Microscopy
DAB Cytochemistry.
After internalization of Tf-HRP, cells were fixed with a mixture of 2% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Cells were postfixed for 2 h in 0.5% OsO4 and 1.25% ferrocyanure in distilled water at 4°C in the dark, dehydrated in ethanol, and embedded in Epon. Ultrathin sections were contrasted with Reynolds buffer for 2 min and viewed in a Philips (Eindoven, The Netherlands) CM120 transmission electron microscope.
Ultracryomicrotomy.
Cells were fixed for 1 h with 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, processed for ultracryomicrotomy, and immunogold labeled as described (Raposo et al., 1997). All the antibodies were visualized with protein A-gold conjugates (purchased from J.W. Slot, Utrecht University, Utrecht, The Netherlands). For quantification of immunogold labeling, the number of gold particles labeling particular structures or the number of labeled compartments was counted directly under the electron microscope. In each case 40–50 cell profiles were analyzed from two independent experiments.
RESULTS
To analyze the pathway of MHC class II transport from endosomes to the endocytic pathway, we designed an original experimental approach based on 1) the accumulation of new MHC class II molecules in the early exocytic pathway by incubation of the cells at 19°C, to synchronize subsequent MHC class II transport steps; and 2) the functional inactivation of early endosomes using Tf-HRP conjugates and the HRP-insoluble substrate DAB. Because the distribution and localization of MHC class II molecules in human and murine B cells are distinct (MIICs and CIIVs, respectively), we decided to analyze the effect of early endosome inactivation in both cell systems in parallel.
Intracellular Accumulation of MHC Class II Molecules at 19°C
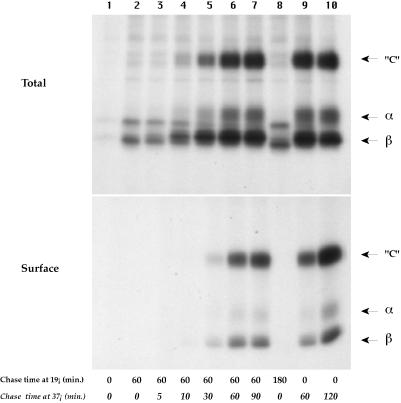
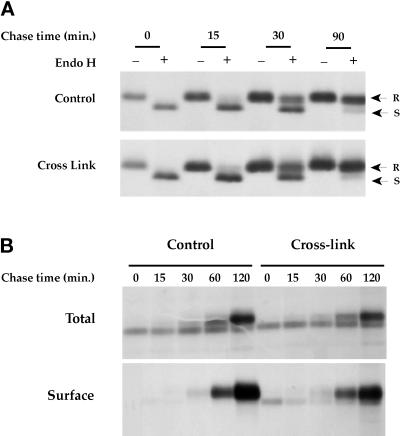
We first analyzed the effect of a 19°C incubation on MHC class II processing and transport to the cell surface. Mouse B4.14 cells were metabolically labeled for 20 min, chased for 60 min at 19°C, and shifted to 37°C for various periods. After cell surface biotinylation and lysis, class II molecules were immunoprecipitated with Y3P, an mAb that recognizes mature, Ii chain-free MHC class II molecules. The immunoprecipitated molecules were analyzed on SDS-PAGE without boiling to detect SDS-resistant compact forms (“C”), which migrate at a molecular mass of 60 kDa and correspond to peptide-loaded αβ-dimers (Germain and Hendrix, 1991).
As shown in Figure 1, after a 20-min pulse, few MHC class II molecules are detected with the Y3P mAb. In control cells, pulsed and chased at 37°C, mature SDS-stable complexes are generated within 60 min after the chase (Total). After a 120-min chase, the entire bulk of processed MHC class II molecules is present at the cell surface (Total and Surface). Incubating the cells at 19°C strongly inhibited the processing of class II molecules: after 60 min of 19°C chase, the amount of αβ complexes immunoprecipitable by Y3P was very low, and this did not change with an additional 120 min of 19°C chase. Moreover, no newly synthesized class II molecules were detected at the cell surface, indicating that during the 19°C incubation the MHC class II molecules were retained intracellularly. Similar results were obtained with human Laz-B29 cells (see below), in which no αβ peptide dimers are detected after 2 h of 19°C chase (our unpublished results).
Figure 1.
Incubation at 19°C reversibly blocks MHC class II molecule maturation. B4-14 cells were pulsed for 20 min with [35S]methionine and chased at 19°C for 60 min (1st through 7th lanes) or for 180 min (8th lane) and shifted to 37°C for the indicated times. Positive control cells (1st, 9th, and 10th lanes) were directly chased at 37°C for the indicated times. After cell lysis, I-Ab molecules were immunoprecipitated with the Y3P mAb. The samples were not boiled before SDS-PAGE analysis. Labeled class II molecules were not detected after the 20-min pulse (1st lane) because the Y3P mAb used for immunoprecipitation does not detect immature αβ dimmers complexed with intact Ii chain.
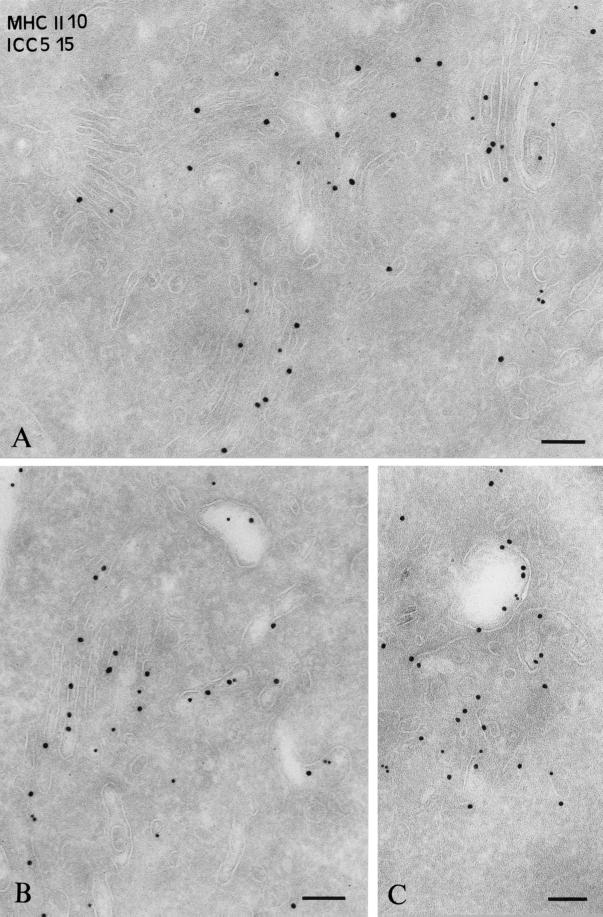
We then analyzed the compartments where αβ-Ii complexes accumulate after incubation at 19°C for 2 h in B-EBV cells. Ultrathin cryosections were double immunogold labeled with anti-HLA-DR and anti-Ii chain (ICC5) antibodies and analyzed by immunoelectron microscopy. As shown in Figure 2A, abundant Ii chain–associated MHC class II molecules were observed in the Golgi apparatus. This accumulation was evident in most cells.
Figure 2.
Immunogold localization of αβ-Ii complexes after 2 h of 19°C incubation and upon shifting to 37°C. Ultrathin cryosections of Laz cells were immunogold labeled with an anti-αβ-DR rabbit serum (PAG-10) and a rabbit Ab directed against the C-terminal domain of the Ii chain (ICC5) (PAG-15). (A) Laz cells incubated at 19°C for 2h. Intense labeling is observed for the Ii chain (PAG 15) and MHC class II (PAG 10) in the Golgi apparatus. (B and C) Cells incubated at 19°C for 2 h and then shifted to 37°C for 45 min with Ii chain (PAG 15) and MHC class II (PAG 10) are visualized in electron-lucent vesicles and tubules apposed to the Golgi apparatus (B) as well as in endosomes (C). Bar, 100 nm.
When localized by immunoelectron microscopy, abundant Ii chain–associated MHC class II molecules were detected in the ER and Golgi stacks in both human (Figure 2A) and murine B cells (our unpublished results). Moreover, after a 10-min pulse at 37°C and a 2-h chase at 19°C, most αβIi complexes were still sensitive to endoglucosaminidase H (endo H) (our unpublished results). Thus, αβIi complexes accumulated in the ER and Golgi apparatus at 19°C. When shifted to 37°C, class II molecules acquire endo H resistance with the same kinetics as control cells that were directly chased at 37°C (our unpublished results).
Transport of Class II Molecules upon Shifting to 37°C
We next analyzed the transport pathway of class II molecules between the TGN and endocytic compartments using the 19°C block and shifting to 37°C. As shown in Figure 1, when shifted to 37°C, αβ dimers matured into SDS-stable “C” forms within 30–60 min and required 30 min more to reach the plasma membrane. These kinetics of maturation and transport of class II molecules to the cell surface observed upon shifting to 37°C are similar to those of control cells that were pulsed and chased at 37°C and to our previously published results (Brachet et al., 1997; Théry et al., 1998). Similar results were obtained using human EBV-B cells, in which mature MHC class II molecules appeared at the cell surface within 2 h of shifting to 37°C (which is the normal delay required for class II molecule processing in these cells; our unpublished data).
We examined the distribution of MHC class II and Ii chain in cells chased for 45 min at 37°C after the 19°C block. In approximately half of cells, labeling was still detected in the Golgi. Interestingly, a significant amount of Ii chain and class II molecules was detected in small vesicles and tubules closely apposed to the Golgi apparatus (Figure 2B) and, to a greater extent, in compartments morphologically similar to early endosomes (they display an electron-lucent bulk and very few internal vesicles; Figure 2, B and C). In control cells, these structures did not stain for HLA-DR or Ii (our unpublished results) (Peters et al., 1995).
In B-EBV cells, transport from the TGN to endocytic compartments does not occur via the plasma membrane, because <5% of the αβIi complexes can be detected in pulse–chase experiments with surface biotinylation using the mAb DA6147 (Saudrais et al., 1998; our unpublished results). This amount remained unchanged when the cells had been incubated at 19°C for 2 h and shifted to 37°C (our unpublished results). Thus, the αβIi complexes found in early endosomal-like compartments after the 19°C block and a 45-min chase at 37°C are most likely directly derived from the TGN. Altogether, these results suggest that αβIi complexes accumulated in the Golgi apparatus at 19°C were transported to early endosomes upon shifting to 37°C, in all likelihood en route to later endocytic compartments.
Inactivation of Early Endosomes: Intracellular Localization of Tf-HRP Complexes
To investigate the putative functions of early endosomes in the transport of αβIi complexes from the TGN to peptide-loading compartments, we attempted to inactivate in vivo Tf-positive compartments using Tf coupled to HRP and its polymerizable substrate DAB. Internalization of HRP-Tf was performed for 2 h at 19°C in both human and murine B cells. When incubated at this temperature, the cells still internalized external components and slowly recycled them to the plasma membrane, but transport of internalized molecules from early to late endosomes was inhibited (our unpublished results). Thus, internalizing HRP-Tf at 19°C specifically loaded early endosomes with HRP and induced the accumulation of MHC class II molecules in the ER and Golgi stacks.
The intracellular localization of HRP-Tf complexes was determined by DAB cytochemistry before ultrathin sectioning of Epon-embedded cells. As shown in Figure 3, in both B4-14 and Laz-B29 cells, DAB precipitates were detected in tubulovesicular structures with typical morphology of early endocytic compartments (Marsh et al., 1986; Hopkins et al., 1990; Gruenberg and Maxfield, 1995). Note that HRP was detected neither in the Golgi apparatus nor in multivesicular endosomes (Figure 3). Not all early endosomes were filled with DAB precipitates (Figure 3B). However, the proportion of such empty early compartments was low compared with the DAB-positive ones.
Figure 3.
Morphology of the HRP-containing compartments. Cells that had internalized HRP-Tf for 2 h at 19°C were processed for HRP detection using DAB and H2O2 and embedded in Epon. Ultrathin sections were then analyzed by electron microscopy. (A and B) B4.14 cells. (C and D) Laz cells. DAB reaction precipitates are observed in tubulovesicular structures. Bar, 500 nm.
To evaluate the proportion of MHC class II-containing compartments that also contained HRP, the compartments where Tf-HRP accumulates after a 2-h incubation at 19°C in mouse B lymphoma and human B-EBV cells were analyzed qualitatively and quantitatively by immunogold labeling on ultrathin cryosections. In human cells, class II molecules were present at the cell surface and accumulated in multivesicular and multilaminar compartments as previously described (Peters et al., 1995; Kleijmeer et al., 1997). As depicted in Table 1, a quantitative analysis revealed that only 8% of these class II compartments also contained HRP. Most HRP-Tf was detected in tubulovesicular structures, often localized near the plasma membrane, which morphologically resemble early endosomes. Only 17% of these compartments also contained MHC class II molecules (Table 1). In mouse B4-14 cells, class II molecules were predominantly localized at the plasma membrane and present intracellularly in tubulovesicular structures already described (Brachet et al., 1997), with 25% of these structures being accessible to HRP (Table 1). However, the majority of HRP-Tf was found in MHC class II–negative tubulovesicular compartments resembling early endosomes. In both cell types, HRP-Tf was absent from Lamp1-positive compartments (our unpublished results).
Table 1.
Distribution of HRP and class II molecules in Laz and B4.14 cells incubated for 2 h at 19°C in the presence of HRP-Tf
| Molecule | Laz-B29
|
B4.14
|
||
|---|---|---|---|---|
| Multivesicular and multilamellar compartments | Early endocytic compartments | Multivesicular compartments | Early endocytic compartments | |
| MHC class II (%) | 83 | 17 | 84.5 | 15.5 |
| HRP (%) | 8 | 92 | 22.2 | 77.8 |
Ultrathin cryosections were immunogold labeled with the anti-αβ-DR Ab (PAG-10; Laz cells) or with the anti-class II mAb M5.114 (PAG-10; B4.14 cells) and with the anti-HRP Ab (PAG-15). Early or late endosomal structures containing either one of the labeled molecules or both were counted, and the distribution of the two markers in each type of compartment was determined. In each case, 50 cell profiles were analyzed.
These results show that after 2 h of internalization at 19°C, the bulk of Tf-HRP was localized in early endosomes and inefficiently reached later endocytic compartments. Importantly, even after 2 h of internalization, little Tf-HRP was found in MHC class II–containing compartments under these conditions.
In Vivo Cross-Linking of Early Endosomes
To inactivate early endosomes, the cells were incubated for 2 h with HRP-Tf at 19°C. Tf-HRP–loaded compartments were then inactivated with DAB, an HRP substrate that polymerises in the presence of H2O2 and inhibits the budding and fusion properties of the compartments in which it accumulates (Futter et al., 1996; Pond and Watts, 1997). Inactivation of early endosomes should not affect internalization from the plasma membrane, but it is expected to block transport to lysosomes and protein degradation. To test these two transport steps, we used murine B lymphoma cells expressing recombinant FcγRIIb2 (A6B9 cells), which we have previously shown to mediate efficient ligand internalization and degradation (Amigorena et al., 1992).
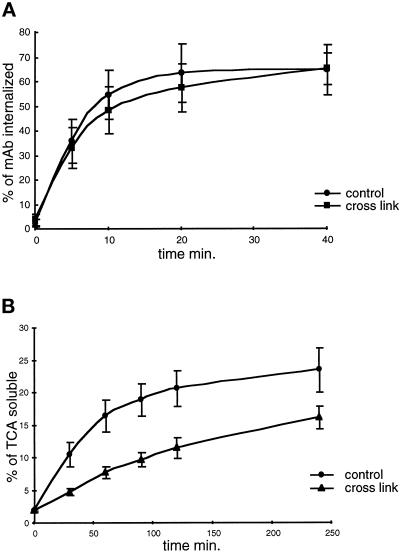
The ability of A6B9 cells to internalize iodinated anti-FcγRIIb2 (2.4G2) was measured first. As shown in Figure 4A, the internalization of 2.4G2 mAb was rapid and reached a plateau of 60–70% internalized radioactivity after 20 min of incubation at 37°C. This process was not affected by DAB cross-linking of early endosomes (Figure 4A). We obtained similar results in human EBV-B cells expressing recombinant FcγRIIb2-B29 cytoplasmic tail chimeras (Laz-B29 cells) (our unpublished data). Thus, inactivation of early endosomes had no effect on receptor-mediated endocytosis.
Figure 4.
Early endosome inactivation has no effect on ligand internalization but reduces their degradation. A6B9 cells were processed for early endosome inactivation. Control cells were submitted to the same treatment as cross-linked cells but were incubated with non–HRP-coupled Tf before testing FcγRIIb2-mediated ligand internalization and degradation. (A) 125I-2.4G2 kinetics of internalization in both control (black circles) and cross-linked cells (black squares); (b) Degradation of 125I-DNP-BSA-anti-DNP ICs determined by measuring the percentage of 20% TCA-soluble radioactivity. Each result shows the average obtained with four independent experiments.
We then measured the effect of early endosomes cross-linking on degradation of ligands internalized by FcγRIIb2. Because the 2.4G2 mAb is particularly resistant to proteolysis, we used iodinated DNP-BSA coupled with rabbit anti-DNP antibodies (ICs), which bind Fc receptors and are much more sensitive to proteolysis (Mellman and Plutner, 1984). After inactivation of early endosomes, the ICs were internalized for 15 min, the cells were incubated at 37°C for various times, and the percentage of TCA-soluble radioactivity present in the culture medium was measured. The degradation of BSA was inhibited by 40–50% by the inactivation of early endosomes (Figure 4B), suggesting that transport of internalized ligands to lysosomes was delayed.
Transport of Newly Synthesized Transmembrane Proteins from the ER to the Golgi Apparatus and to the Plasma Membrane
To control the integrity of the protein secretion machinery in the cross-linked cells, we first investigated the effect of early endosomes cross-linking on ER to Golgi transport, as detected by the acquisition of resistance of newly synthesized proteins to endo H digestion. Laz-B29 cells were pulsed and chased for various periods before immunoprecipitation of TfR. The precipitates were then treated with endoglycosilase H, which digests high mannose residues before, but not after, Golgi processing. As shown in Figure 5A, the kinetic and efficacy of appearance of endo H–resistant TfR were not modified by early endosome inactivation. Similar results were obtained in mouse B4-14 cells (our unpublished results) and with MHC class I and II molecules in both human and mouse cells (our unpublished results). Therefore, early endosome cross-linking does not affect protein export from the ER.
Figure 5.
Early endosome inactivation has no effect on the secretory pathway. Laz-B29 (A) or B4.14 (B) cells pulsed for 20 min with [35S]methionine were processed (cross-link) or not (control) for early endosome inactivation and chased at 37°C for the indicated times. (A) TfR was immunoprecipitated and treated or not treated with endo H before analysis on SDS-PAGE. (B) After each chase period, the cell surface was biotinylated, and MHC class I molecules were immunoprecipitated. Cell surface MHC class I molecules were isolated using streptavidin–agarose. Samples were then boiled and analyzed on SDS-PAGE. Early endosome cross-linking has no effect on ER to Golgi to plasma membrane transport.
We next investigated the kinetic transport to the cell surface of MHC class I molecules, which are known to be directly targeted from the TGN to the cell surface (Neefjes et al., 1990). Cells were pulsed, processed to inactivate early endosomes, and chased for various periods at 37°C. After cell surface biotinylation, the cells were lysed, and total and surface MHC class I molecules were immunoprecipitated and analyzed on SDS-PAGE. As shown in Figure 5B, MHC class I molecules in control cells matured within 60–120 min of the chase and were very rapidly transported to the plasma membrane. We did not detect any significant difference in the kinetics of MHC class I maturation and cell surface delivery when early endosomes were cross-linked (Figure 5B). Similar results were obtained in Laz-B29 cells (our unpublished data). Altogether, these result show that cross-linking of early endosomes did not affect the secretory pathway.
MHC Class II Peptide Loading and Cell Surface Transport
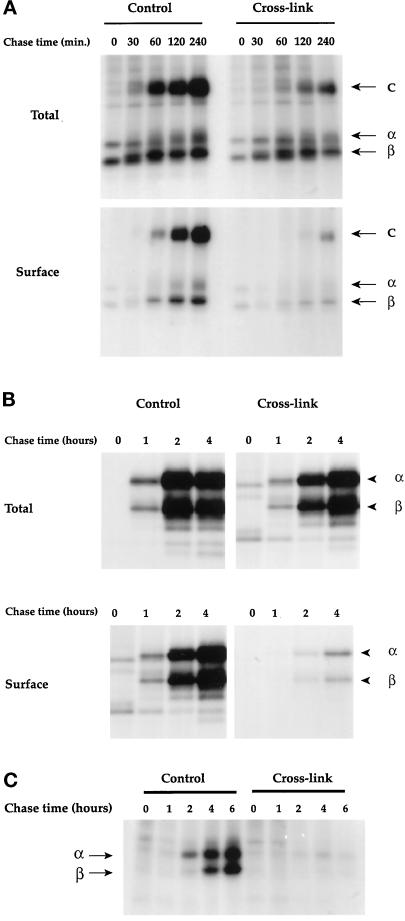
To evaluate the role of early endosomes in the maturation and transport of MHC class II molecules, cells were pulsed and processed (or not) to inactivate early endosomes before chasing for various periods. Cell surface molecules were biotinylated before lysis, and MHC class II molecules were immunoprecipitated. In murine B4.14 cells, samples were analyzed on SDS-PAGE without boiling to detect the SDS-resistant compact dimers, which migrate at a molecular mass of 60 kDa and correspond to peptide-loaded αβ-dimers. As shown in Figure 6A, no SDS-stable dimers (C) were observed at time 0 both in control and cross-linked cells. This confirms that, during the 19°C incubation, the processing of newly synthesized class II molecules was blocked. In control cells, SDS-stable dimers (C) were generated within 30–60 min of chase at 37°C (Figure 6A, upper panels). In cross-linked cells, the generation of SDS-stable αβ dimers was reproducibly delayed by 60–90 min and reduced by >50% compared with non–cross-linked cells (Figure 6A, upper panels).
Figure 6.
Early endosome inactivation delays MHC class II molecule maturation and transport to the cell surface. Cells were processed as for Figure 5. MHC class II molecules were immunoprecipitated with the Y3P mAb in B4.14 and T2bb cells and with the L243 mAb in Laz cells. (A) B4.14 cells; the samples were not boiled before SDS-PAGE analysis to detect the compact mature forms of class II molecules. (B) Laz cells; because the L243 mAb stronglyassociates with the class II molecules, samples were boiled before analysis to dissociate class II-L243 aggregates. No class II molecules are detected before 1 h of chase in control cells and 2 h in cross-linked cells, because L243 mAb does not detect immature αβ-Ii complexes. (C) T2bb cells; samples were boiled before analysis.
Similar results were obtained with human B-EBV cells. In these cells, SDS-stable αβ dimers were difficult to visualize. We therefore used the mAb L243, which detects exclusively mature Ii chain-free αβ dimers. As shown in Figure 6B, class II molecules in control cells were processed within 1–2 h of the chase and reached a plateau between 2 and 4 h. After 2 h of chase, the amount of mature class II molecules in cross-linked cells was ∼30% of that precipitated in control cells, indicating that the processing of class II molecules was also delayed by 60–90 min in B-EBV cells.
Transport of mature MHC class II molecules to the plasma membrane was also affected by the cross-linking in both murine and human cells. In B4.14 cells, αβ-peptide complexes were transported to the plasma membrane within 30 min and accumulated at the cell surface (Figure 6A, lower left panel). Inactivation of early endosomes strongly delayed transport of MHC class II molecules to the plasma membrane (Figure 6A, lower right panel). Delivery of MHC class II molecules was also severely impaired (Figure 6A, lower panels). Quantification of the gels revealed that 89% of the total SDS-stable class II dimers are at the cell surface after a 4-h chase in untreated cells, whereas only 60% of the total SDS-stable dimers are at the cell surface in cross-linked cells. In Laz-B29 cells, early endosome inactivation had the same impact on transport of class II molecules to the cell surface (Figure 6B, lower panel). In control cells, the αβ-peptide complexes generated after 2 h needed an additional 2 h to reach the plasma membrane. Upon inactivation of early endosomes, we detected almost no processed class II molecules at the cell surface, despite their presence in the cells. These results suggest that early endosomes may also be involved in MHC class II bulk transport to the cell surface, although functional T cell assays indicated that transport of a particular T cell epitope is not (Pond and Watts, 1997).
MHC class II final maturation requires the removal of the Ii chain peptide CLIP and its replacement by an antigenic peptide, a step that is mediated by HLA-DM molecules (Fling et al., 1994; Denzin and Cresswell, 1995; Roche, 1995). Because the inactivation of early endosomes partially inhibited the degradation of internalized ligands, the observed inhibition of peptide loading onto MHC class II could also be an indirect consequence of the effect of early endosome inactivation on the delivery or generation of antigenic peptides. We tested this possibility using the HLA-DM-negative T2 cells expressing IAb molecules. Indeed, it has previously been shown that in the absence of HLA-DM, IAb molecules are loaded exclusively with the Ii chain-derived peptide CLIP, which remains associated with IAb molecules after Ii degradation (Denzin et al., 1994). IAb-expressing T2 cells were processed as for Figure 6, and IAb molecules were immunoprecipitated. As shown in Figure 6C, in control cells, mature MHC class II molecules were detected after a 2-h chase and accumulate within 6 h. Early endosome cross-linking totally abolished the appearance of mature αβ dimers within 6 h of chase (Figure 6C). Therefore, even though MHC class II maturation in these cells is independent of antigenic peptides and HLA-DM, early endosome ablation inhibited maturation, indicating that this effect of the cross-linking is due to a direct interference with MHC class II intracellular transport.
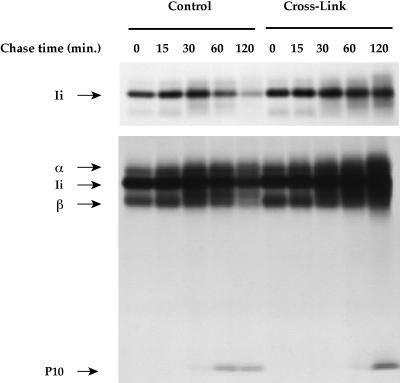
Effect of Early Endosome Inactivation on Ii Chain Degradation
The results presented thus far suggest that early endosomes are the site of entry of Ii chain-associated αβ dimers in the endocytic pathway. If this was the case, then cross-linking of Tf-containing compartments should prevent or delay delivery of MHC class II to endosomes, delaying Ii chain degradation. To test this possibility, B4.14 cells were processed as for Figure 4, and class II molecules were precipitated with rabbit anti-MHC class II polyclonal antibodies (which very efficiently bind to Ii chain-associated class II molecules) and analyzed on SDS-PAGE. A short exposure of the gel to film is presented in Figure 7, upper panel. Most of the MHC class II-associated Ii chain in control cells is degraded within 2 h of chase. Early endosome cross-linking strongly delayed Ii chain degradation (Figure 7, upper panel). A longer exposure of the gel (Figure 7, lower panel) shows that in control cells, the Ii chain degradation product Ii-p10 appears within 60 min of the chase. In early endosome cross-linked cells, Ii-p10 generation was delayed by 1 h. Therefore, early endosome inactivation delayed Ii chain degradation, indicating that delivery of MHC class II to the endocytic pathway requires functional early endosomes.
Figure 7.
Early endosome inactivation strongly delays the degradation of αβ dimer-associated Ii chain. B4.14 cells were processed as for Figure 6. After cell lysis, class II molecules were immunoprecipitated with a rabbit anti-class II serum that has a strong affinity for immature αβ dimers. Samples were boiled before SDS-PAGE analysis. Upper panel, autoradiography after a short exposure of the gel (4 d). Lower panel, longer exposure of the same gel (2 wk).
Tansport of αβ-Ii Complexes from the TGN to the Endocytic Pathway
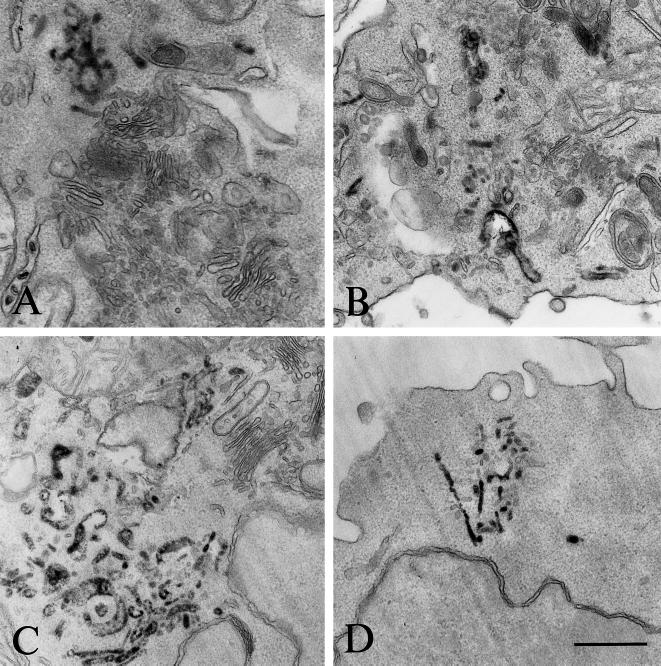
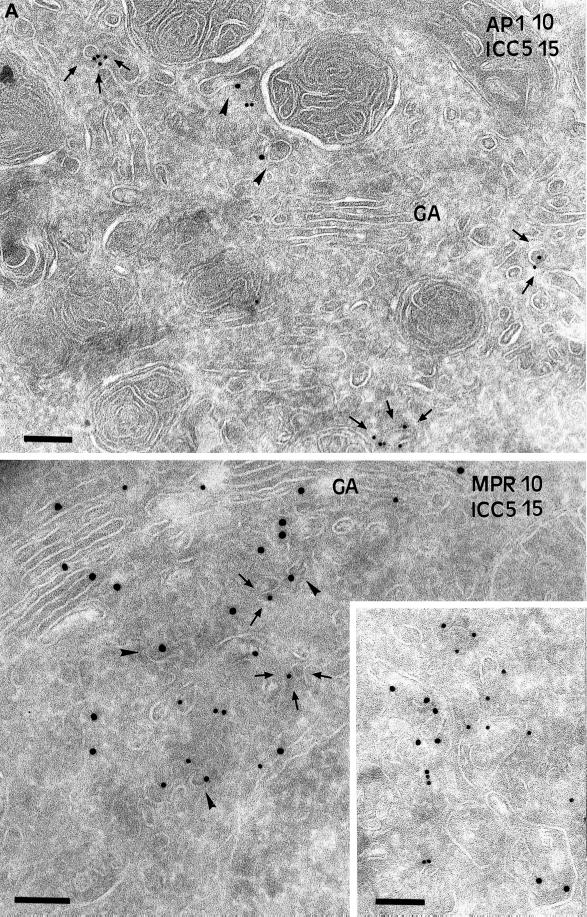
When early endosomes were inactivated, the arrival of MHC class II molecules to endosomes was delayed, presumably because putative transport vesicles delivering Ii chain-associated MHC class II molecules from the TGN may not fuse with the cross-linked early endosomes any longer. To investigate this possibility, B-EBV cells were processed for early endosome inactivation as before and further incubated for 45 min at 37°C to allow MHC class II transport out of the TGN and accumulation in transport intermediates. Then ultrathin cryosections of these cells were immunogold labeled and analyzed qualitatively and quantitatively at the electron microscopic level. As already shown in Figure 2B, in cells incubated at 19°C for 2 h and then at 37°C for 45 min, Ii chain is detected in vesicles and tubules near the Golgi apparatus. As shown in Figure 8A, in cross-linked cells Ii chain also accumulates in vesicles and tubules in the TGN region. The number of Ii chain-positive vesicles per cell was quantified (see MATERIALS AND METHODS) in untreated cells, in cells incubated at 19°C for 2 h and then at 37°C for 45 min, or in cells treated in the same way but whose early endosomes had been inactivated after the 19°C incubation. Incubation at 19°C increased the number of Ii-positive vesicles by threefold (an average of one Ii chain-positive vesicle per cell vs. three in 19°C-treated cells). Endosome cross-linking followed by a 45-min chase caused a further two- to threefold increase (five Ii chain-positive vesicles per cell).
Figure 8.
Immunogold localization of Ii chain in cross-linked cells. Laz cells were incubated at 19°C for 2 h in the presence of Tf-HRP and processed for early endosomes inactivation as before. The cells were then chased at 37°C for 45 min to induce accumulation of MHC class II in compartments en route to the endocytic pathway. Ultrathin cryosections were immunogold labeled with the anti-Ii chain Ab ICC5 (PAG15) and with the anti-γ-adaptin mAb (PAG10; upper panel) or with the anti-46-kDa M6PR Ab (PAG10; lower panel). Upper panel, note that the Ii-positive vesicles (PAG 15) are distinct from the γ-adaptin (PAG 10)–labeled vesicles. Lower panel, the vesicles and tubulovesicular structures (inset) labeled with the anti-Ii antibody are not labeled with the anti-46-kDa M6PR antibody. Bar, 200 nm.
To characterize these vesicles, sections of cells cross-linked and incubated at 37°C for 45 min were double immunogold labeled with anti-Ii chain antibody and either anti-γ-adaptin antibodies (AP1; Figure 8A, upper panel) or anti-M6PR antibodies (Figure 8A, lower panel).
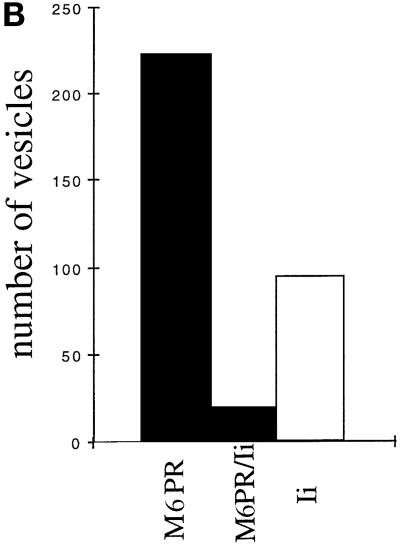
These structures were devoid of γ-adaptin (Figure 8A, upper panel). Quantification of the gold labeling (see MATERIALS AND METHODS) in untreated, 19°C-treated, and cross-linked (and 37°C chased) cells revealed that 20, 18, and 21%, respectively, of the Ii chain-positive vesicles were also labeled with the anti-γ adaptin antibody. Importantly, the γ-adaptin-positive vesicles were only faintly labeled by the anti-Ii antibodies (one gold particle per vesicle), whereas the vesicles that contained more Ii chain (two or more gold particles per vesicle) were γ-adaptin negative. These results suggest that MHC class II and Ii chains are sorted at the TGN by an AP1-independent mechanism. However, because Ii chain-positive vesicles may have lost their coat, we next analyzed the presence of the small MPR (46 kDa), a membrane receptor sorted by the AP1 pathway. As shown in Figure 8A, lower panel, MPR and Ii chain antibodies labeled different vesicular structures in cells cross-linked and chased at 37°C. Quantification of the labeling is shown in Figure 8B. Only 8% of the M6PR-positive vesicles contained the Ii chain, confirming that MHC class II-Ii chain complexes do not use the AP1 pathway to exit the TGN. The Ii chain-positive vesicles were not labeled by anti-β cop antibodies, indicating that they are not implicated in intra-Golgi transport (our unpublished results). Therefore, most Ii chain was found in vesicles and tubules different from those involved in the AP1-M6PR pathway, suggesting that transport of MHC class II from the TGN to the endosomes uses another pathway. These results are consistent with the results of Glickman et al. (1996) and Liu et al. (1998), who showed that MHC class II transport is independent of AP1 and clathrin.
DISCUSSION
We present functional and morphological results indicating that 1) Tf-positive compartments represent the site of entry of MHC class II molecules into the endocytic pathway; and 2) exit of Ii chain-associated MHC class II molecules from the TGN does not occur by AP1-clathrin–coated buds and vesicles.
Early endosome inactivation was achieved by loading of the cells with Tf-HRP complexes. Loading was performed at 19°C, a temperature at which transport from early to late endosomes is inefficient. As a result, selective accumulation of Tf-HRP in early parts of the endocytic pathway was obtained. In vivo cross-linking of early endosomes was then induced by treating the cells with DAB (an HRP insoluble substrate) in the presence of H2O2. Although indispensable for the cross-linking reaction, H2O2 is also toxic for the cells. Only at extremely low H2O2 concentrations (0.003%) could we obtain cross-linking of early endosomes in the absence of detectable cellular toxicity. Under these conditions, protein synthesis and translocation into the ER (our unpublished results), transport from the ER to the Golgi apparatus (Figure 5) and from the Golgi to the plasma membrane (Figure 5), and internalization (Figure 4) were not affected, indicating that the treatment was not toxic to the cells.
The selectivity of the cross-linking for Tf-positive compartments was established by showing that inactivation of early endosomes did not affect receptor-mediated ligand internalization, whereas transport of internalized proteins to lysosomes was delayed. Using a similar approach, Futter et al. (1996) obtained selective cross-linking of lysosomes, as did Pond and Watts (1997) for Tf-positive endosomes. In both cases, cross-linking of the compartments in living cells was selective and resulted in the block of the fusogenic properties of the compartments.
We show that under conditions of selective early endosome cross-linking, maturation of MHC class II molecules and Ii chain degradation were delayed. Ii chain is extremely sensitive to degradation and is believed to undergo proteolytic cleavage as soon as it reaches endosomes (Cresswell, 1996). The delay in its degradation after early endosome inactivation suggests that Tf-positive compartments, as opposed to late multivesicular compartments, represent the first site of MHC class II entry into the endocytic pathway. Early endosomes receive membrane compounds from two main origins: the plasma membrane and the TGN (Gruenberg and Maxfield, 1995). In murine B lymphoma cells and human EBV-transformed B cells, <5% of newly synthesized MHC class II molecules pass through the plasma membrane en route to peptide-loading compartments (Roche et al., 1993). We checked that after early endosome cross-linking the proportion of MHC class II molecules that transit through the plasma membrane does not increase. Therefore, the delay in Ii chain degradation must reflect a delay in transport of newly synthesized MHC class II molecules from the TGN to early endosomes.
This possibility is in accord with several reports showing that MHC class II and Ii chains colocalize with TfR before being transferred to later endocytic compartments (Cresswell, 1985; Pieters et al., 1991; Romagnoli et al., 1993; Castellino and Germain, 1995; Warmerdam et al., 1996). However, in these studies, the immediate origin of these MHC class II molecules (plasma membrane or TGN) was unclear, because the proportion of MHC class II molecules passing through the plasma membrane was not always determined. In normal spleen B-lymphocytes, TfR-positive early endosomes contain MHC class II molecules (Castellino and Germain, 1995), but also in this study it was unclear whether these molecules arose from the TGN or the plasma membrane.
In human EBV-B lymphocytes, virtually no MHC class II is found in TfR-positive early endosomes (Peters et al., 1995). In mouse B lymphoma cells and human EBV-B lymphocytes, a detailed immunoelectron microscopy analysis showed recently that Ii chain-associated MHC class II molecules accumulate in an intermediate population of multivesicular endosomes, which are negative for TfR (Kleijmeer et al., 1997). These authors proposed that this particular endosome population represents the first site of MHC class II entry into the endocytic pathway. Our results suggest that MHC class II molecules actually enter the endocytic pathway before multivesicular MHC class II-positive late endosomes. The absence of staining for MHC class II and Ii chain in early endosomes probably results from the very short time of residence of membrane proteins in early endocytic compartments (Gruenberg and Maxfield, 1995). Nevertheless, we cannot exclude the possibility that low amounts of Tf-HRP, which may have escaped immunodetection, actually reach and partially inactivate late endosomes. This is, however, quite unlikely, because the detection of HRP is very sensitive and does not reveal any HRP in multivesicular compartments.
Early endosome inactivation also provided further insight into the pathway of MHC class II transport out of the TGN, which, until now, remained elusive. It was recently shown that the Ii chain cytosolic tail may recruit γ-adaptin in vitro, suggesting that MHC class II molecules use the AP1/M6PR pathway of transport to endosomes (Salamero et al., 1996; Rodionov and Bakke, 1998). However, these in vitro experiments failed to demonstrate γ-adaptin recruitment in the TGN, rather than endosomes, in which AP1 is also known to operate (Mallard et al., 1999). In addition, despite extensive analysis, very little colocalization between MHC class II or Ii chain and γ-adaptin in the TGN is found by electron microscopy. Furthermore, morphological analysis in M6PR-negative cells (I cell disease), led to the conclusion that MHC class II may exit the TGN through non–γ-adaptin–mediated transport (Glickman et al., 1996). However, the absence of colocalization of MHC class II with M6PR and γ-adaptin may also be due to the development of alternative TGN to endosome pathways in both I cell disease and early endosome cross-linked cells. In the latter case, it is also possible that, despite their localization to the TGN region of the cytoplasm, the vesicles that accumulate in cross-linked cells are not directly derived from the TGN.
In any case, our results strongly support the possibility previously proposed by others (Glickman et al., 1996; Liu et al., 1998) that most αβIi complexes do not use the AP1 pathway to reach the endocytic pathway. Upon early endosome inactivation, Ii chain degradation was delayed (probably reflecting a delay in delivery to early endosomes), and Ii chain-associated MHC class II molecules accumulated in M6PR-, γ-adaptin–negative vesicles. The association of the functional and morphological data strongly indicates that these vesicles mediate transport from the TGN to endosomes. MHC class II accumulation in these vesicles was probably not an artifactual consequence of the cross-linking, because it only affects post-TGN transport events. The vesicles in which Ii chain accumulated after cross-linking did not label for β-cop (our unpublished results), indicating that they are not Golgi transport vesicles.
However, we cannot exclude the possibility that MHC class II accumulation in the TGN (by the 19°C incubation) causes overloading of the γ adaptin pathway and accumulation in these vesicles. The fact that the kinetics of Ii degradation and peptide loading observed after reincubation of the cells at 37°C was normal argues against this hypothesis, however. In addition, M6PR-negative vesicles stained more strongly for Ii chain than the M6PR-positive ones, which is also inconsistent with the possibility of overloading of the AP1 pathway.
Taken together, our results indicate that most MHC class II molecules are not transported from the TGN to endosomes by the M6PR-AP1 pathway. Another pathway for the transport from the TGN to endosomes, which uses a novel family of adaptor molecules, AP3, has been described. However, although the overall level of AP3 immunodetection in B cells was very low, the Ii chain-positive vesicles that accumulated after early endosome cross-linking did not label for AP3 (our unpublished results). Because we may not exclude that these vesicles have already lost their coats, the involvement of AP3 in MHC class II sorting in the TGN needs to be examined in further detail.
ACKNOWLEDGMENTS
We thank C. Bonnerot, B. Goud, and J. Salamero for helpful discussions, M. Robinson for the anti-AP3 antibodies, N.S. Braunstein for the T2bb cells, H. Ploegh for the anti-HLA-DR antibodies, D. Meur for photographical assistance, D. Tenza and D. Lankar for technical help, and M. Johnston for critical reading of the manuscript. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Association pour la Recherche Contre le Cancer, Ligne de Lutte Contre le Cancer, and the European Community. V.B. is supported by Ministére de la Recherche et la Technologie.
REFERENCES
- Amigorena S, Bonnerot C, Drake J, Choquet D, Hunziker W, Guillet J, Webster P, Sautes C, Mellman I, Fridman W. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Bénaroch PJ, Yilla M, Raposo MG, Ito K, Miwa K, Geuze HJ, Ploegh HL. How MHC class II molecules reach the endocytic pathway. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet V, Raposo G, Amigorena S, Mellman I. Invariant chain controls the transport of MHC class II molecules to and from lysosomes. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Choquet D, Ku G, Cassard S, Malissen B, Korn H, Fridman W, Bonnerot C. Different patterns of calcium signaling triggered through two components of the B lymphocyte antigen receptor. J Biol Chem. 1994;269:6491–6497. [PubMed] [Google Scholar]
- Cresswell P. Intracellular class II HLA antigens are accessible to transferrin-neuraminidase conjugates internalized by receptor-mediates endocytosis. Proc Natl Acad Sci USA. 1985;82:8188–8192. doi: 10.1073/pnas.82.23.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. doi: 10.1016/s0092-8674(00)81025-9. [DOI] [PubMed] [Google Scholar]
- Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha/beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Denzin LK, Robbins NF, Carboy-Newcomb C, Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Eastman S, Deftos M, DeRoos P, Hsu D, Teyton L, Braunstein N, Hackett C, Rudensky A. A study of complexes of class II invariant chain peptide: major histocompatibility complex class II molecules using a complex-specific monoclonal antibody. Eur J Immunol. 1996;26:385–393. doi: 10.1002/eji.1830260218. [DOI] [PubMed] [Google Scholar]
- Fling SP, Arp B, Pious D. HLA-DMA and-DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins C. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Germain RN, Hendrix LR. MHC class II structure, occupancy and surface expression determined by postendoplasmic reticulum antigen binding. Nature. 1991;353:134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- Glickman JN, Morton PA, Slot JW, Kornfeld S, Geuze HJ. The biogenesis of MHC class II compartment in human I-cell disease B lymphoblasts. J Cell Biol. 1996;132:769–785. doi: 10.1083/jcb.132.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield F. Membrane transport in the endocytic pathway. Curr Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Guy K, Van Heyningen V, Cohen BB, Deane DL, Steel CM. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982;12:942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:318–319. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Conrad PJ, Lerner EA, Babich J, Wettstein P, Murphy DB. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: possible role of T cell bound Ia antigens as targets of immunoregulatory T cells. J Immunol. 1984;132:662–667. [PubMed] [Google Scholar]
- Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropshofer H, Hammerling GJ, Vogt AB. How HLA-DM edits the MHC class II peptide repertoire: survival of the fittest? Immunol Today. 1997;18:77–82. doi: 10.1016/s0167-5699(97)01006-2. [DOI] [PubMed] [Google Scholar]
- Liu S, Marks MS, Brodsky F. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J Cell Biol. 1998;140:1023–1037. doi: 10.1083/jcb.140.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport J. Cell Biol. 1999;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Griffiths G, Dean GE, Mellman I, Helenius A. Three-dimensional structure of endosomes in BHK-21 cells. Proc Natl Acad Sci USA. 1986;83:2899–2903. doi: 10.1073/pnas.83.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984;98:1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton PA, Zacheis ML, Giacoletto KS, Manning JA, Schwartz BD. Delivery of nascent MHC class II-invariant chain by cystein proteases precedes peptide binding in B-lymphoblastoid cells. J Immunol. 1995;154:137–150. [PubMed] [Google Scholar]
- Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Cowles C, Emr S. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Peters P, Raposo G, Neefjes JJ, Oorschot V, Leijendekker RL, Geuze HJ, Ploegh HL. Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes. J Exp Med. 1995;182:325–334. doi: 10.1084/jem.182.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Pierre P, Denzin LK, Hammond C, Drake JR, Amigorena P, Cresswell P, Mellman I. HLA-DM is localized to conventional and unconventional MHC class II-containing compartments. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Pieters J, Horstmann H, Bakke O, Griffiths G, Lipp J. Intracellular transport and localization of major histocompatibility complex class II molecules and associated invariant chain. J Cell Biol. 1991;115:1213–1223. doi: 10.1083/jcb.115.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L, Watts C. Characterization of transport of newly assembled T cell-stimulatory MHC class II-peptide complexes form MHC class II compartments to the cell surface. J Immunol. 1997;159:543–553. [PubMed] [Google Scholar]
- Raposo G, Kleijmeer MJ, Posthuma G, Slot JW, Geuze HJ. Immunogold labeling of ultrathin cryosections: application in immunology. In: Blackwell I, editor. Handbook of Experimental Immunology. 5th ed. Cambridge, MA: Elsevier; 1997. pp. 1–11. [Google Scholar]
- Robinson MS. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- Roche P. HLA-DM an in vivo facilitator of MHC class II peptide loading. Immunity. 1995;3:259–262. doi: 10.1016/1074-7613(95)90111-6. [DOI] [PubMed] [Google Scholar]
- Roche P, Teletski C, Stang E, Bakke O, Long E. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DG, Bakke O. Medium chains of adaptor complexes AP-1 and AP-2 recognize Leucine-based sorting signals from the invariant chain. J Biol Chem. 1998;273:6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- Romagnoli P, Layet C, Yewdell J, Bakke O, Germain RN. Relationship between invariant chain expression and major histocompatibility complex class II transport into early and late endocytic compartments. J Exp Med. 1993;177:583–596. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamero J, Le Borgne R, Saudrais C, Goud B, Hoflack B. Expression of major histocompatibility complex class II molecules in Hela cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on Golgi membranes. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Saudrais C, Spehner D, de la Salle H, Bohbot A, Cazenave JP, Goud B, Salamero J. Intracellular pathway for the generation of functional MHC class II peptide complexes in immature human dendritic cells. J Immunol. 1998;160:2597–2607. [PubMed] [Google Scholar]
- Shackelford D, Lampson L, Strominger J. Analysis of HLA antigens by using monoclonal antibodies: recognition of conformational difference in biosynthetic intermediates. J Immunol. 1981;127:1403–1410. [PubMed] [Google Scholar]
- Théry C, Brachet V, Regnault A, Rescigno M, Ricardi-Castagnoli P, Bonnerot C, Amigorena S. MHC class II transport from lysosomal compartments to the cell surface is determined by stable peptide binding but not by the cytosolic domains of the alpha- and beta-chains. J Immunol. 1998;161:2106–2113. [PubMed] [Google Scholar]
- Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Unkeless JC. Characterization of monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmerdam PAM, Long EO, Roche PA. Isoforms of the invariant chain regulate transport of MHC class II molecules to antigen processing compartments. J Cell Biol. 1996;133:281–291. doi: 10.1083/jcb.133.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]