Abstract
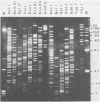
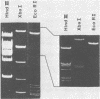
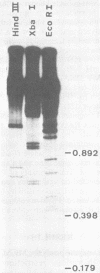
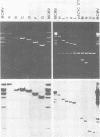
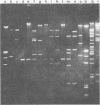
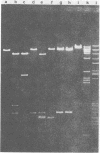
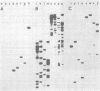
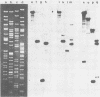
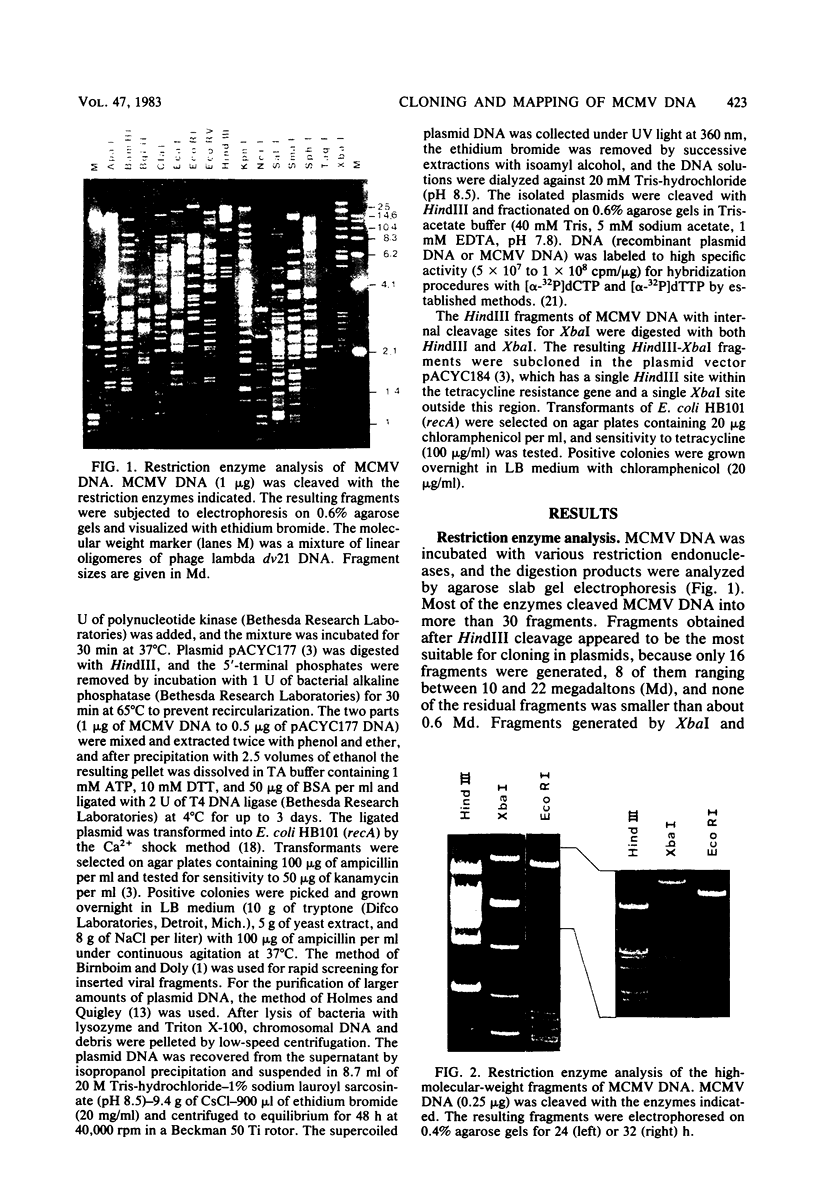
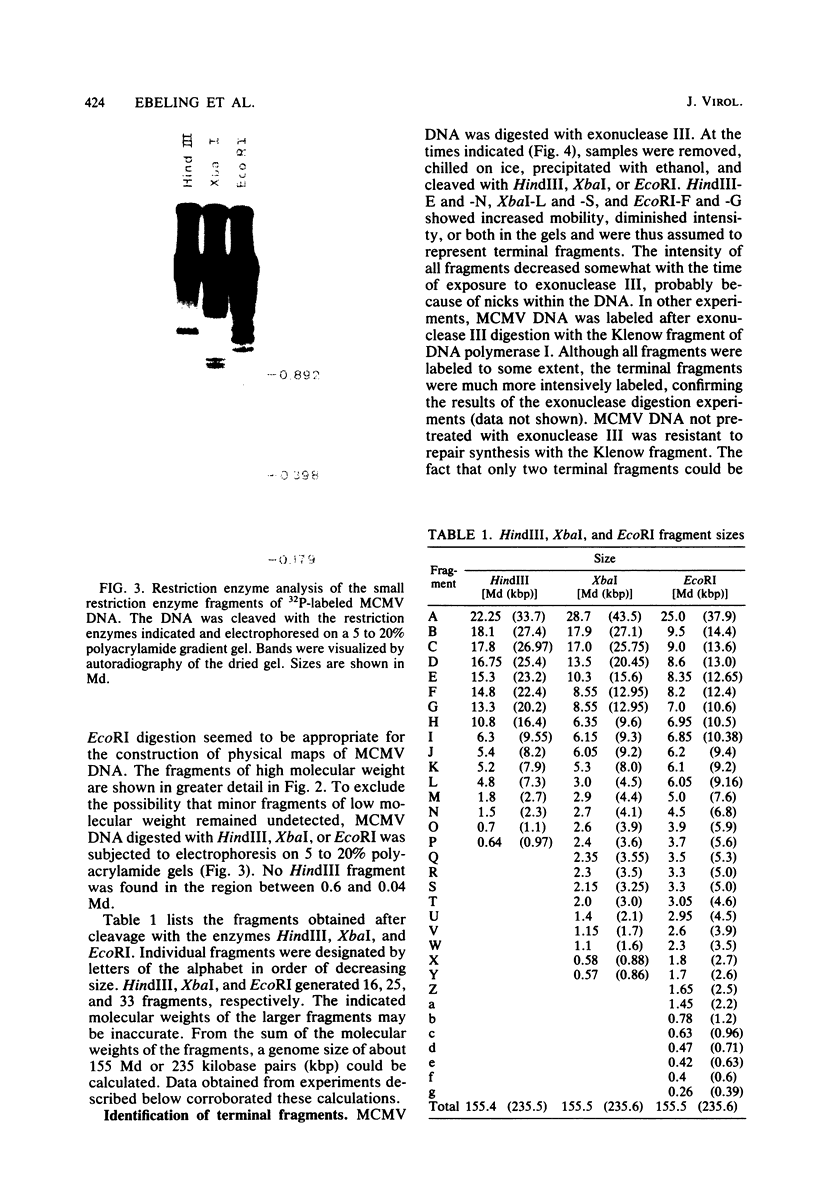
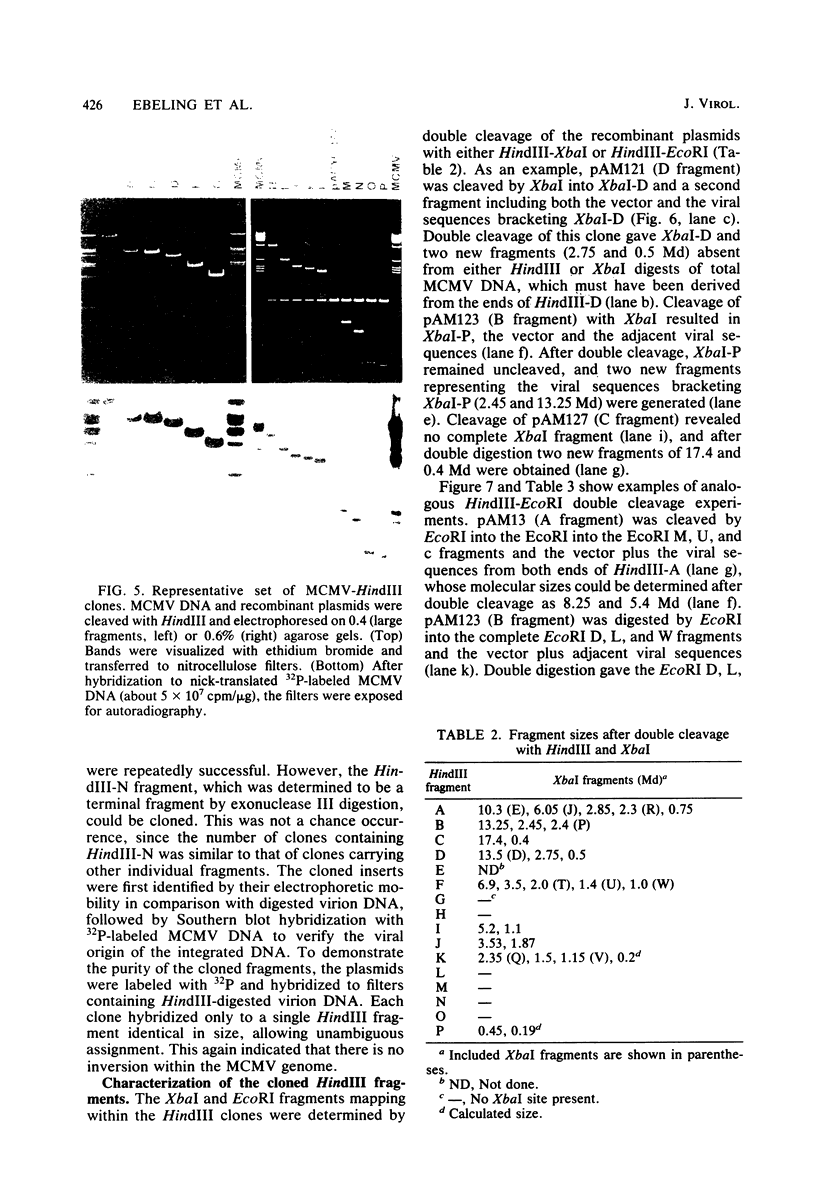
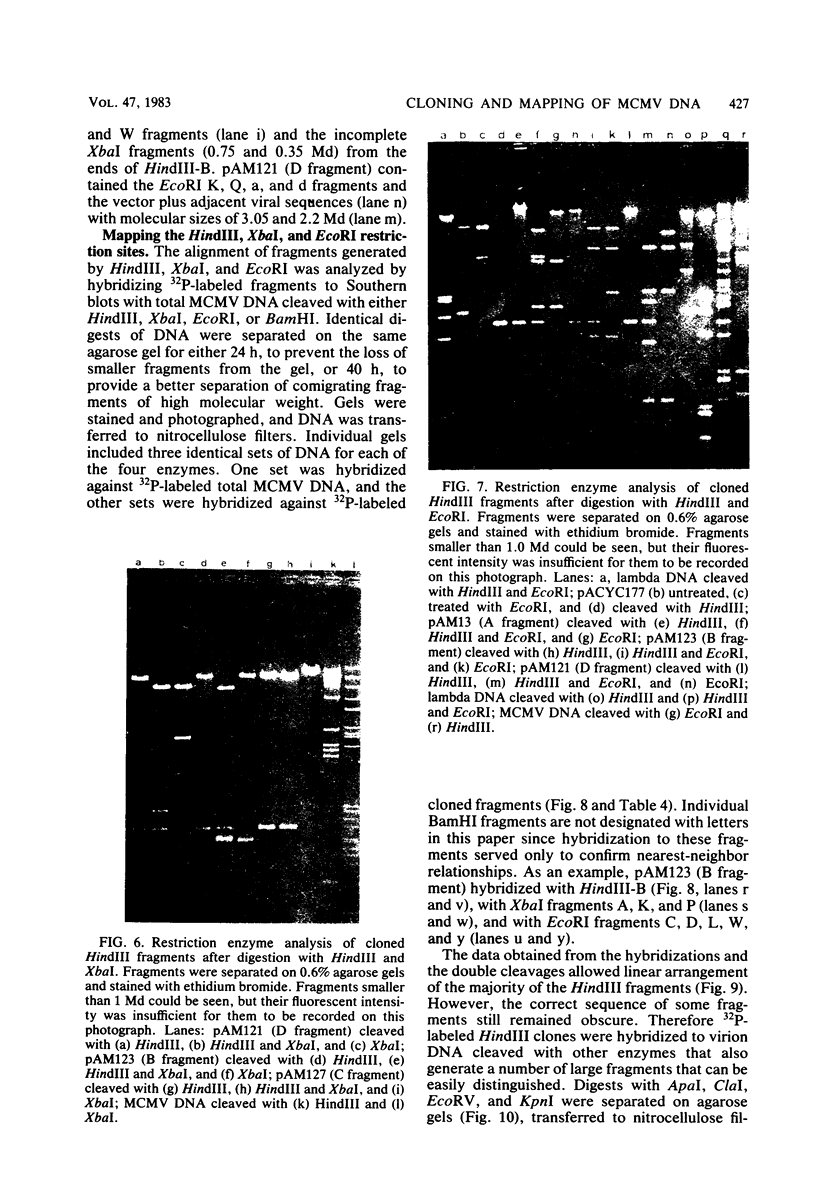
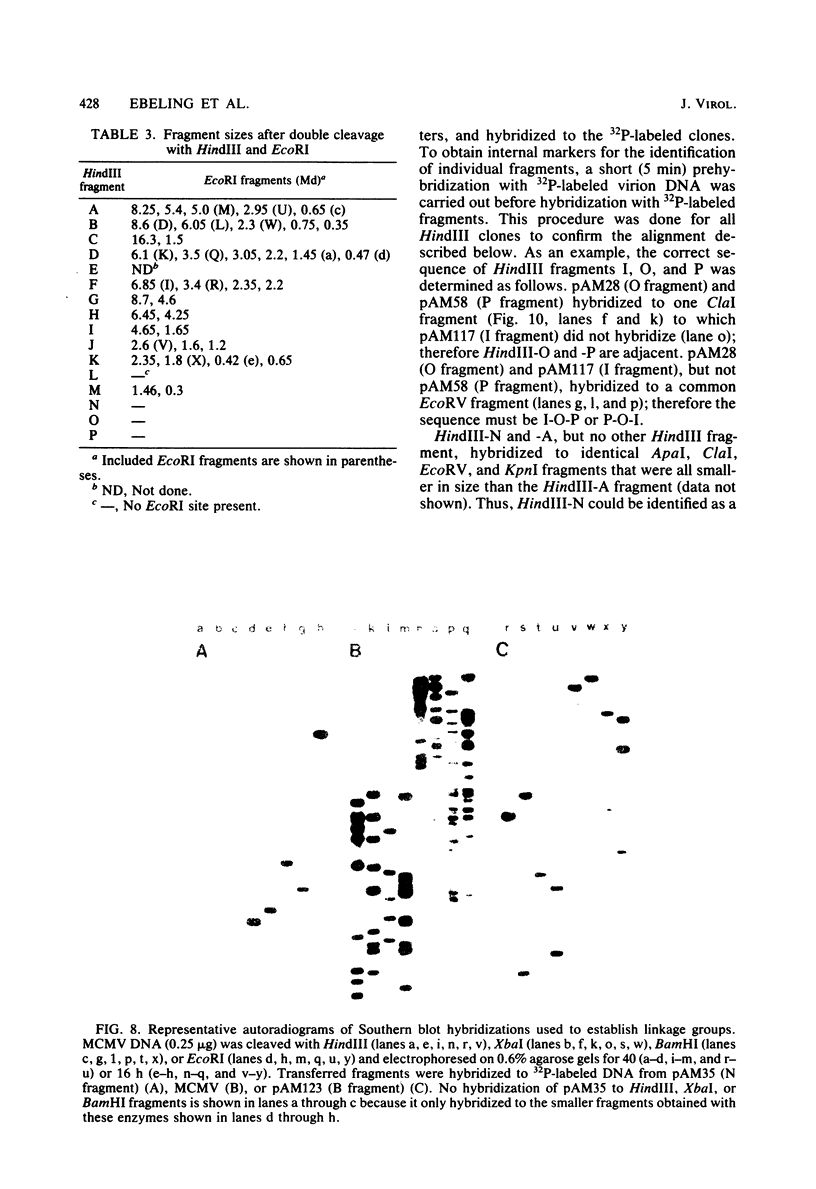
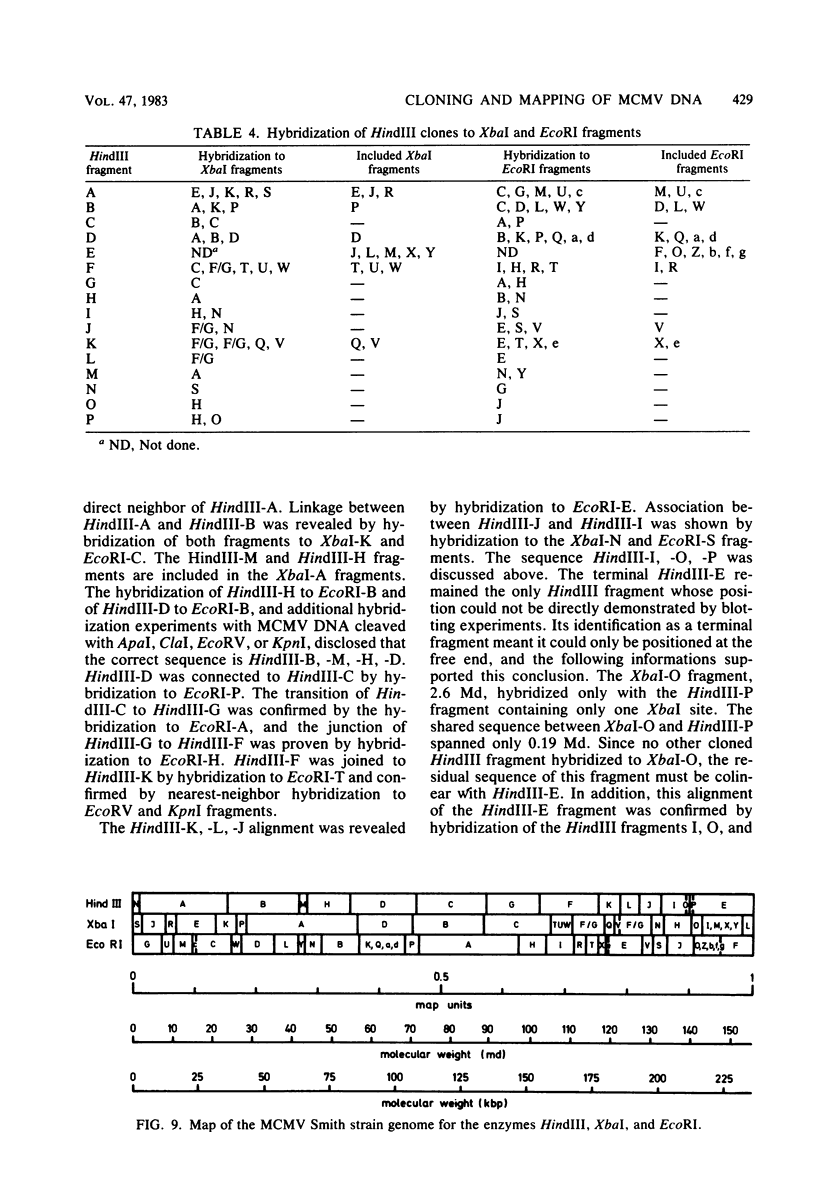
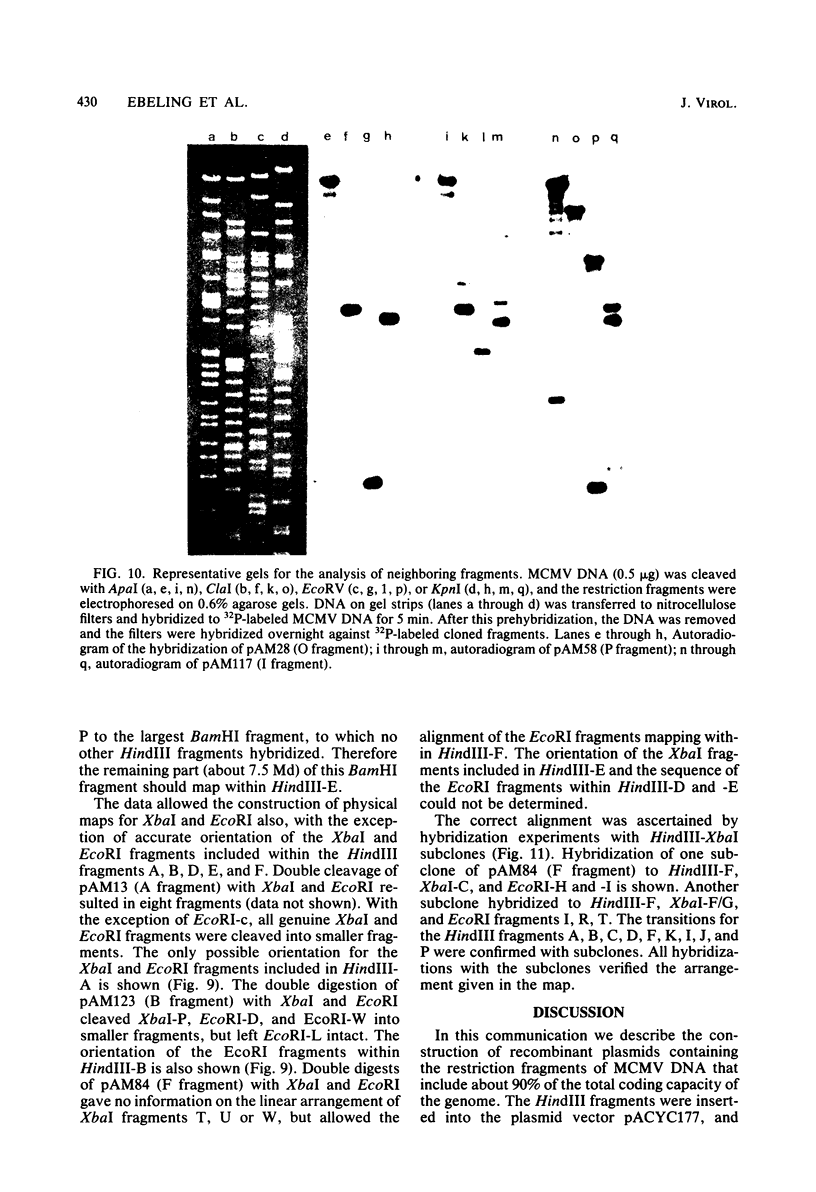
Murine cytomegalovirus (MCMV) Smith strain DNA is cleaved by restriction endonuclease HindIII into 16 fragments, ranging in size from 0.64 to 22.25 megadaltons. Of the 16 HindIII fragments, 15 were cloned in plasmid pACYC177 in Escherichia coli HB101 (recA). The recombinant plasmid clones were characterized by cleavage with the enzymes XbaI and EcoRI. In addition, fragments generated by double digestion of cloned fragments with HindIII and XbaI were inserted into the plasmid vector pACYC184. The results obtained after hybridization of 32P-labeled cloned fragments to Southern blots of MCMV DNA cleaved with HindIII, XbaI, EcoRI, BamHI, ApaI, ClaI, EcoRV, or KpnI allowed us to construct complete physical maps of the viral DNA for the restriction endonucleases HindIII, XbaI, and EcoRI. On the basis of the cloning and mapping experiments, it was calculated that the MCMV genome spans about 235 kilobase pairs, corresponding to a molecular weight of 155,000,000. All fragments were found to be present in equimolar concentrations, and no cross-hybridization between any of the fragments was seen. We conclude that the MCMV DNA molecule consists of a long unique sequence without large terminal or internal repeat regions. Thus, the structural organization of the MCMV genome is fundamentally different from that of the human cytomegalovirus or herpes simplex virus genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman S., Lacasa M., Sheldrick P. Physical Map of the Channel Catfish Virus Genome: Location of Sites for Restriction Endonucleases EcoRI, HindIII, HpaI, and XbaI. J Virol. 1979 Jul;31(1):73–85. doi: 10.1128/jvi.31.1.73-85.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Ebeling A., Keil G., Nowak B., Fleckenstein B., Berthelot N., Sheldrick P. Genome structure and virion polypeptides of the primate herpesviruses Herpesvirus aotus types 1 and 3: comparison with human cytomegalovirus. J Virol. 1983 Feb;45(2):715–726. doi: 10.1128/jvi.45.2.715-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci U S A. 1982 Jan;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982 Apr;18(1):39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., Oram J. D., Downing R. G., Patel K. Human cytomegalovirus DNA: BamHI, EcoRI and PstI restriction endonuclease cleavage maps. Gene. 1982 Jun;18(3):355–360. doi: 10.1016/0378-1119(82)90174-3. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976 Jul 1;72(1):235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Downing R. G., Akrigg A., Dollery A. A., Duggleby C. J., Wilkinson G. W., Greenaway P. J. Use of recombinant plasmids to investigate the structure of the human cytomegalovirus genome. J Gen Virol. 1982 Mar;59(Pt 1):111–129. doi: 10.1099/0022-1317-59-1-111. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Gene. 1978 Nov;4(3):183–194. doi: 10.1016/0378-1119(78)90017-3. [DOI] [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Grafstrom R. H., Hill C. W. Terminal fragments of herpes simplex virus DNA produced by restriction endonuclease. Biochem Biophys Res Commun. 1975 Nov 17;67(2):556–561. doi: 10.1016/0006-291x(75)90848-7. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Cortini R. Sequence arrangement in herpes simplex virus type 1 DNA: identification of terminal fragments in restriction endonuclease digests and evidence for inversions in redundant and unique sequences. J Virol. 1976 Oct;20(1):211–221. doi: 10.1128/jvi.20.1.211-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]