Abstract
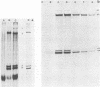
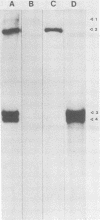
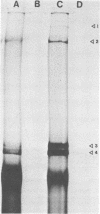
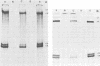
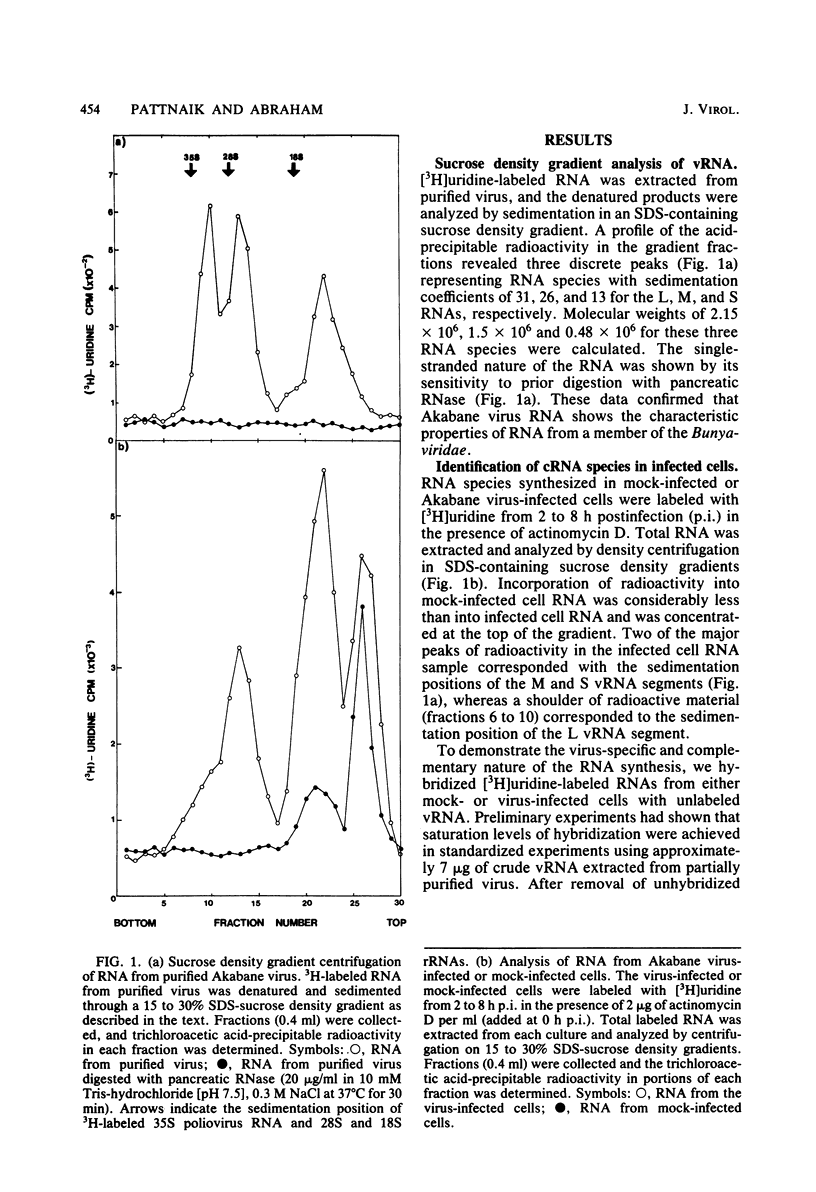
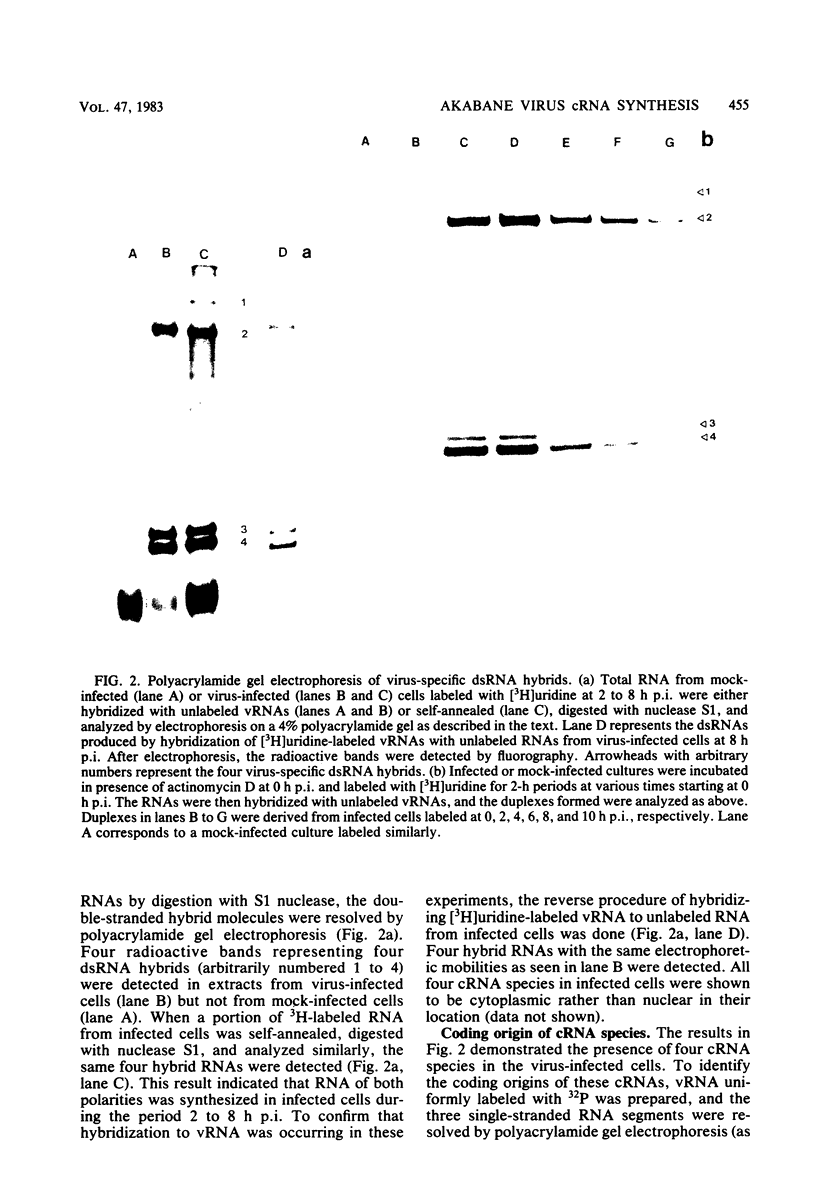
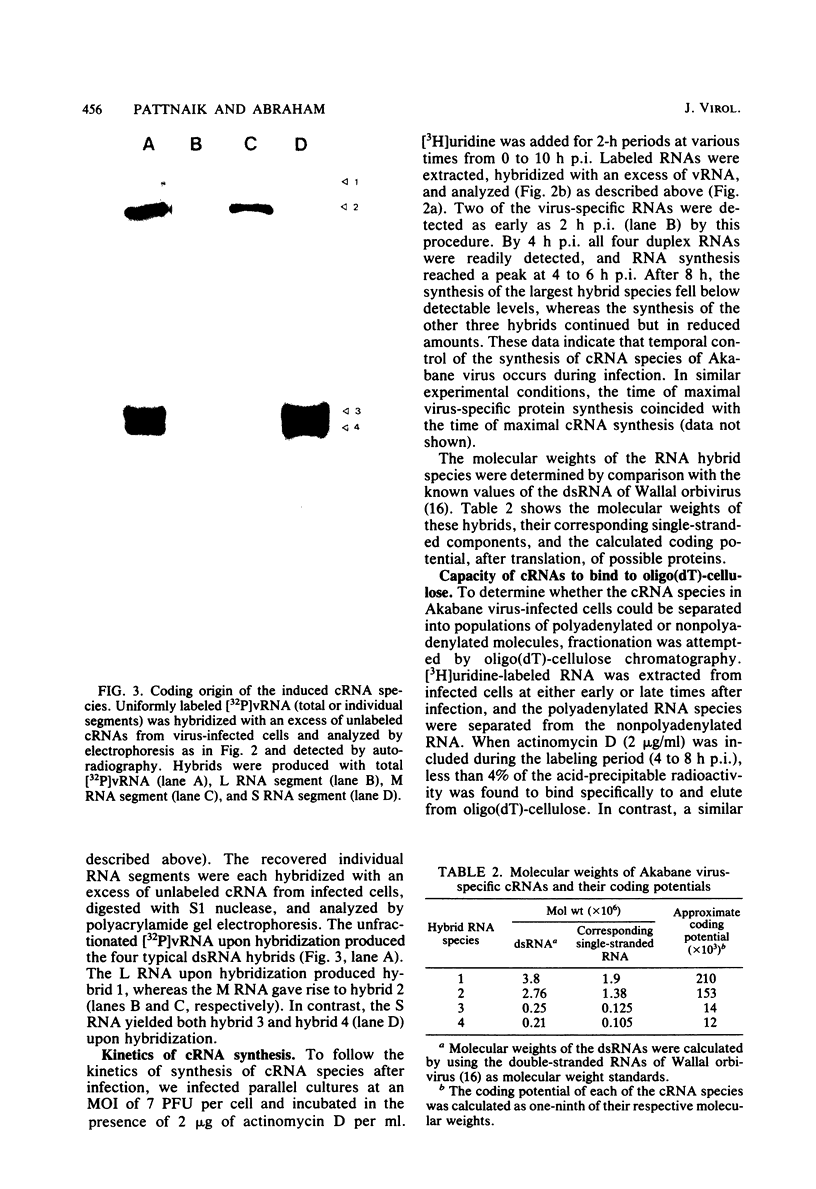
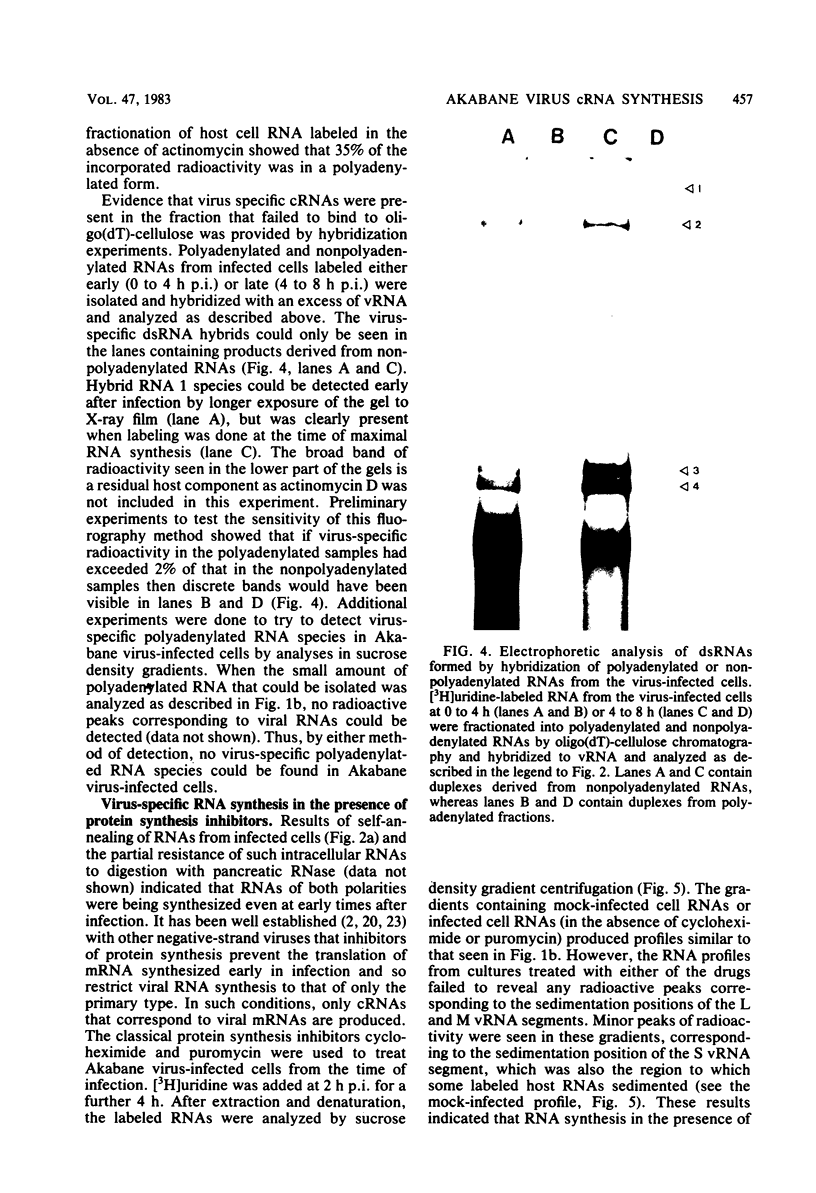
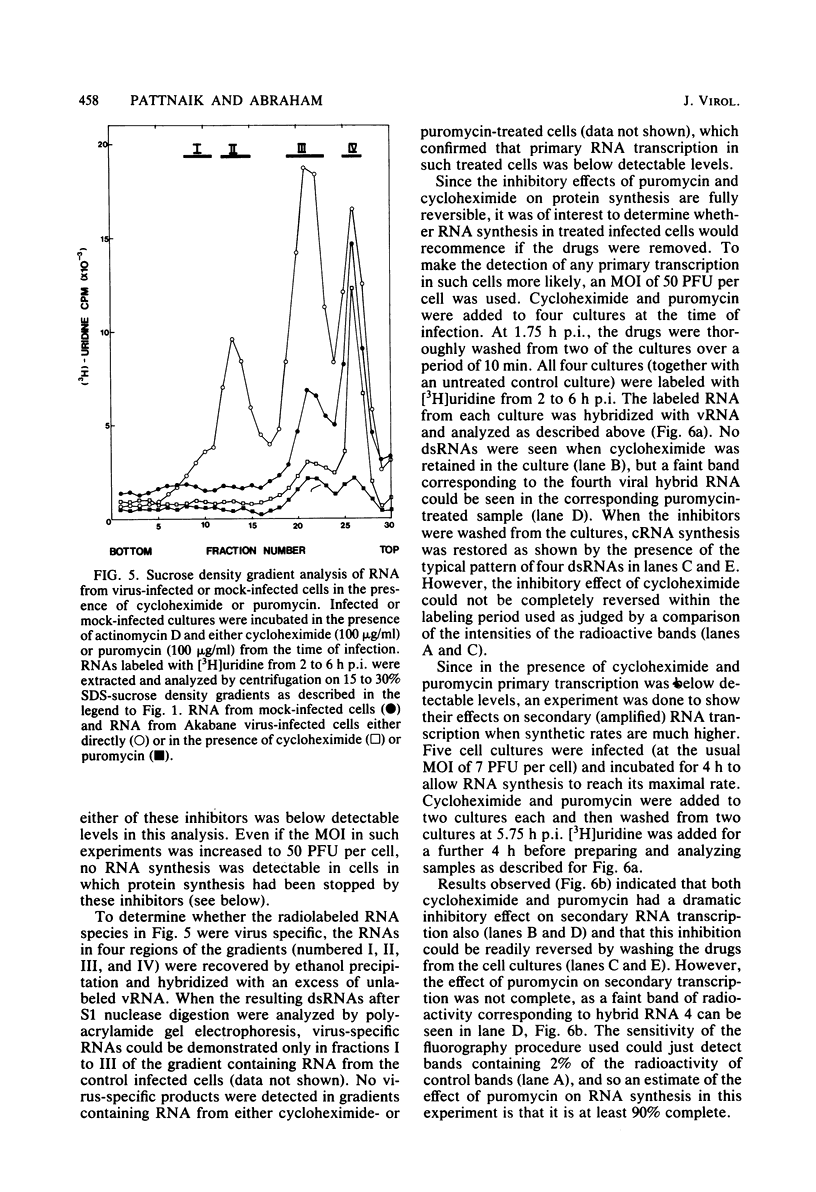
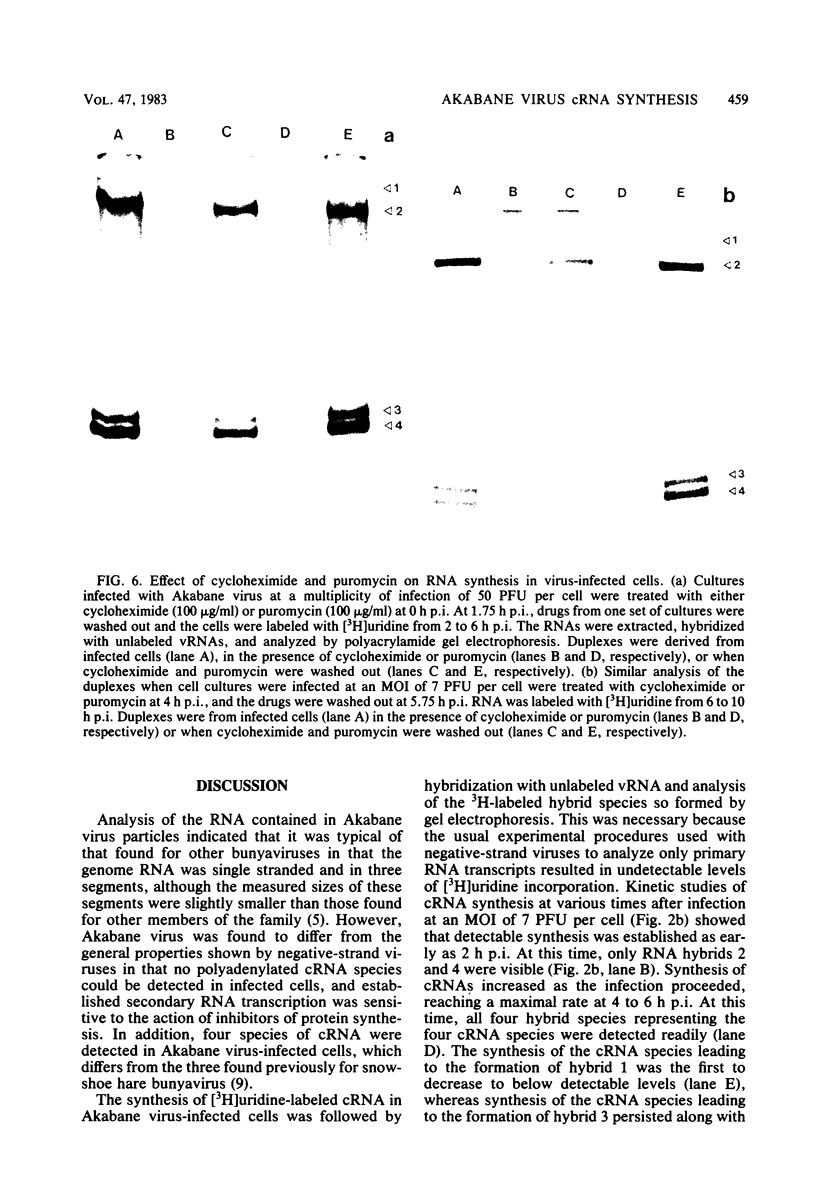
The analysis of RNA extracted from purified Akabane virus demonstrated the presence of three size classes of single-stranded RNAs with sedimentation coefficients of 31S (large, L), 26S (medium, M), and 13S (small, S). Molecular weights of these RNA species were estimated to be 2.15 X 10(6), 1.5 X 10(6), and 0.48 X 10(6) for the L, M, and S RNAs, respectively. Hybridization analysis involving viral genomic RNA and RNA from virus-infected cells resulted in the identification of four virus-specific cRNA species in infected cells. These cRNAs were found to be nonpolyadenylated by their inability to bind to oligodeoxythymidylate-cellulose. Kinetic analysis of cRNA synthesis in infected cells at various times postinfection suggested that cRNA synthesis could be detected as early as 2 h postinfection and that maximal synthesis occurred at 4 to 6 h postinfection. The RNAs synthesized in infected cells could be partially resolved by sucrose density gradient centrifugation. The RNA fraction that cosedimented with the S segment of viral genomic RNA yielded two duplex RNA species when hybridized with viral genomic RNA, suggesting the presence of two small cRNA species. Specific hybridization with individual viral genomic RNAs confirmed that two species of cRNA are coded by the S RNA segment. Analysis of cRNA synthesis in the presence of the protein synthesis inhibitors cycloheximide and puromycin indicated that cycloheximide completely inhibited virus-specific RNA synthesis early and late in infection, whereas a very low level of synthesis occurred in the presence of puromycin. The inhibitory effects of these drugs were found to be reversible when the drugs were washed from the cells. It is concluded that continued protein synthesis is required for cRNA synthesis to proceed in Akabane virus-infected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. The effect of ultraviolet radiation on the primary transcription of influenza virus messenger RNAs. Virology. 1979 Aug;97(1):177–182. doi: 10.1016/0042-6822(79)90384-2. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Beaty B. J., Shope R. E. Recombination and gene coding assignments of bunyaviruses and arenaviruses. Ann N Y Acad Sci. 1980;354:84–106. doi: 10.1111/j.1749-6632.1980.tb27960.x. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Bishop D. H. Genetic potential of bunyaviruses. Curr Top Microbiol Immunol. 1979;86:1–33. doi: 10.1007/978-3-642-67341-2_1. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Gould K. G., Akashi H., Clerx-van Haaster C. M. The complete sequence and coding content of snowshoe hare bunyavirus small (S) viral RNA species. Nucleic Acids Res. 1982 Jun 25;10(12):3703–3713. doi: 10.1093/nar/10.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cash P., Vezza A. C., Gentsch J. R., Bishop D. H. Genome complexities of the three mRNA species of snowshoe hare bunyavirus and in vitro translation of S mRNA to viral N polypeptide. J Virol. 1979 Sep;31(3):685–694. doi: 10.1128/jvi.31.3.685-694.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Bishop D. H. Identification of virus-coded nonstructural polypeptides in bunyavirus-infected cells. J Virol. 1982 Feb;41(2):643–648. doi: 10.1128/jvi.41.2.643-648.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. H. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol. 1978 Oct;28(1):417–419. doi: 10.1128/jvi.28.1.417-419.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. L. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J Virol. 1979 Jun;30(3):767–770. doi: 10.1128/jvi.30.3.767-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F., Shope R. E., Calisher C. E., Nathanson N. Characterization of monoclonal antibodies against the G1 and N proteins of LaCrosse and Tahyna, two California serogroup bunyaviruses. Virology. 1982 Jul 15;120(1):42–53. doi: 10.1016/0042-6822(82)90005-8. [DOI] [PubMed] [Google Scholar]
- Gorman B. M., Taylor J., Walker P. J., Young P. R. The isolation of recombinants between related orbiviruses. J Gen Virol. 1978 Nov;41(2):333–342. doi: 10.1099/0022-1317-41-2-333. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Lyons M. J. Bunyamwera virus. I. The molecular complexity of the virion RNA. Virology. 1977 Oct 1;82(1):37–47. doi: 10.1016/0042-6822(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Murphy F. A., Palmer E. L. Structural proteins of La Crosse virus. J Virol. 1976 Sep;19(3):985–997. doi: 10.1128/jvi.19.3.985-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Short N. J., Meek A. D., Dalgarno L. Seven infection-specific polypeptides in BHK cells infected with Bunyamwera virus. J Virol. 1982 Sep;43(3):840–843. doi: 10.1128/jvi.43.3.840-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Repik P. M., Cash P., Bishop D. H. In vivo transcription and protein synthesis capabilities of bunyaviruses: wild-type snowshoe hare virus and its temperature-sensitive group I, group II, and group I/II mutants. J Virol. 1979 Aug;31(2):426–436. doi: 10.1128/jvi.31.2.426-436.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]