Abstract
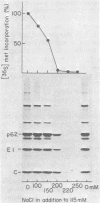
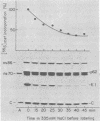
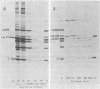
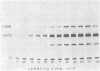
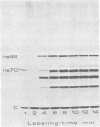
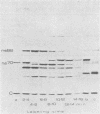
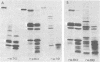
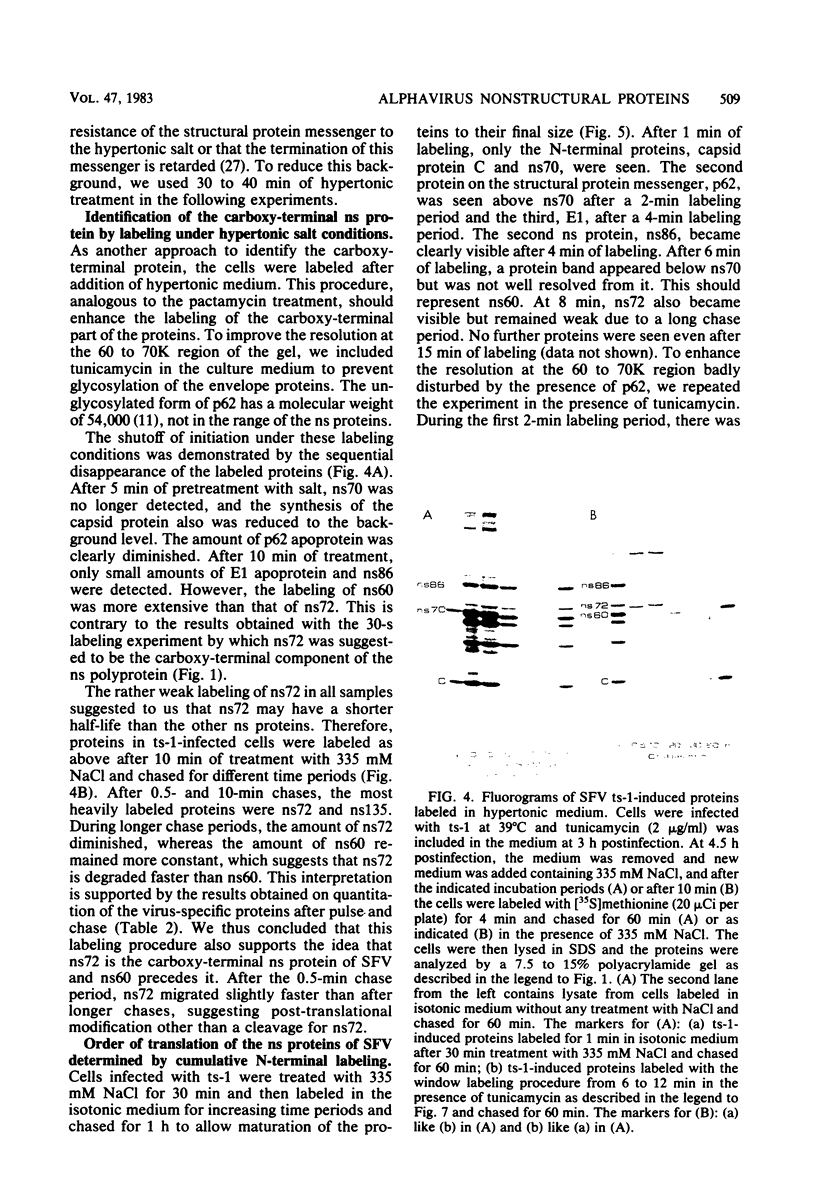
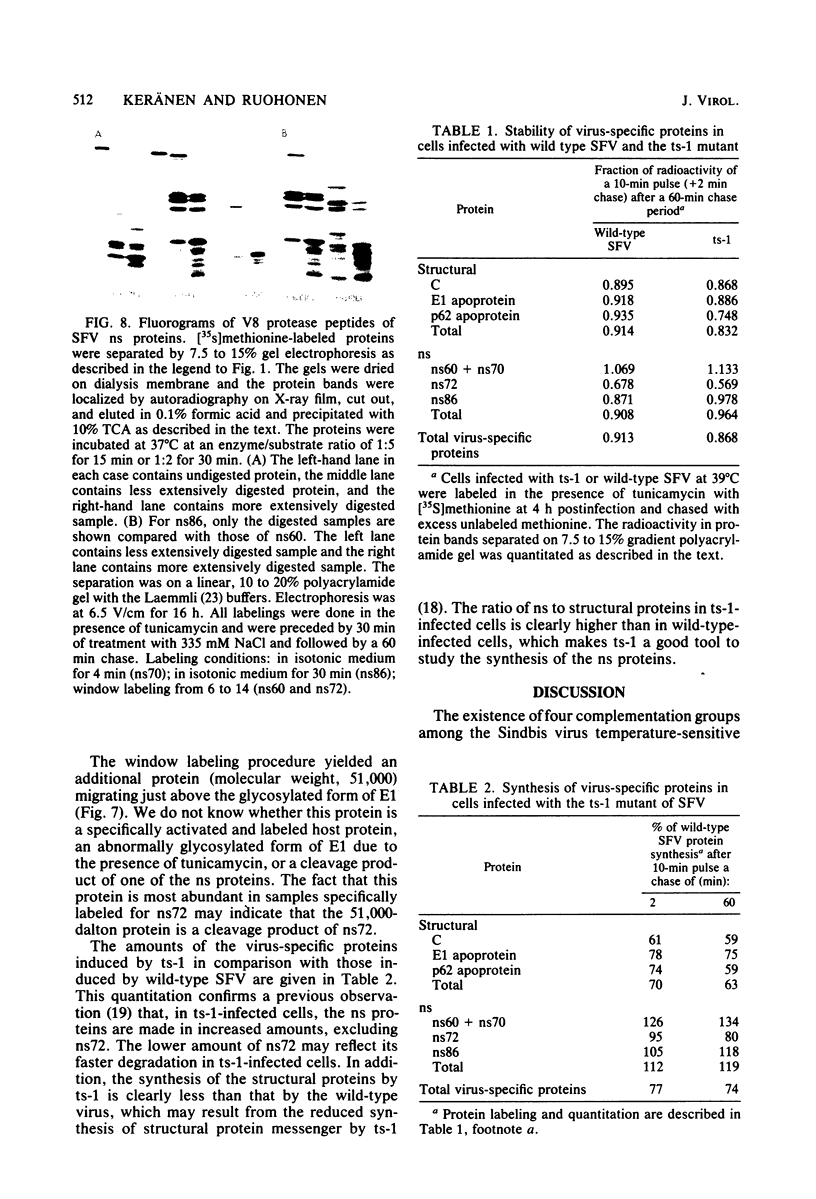
The synthesis of the nonstructural (ns) proteins of Semliki Forest virus was studied in vivo. The fourth ns protein, ns60, was identified and isolated. The order of translation (NH2-ns70-ns86-ns60-ns72-COOH) was determined by using various labeling procedures after or in the presence of a hypertonic block of translation initiation. A sequential labeling procedure was devised to specifically label defined segments of the polyprotein. The specific labeling procedures allowed isolation of the four ns proteins in radiochemically pure form by gradient polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. The four ns proteins were shown to have different primary structures by digestion with V8 protease of Staphylococcus aureus. The processing of the ns polyprotein and the stability of the mature ns proteins were studied by pulse-chase experiments. The cleavage of each of the proteins from the polyprotein took place within 2 to 3 min after the translation of the polypeptide chain. The N-terminal protein, ns70, appeared in its mature form later than ns86, which follows it in the polyprotein, suggesting that ns70 undergoes a post-translational modification. The migration of the C-terminal protein, ns72, immediately after a pulse was slightly faster than after a chase, suggesting that ns72 also undergoes a post-translational modification other than a cleavage. The half-life of ns72 was shorter than that of the other ns proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of Sindbis virus nonstructural polypeptides in chicken embryo fibroblasts. J Virol. 1977 May;22(2):420–429. doi: 10.1128/jvi.22.2.420-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Sequential translation of capsid and membrane protein genes of alphaviruses. Nature. 1975 Apr 3;254(5499):454–455. doi: 10.1038/254454a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins P. L., Fuller F. J., Marcus P. I., Hightower L. E., Ball L. A. Synthesis and processing of Sindbis virus nonstructural proteins in vitro. Virology. 1982 Apr 30;118(2):363–379. doi: 10.1016/0042-6822(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fuller F. J., Marcus P. I. Sindbis virus. I. Gene order of translation in vivo. Virology. 1980 Dec;107(2):441–451. doi: 10.1016/0042-6822(80)90311-6. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Söderlund H. The amphiphilic membrane glycoproteins of Semliki Forest virus are attached to the lipid bilayer by their COOH-terminal ends. J Mol Biol. 1978 Sep 25;124(3):535–549. doi: 10.1016/0022-2836(78)90186-9. [DOI] [PubMed] [Google Scholar]
- Glanville N., Lachmi B. E., Smith A. E., Käriäinen L. Tryptic peptide mapping of the nonstructural proteins of Semliki Forest virus and their precursors. Biochim Biophys Acta. 1978 May 23;518(3):497–506. doi: 10.1016/0005-2787(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Glanville N., Lachmi B. E. Translation of proteins accounting for the full coding capacity of the Semliki Forest virus 42 S RNA genome. FEBS Lett. 1977 Sep 15;81(2):399–402. doi: 10.1016/0014-5793(77)80563-2. [DOI] [PubMed] [Google Scholar]
- Glanville N., Ranki M., Morser J., Käriäinen L., Smith A. E. Initiation of translation directed by 42S and 26S RNAs from Semliki Forest virus in vitro. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3059–3063. doi: 10.1073/pnas.73.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Proteins synthesized by Semliki Forest virus and its 16 temperature-sensitive mutants. J Virol. 1975 Aug;16(2):388–396. doi: 10.1128/jvi.16.2.388-396.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Sawicki D., Gomatos P. J. Cleavage defect in the non-structural polyprotein of Semliki Forest virus has two separate effects on virus RNA synthesis. J Gen Virol. 1978 Jun;39(3):463–473. doi: 10.1099/0022-1317-39-3-463. [DOI] [PubMed] [Google Scholar]
- Lachmi B. E., Glanville N., Keränen S., Läriäinen L. Tryptic peptide analysis on nonstructural and structural precursor proteins from Semliki Forest virus mutant-infected cells. J Virol. 1975 Dec;16(6):1615–1629. doi: 10.1128/jvi.16.6.1615-1629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmi B. E., Käriäinen L. Sequential translation of nonstructural proteins in cells infected with a Semliki Forest virus mutant. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1936–1940. doi: 10.1073/pnas.73.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Ulmanen I., Käriäinen L., Keränen S., Philipson L. Synthesis and processing of Semliki Forest virus-specific nonstructural proteins in vivo and in vitro. Eur J Biochem. 1980 Dec;112(3):461–468. doi: 10.1111/j.1432-1033.1980.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]