Biliary malignant tumours represent less than 1% of all neoplastic processes in North America. Most of them are of adenocarcinomatous type; only 2% of biliary neoplasms are of squamous cell origin. Although 20 cases of squamous cell carcinoma of the biliary tree have been reported, only 6 cases were well documented.1,2 We report a case of primary squamous cell carcinoma at the bifurcation level that may be only the second such case published in the literature.1,3 We describe the clinical presentation and surgical procedure and discuss the current imaging modalities and therapy recommended for the management of this rare condition.
Case report
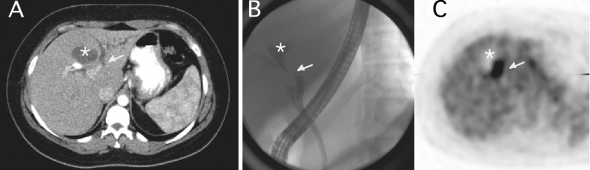
A 28-year-old woman was referred with a 4-week history of continuous, moderate right upper quadrant pain associated with jaundice, as well as weight loss (10 kg over 3 months) and a liver mass identified by ultrasonography. The pain sensation seemed different from previous colicky attacks the patient had experienced before she underwent laparoscopic cholecystectomy 7 years previously. On physical examination, she was obese (body mass index 37.8), with icterus noted over the conjunctivae, oral mucosa and skin. Imaging modalities included computed tomography (CT), positron emission tomography (PET) and endoscopic cholangiopancreatography (ERCP) (Fig. 1).
FIG. 1. Imaging modalities used to highlight a primary squamous cell carcinoma of the bile ducts. (A) CT scan of the liver showed an avascular liver mass with no distortion of the liver parenchyma (*), dilatation of the left biliary system with possible stricture of the left main duct (arrow) and collapse of the common bile duct (not shown). (B) ERCP showed communication of the left biliary system with the cystic mass (*) and a stricture at the bifurcation level with a stricture in the main left duct. The right biliary system is not seen. The cystic duct is visualized. (C) PET findings included a hypoactive region corresponding to the cystic mass (*) and a hyperactive region corresponding to the stricture area (arrow), suggesting malignant disease.
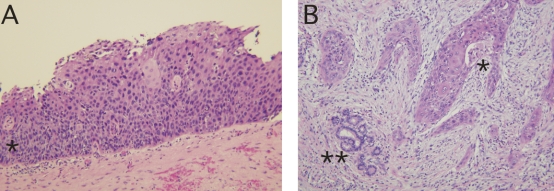
The patient underwent exploratory laparotomy. Intraoperative ultrasonography revealed a cystic lesion measuring 3.5 2.5 cm within the central portion of the liver, anterior to the porta hepatis. Intraoperative cholangiography demonstrated an extensive stricture obliterating the left hepatic duct, with partial occlusion of the right hepatic duct. An extended left lobectomy was done en bloc with the biliary confluence. On frozen-section examination, all margins of the excised specimen were free of malignant cells. Reconstruction was performed with a Roux-en-Y cholangiojejunostomy to 3 second bile duct radicals in the right side. Intraoperatively, radiotherapy was applied to the surgical margins. After this, the patient underwent 6 weeks of image-guided external beam radiation centred on the resection field labelled at surgery. The final pathology described this tumour as an infiltrating, moderately differentiated squamous cell carcinoma associated with severe dysplasia of the bile-duct epithelium (Fig. 2). The patient recovered without complications and was doing well 18 months after the initial surgical procedure, with an unremarkable CT scan.
FIG. 2. Primary squamous cell carcinoma of the bile ducts: pathological findings. (A) Section of a large biliary duct showing high-grade squamous dysplasia (*). (B) Invasive, moderately differentiated squamous cell carcinoma of the biliary tree (*). Note the residual accessory bile ducts without evidence of neoplasia (**) (hematoxylin–eosin stain, original magnification 20×).
Discussion
In our patient, congenital abnormalities (i.e., choledochal cyst, biliary cystadenoma), which may certainly manifest in a young patient, were not evident on previous laparoscopy. Associated conditions such as primary sclerosing cholangitis, hepatolithiasis or liver fluke infestation were not present. The mass consisted of an uncommon primary squamous cell carcinoma of the bile ducts managed by surgical resection and intraoperative radiation of the tissue margins complemented by external beam radiation therapy. At 18 months postoperatively, the patient was doing well, with no evidence of tumour recurrence.
The incidence of biliary tract cancers increases with age; most patients manifesting the disease are in the fifth to seventh decades of life. Primary sclerosing cholangitis and choledochal cyst malignant strictures develop in patients nearly 2 decades younger. Although most cases of gallbladder cancer and cholangiocarcinoma associated with recurrent pyogenic cholangitis are seen in women, the incidence of cholangiocarcinoma is slightly higher in men.1,3 Other associated conditions include Caroli disease and choledocholithiasis. However, the direct causality of some of those risk factors has been questioned, more so for perihilar and extrahepatic biliary malignant disease. Whether gallstones predispose to cholangiocarcinoma is unclear. The incidence of cholelithiasis in patients with cholangiocarcinoma is estimated at 30%, but this rate is also similar to that of control populations without cholangiocarcinoma.1,3
Surgical resection remains the best treatment option in the aim to cure.3–5 Intraoperative radiation of the surgical margins complemented with postoperative external beam radiation is suggested as an option to decrease local recurrence rates. When radiation therapy cannot be initiated during the surgical procedure, demarcation of the operative field may be useful to define the external radiation area. Several observational series suggest improvements in long-term survival with the use of postoperative radiotherapy in such patients.5 Studies of patients with cholangiocarcinoma who received adjuvant chemotherapy without radiation showed no improvement in survival; patients had either complete or incomplete resection of their tumours. Other studies showed improved control of the cholangiocarcinoma growth rate, with both chemotherapy and radiation perhaps acting synergistically. Randomized controlled trials are still needed to ascertain the combined benefit of chemotherapy and radiotherapy.4,5
The documentation of such a rare tumour as primary biliary squamous cell carcinoma calls for a better understanding of its pathogenesis and management for optimal survival rates. We recommend primary surgical treatment with intraoperative radiotherapy of the resection margins followed by postoperative external radiation.
Competing interests: None declared.
Accepted for publication July 3, 2007
Correspondence to: Dr. J. Sanabria, 11100 Euclid Ave., Lakeside 7506 PS-5047, Division of Transplant and HB Surgery, University Hospitals–Case Medical Center, Cleveland, OH 44106; juan.sanabria@uhhospitals.org, juan.sanabria@case.edu
References
- 1.Aranha GV, Reyes CV, Greenlee HB, et al. Squamous cell carcinoma of the proximal bile duct — a case report. J Surg Oncol 1980;15:29-35. [DOI] [PubMed]
- 2.Sewkani A, Kapoor S, Sharma S, et al. Squamous cell carcinoma of the distal common bile duct. JOP 2005;6:162-5. [PubMed]
- 3.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers [review]. N Engl J Med 1999;341:1368-78. Comment in: N Engl J Med 2000;342:663-4. [DOI] [PubMed]
- 4.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8. [DOI] [PMC free article] [PubMed]
- 5.Serafini FM, Sachs D, Bloomston M, et al. Location, not staging, of cholangiocarcinoma determines the role for adjuvant chemoradiation therapy. Am Surg 2001;67:839-43. [PubMed]