Abstract
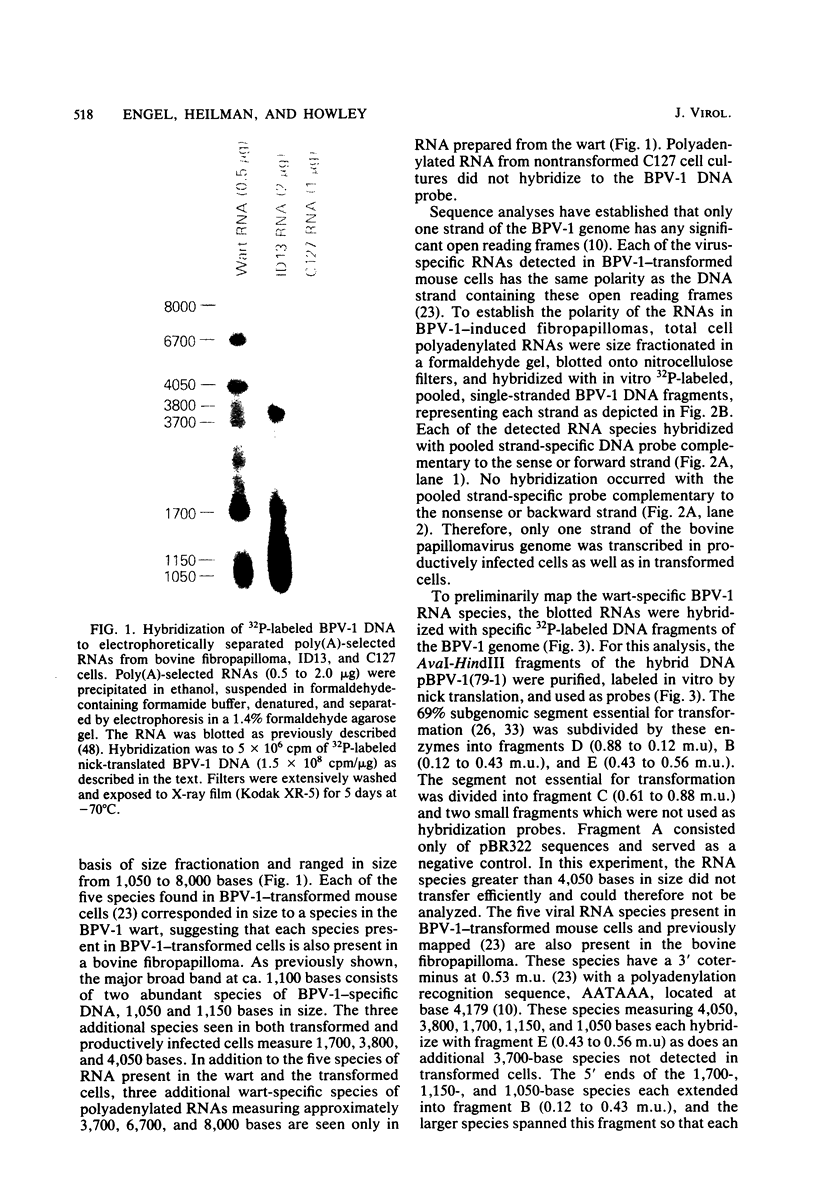
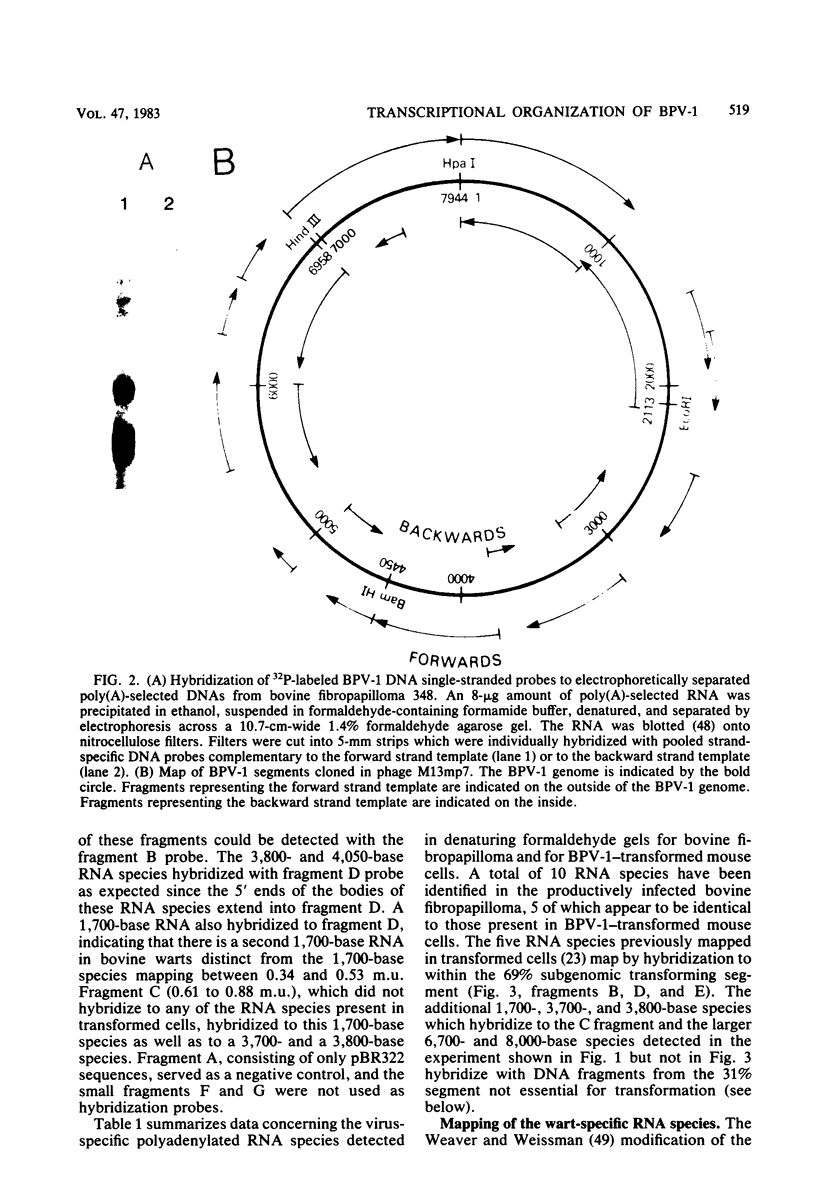
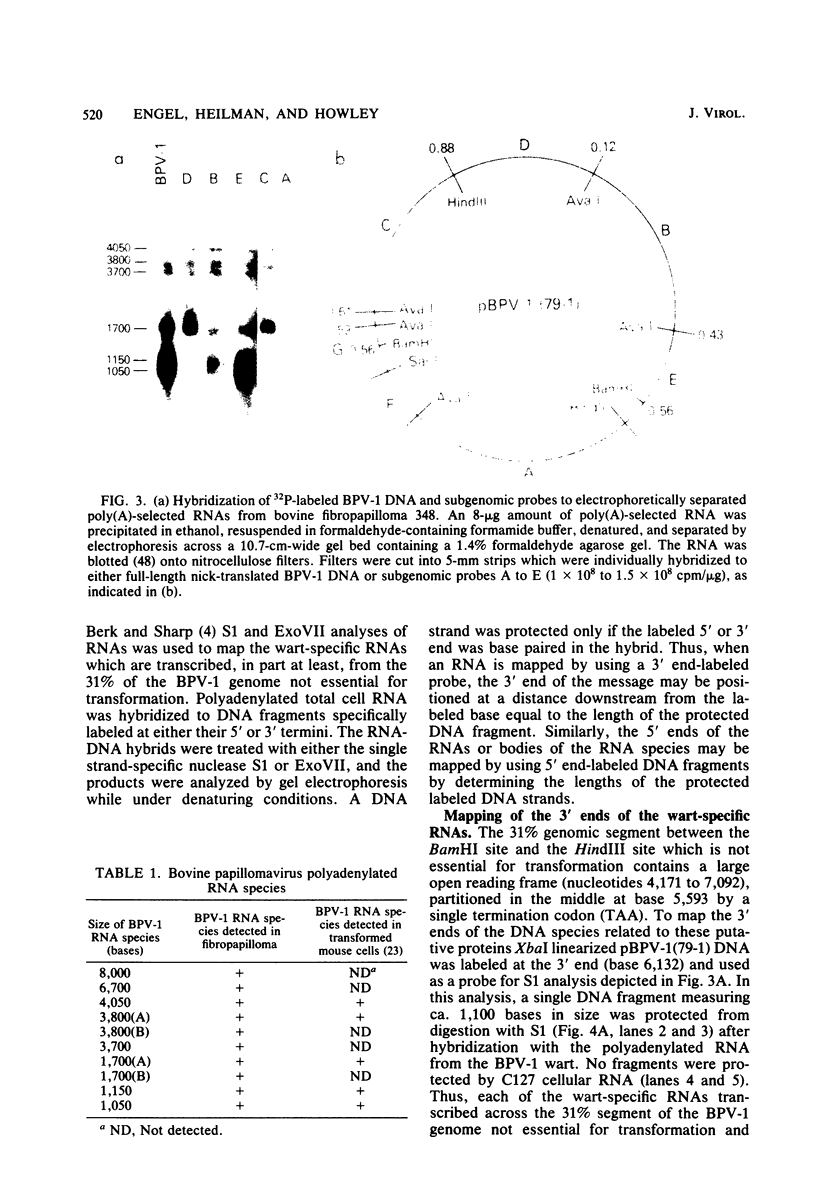
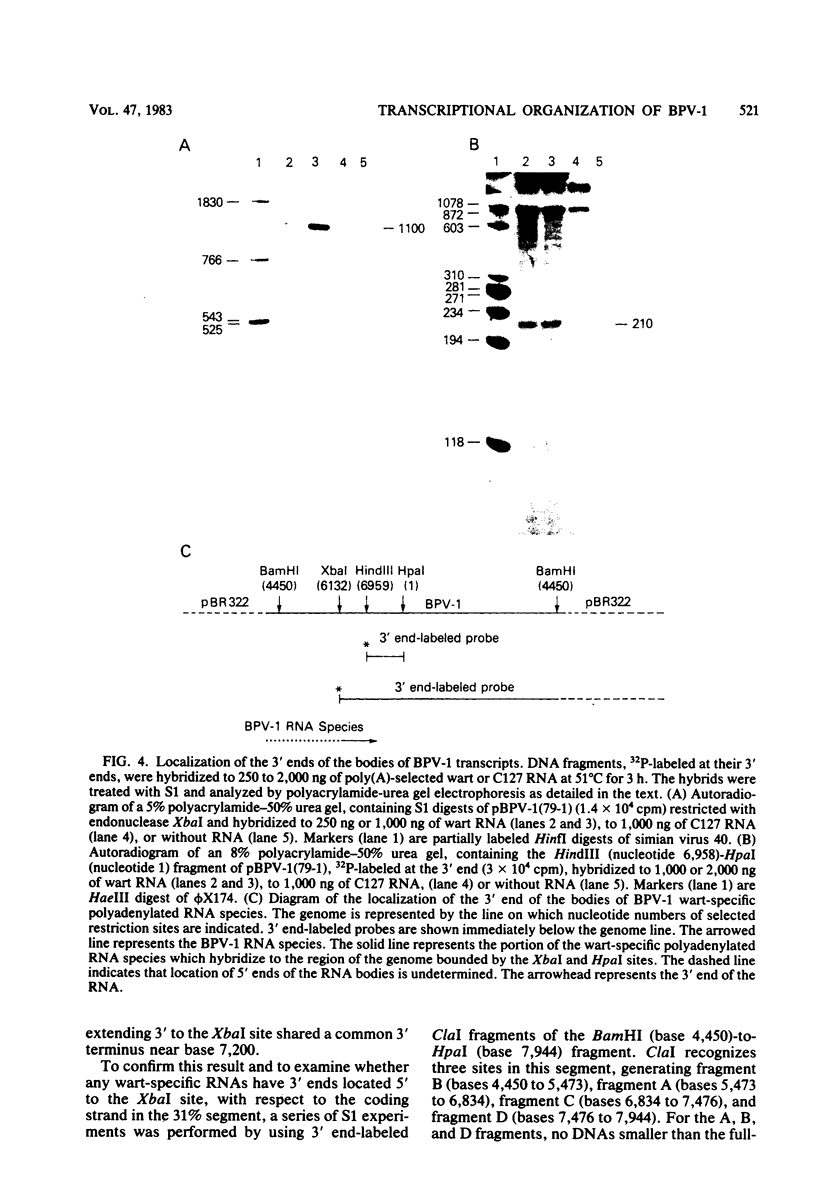
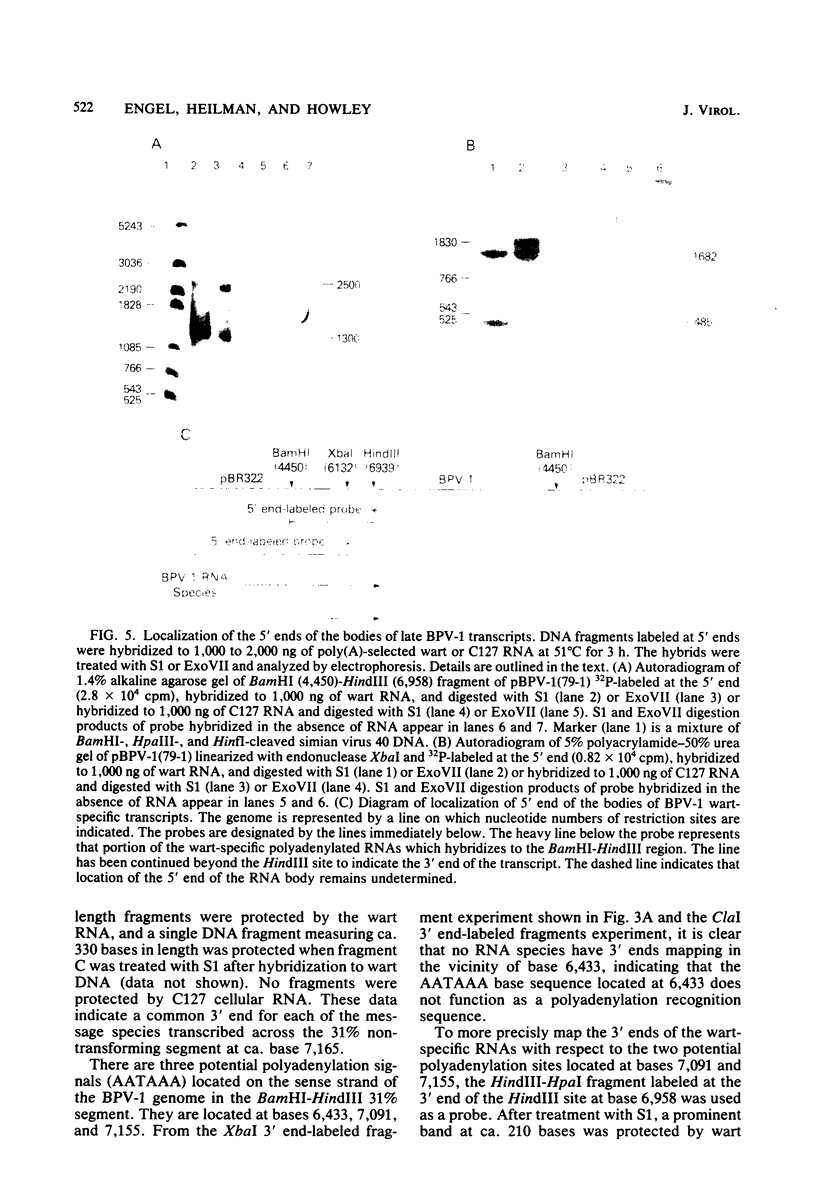
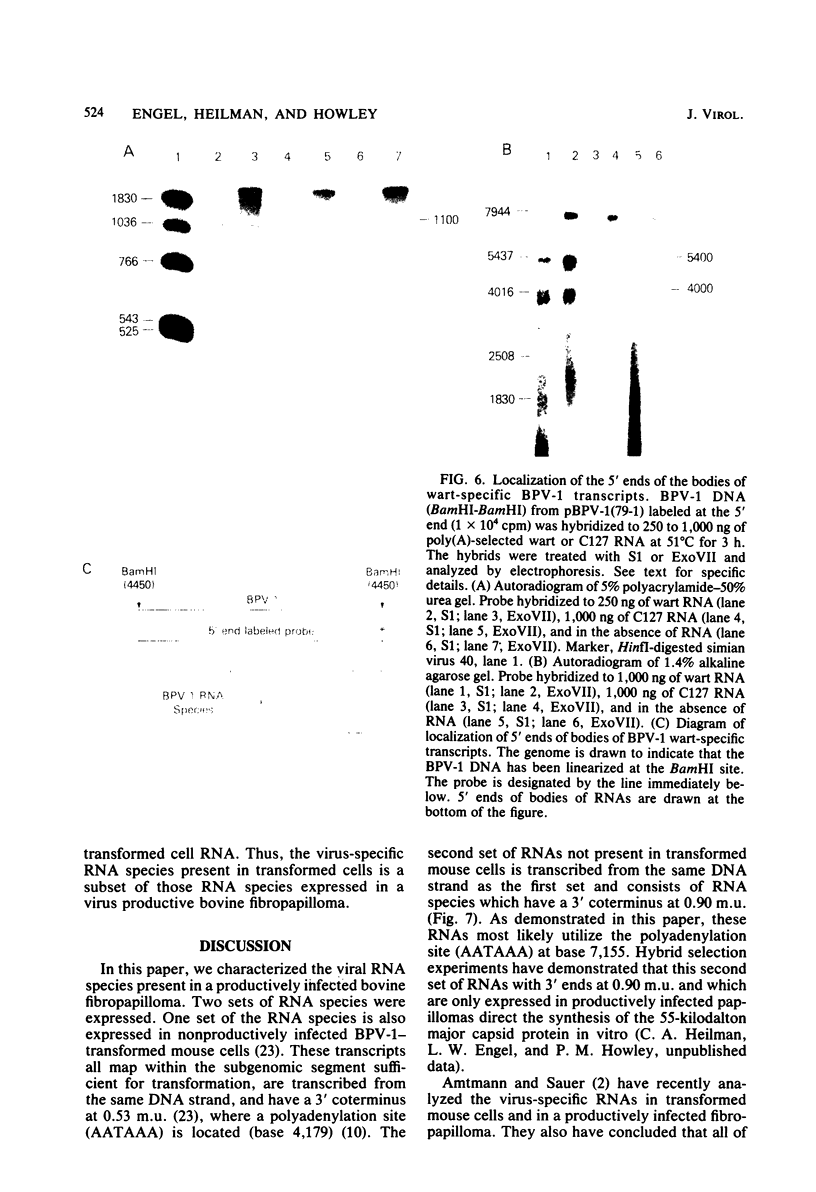
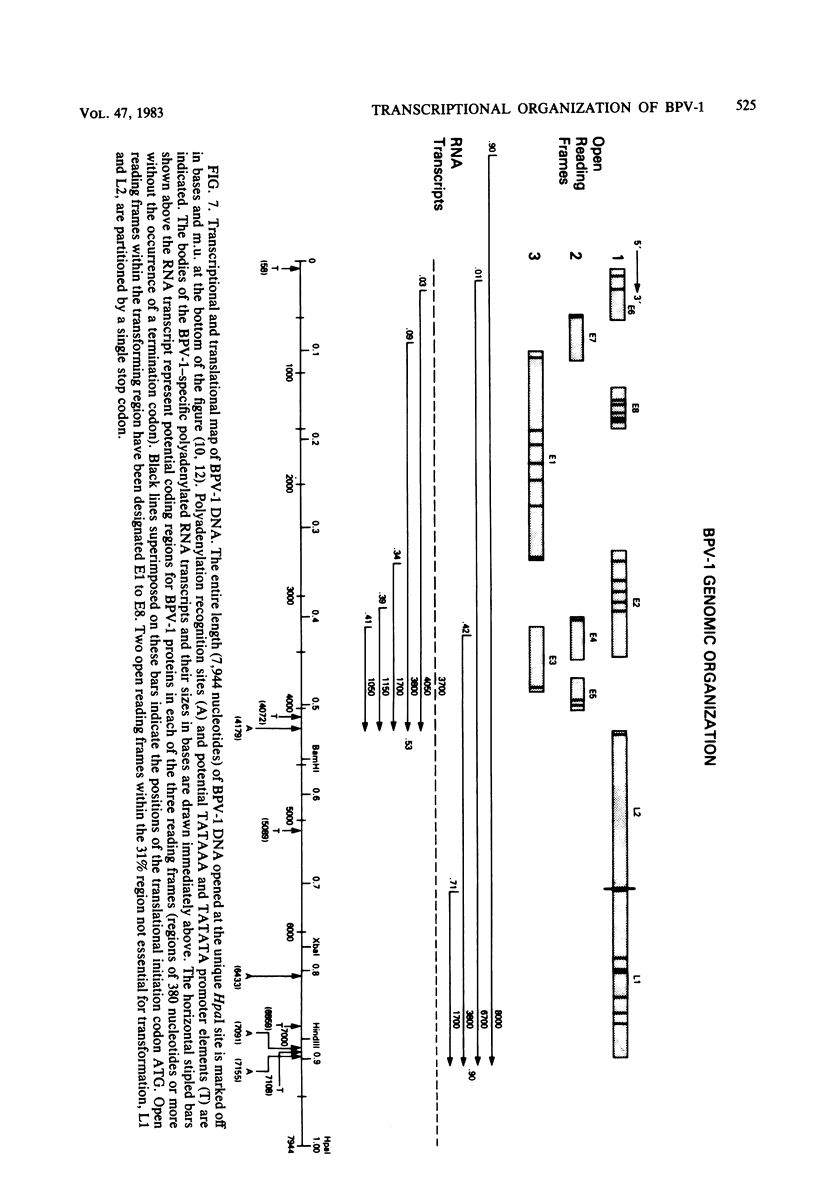
Multiple bovine papillomavirus type 1 (BPV-1)-specific polyadenylated RNA species in a BPV-1-infected bovine fibropapilloma were identified and mapped. All of the RNA species were transcribed from the same DNA strand of the BPV-1 genome. Five RNA species previously identified in BPV-1-transformed mouse cells were also present in the bovine fibropapilloma. These five species measured 1,050, 1,150, 1,700, 3,800, and 4,050 bases, mapped within the 69% transforming segment of the BPV-1 genome, and shared a 3' coterminus at 0.53 map units (m.u.). The 5' ends of the bodies of these distinct transcripts were located at ca. 0.03, 0.09, 0.34, 0.39, and 0.41 m.u. Additional polyadenylated RNA species not present in BPV-1-transformed mouse cells were specific for the BPV-1-infected bovine fibropapilloma and measured 1,700, 3,700, 3,800, 6,700, and 8,000 bases. These wart-specific species shared a 3' coterminus at 0.90 m.u. The 5' termini of the bodies of the 1,700- and 3,800-base species mapped at 0.71 and 0.42 m.u., respectively. Exonuclease VII analysis failed to reveal any internal splicing in these two species; however, the presence of small remote 5' leader sequences could not be ruled out. The 3,700-base species hybridized to DNA fragments from the 69% transforming segment as well as from the 31% nontransforming segment of the BPV-1 genome; however, this species was not precisely mapped. The 5' termini of the two largest RNA species (6,700 and 8,000 bases in size) were located at ca. 0.01 and 0.90 m.u., respectively. Since the 5' ends of these mapped adjacent to a TATAAA sequence which could possibly serve as an element of a transcriptional promoter, it is possible that one or both of these species represent nonspliced precursor RNA molecules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann E., Sauer G. Bovine papilloma virus transcription: polyadenylated RNA species and assessment of the direction of transcription. J Virol. 1982 Jul;43(1):59–66. doi: 10.1128/jvi.43.1.59-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., HARTLEY J. W., ROWE W. P., HUEBNER R. J. TRANSFORMATION OF BOVINE TISSUE CULTURE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1963 Sep 7;199:1016–1018. doi: 10.1038/1991016a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., LEVY J. P., THOMAS M., FRIEDMANN J. C., BERNARD J. SOME PROPERTIES OF BOVINE PAPILLOMA VIRUS. Nature. 1964 Jan 25;201:423–424. doi: 10.1038/201423a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binétruy B., Meneguzzi G., Breathnach R., Cuzin F. Recombinant DNA molecules comprising bovine papilloma virus type 1 DNA linked to plasmid DNA are maintained in a plasmidial state both in rodent fibroblasts and in bacterial cells. EMBO J. 1982;1(5):621–628. doi: 10.1002/j.1460-2075.1982.tb01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F., Favre M., Zoorob R., Fortin D., Orth G. Detection and characterization of viral genomes and search for tumoral antigens in two hamster cell lines derived from tumors induced by bovine papillomavirus type 1. Int J Cancer. 1981 May 15;27(5):693–702. doi: 10.1002/ijc.2910270517. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Engel L. W., Chen E. Y., Yaniv M., Howley P. M. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J Virol. 1983 May;46(2):557–566. doi: 10.1128/jvi.46.2.557-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Molecular cloning, refined physical map and heterogeneity of methylation sites of papilloma virus type 1a DNA. Eur J Biochem. 1980 Aug;109(2):457–461. doi: 10.1111/j.1432-1033.1980.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN J. C., LEVY J. P., LASNERET J., THOMAS M., BOIRON M., BERNARD J. INDUCTION DE FIBROMES SOUS-CUTAN'ES CHEZ LE HAMSTER DOR'E PAR INOCULATION D'EXTRAITS ACELLULAIRES DE PAPILLOMES BOVINS. C R Hebd Seances Acad Sci. 1963 Oct 14;257:2328–2331. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Freese U. K., Schulte P., Pfister H. Papilloma virus-induced tumors contain a virus-specific transcript. Virology. 1982 Feb;117(1):257–261. doi: 10.1016/0042-6822(82)90525-6. [DOI] [PubMed] [Google Scholar]
- Geraldes A. Malignant transformation of hamster cells by cell-free extracts of bovine papillomas (in vitro). Nature. 1969 Jun 28;222(5200):1283–1284. doi: 10.1038/2221283a0. [DOI] [PubMed] [Google Scholar]
- Gibbs E. P., Smale C. J., Lawman M. J. Warts in sheep. Identification of a papilloma virus and transmission of infection to sheep. J Comp Pathol. 1975 Apr;85(2):327–334. doi: 10.1016/0021-9975(75)90075-4. [DOI] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Heilman C. A., Law M. F., Israel M. A., Howley P. M. Cloning of human papilloma virus genomic DNAs and analysis of homologous polynucleotide sequences. J Virol. 1980 Nov;36(2):395–407. doi: 10.1128/jvi.36.2.395-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Olson C. Attempted transmission of warts from man, cattle, and horses and of deer fibroma, to selected hosts. J Invest Dermatol. 1972 Jun;58(6):366–368. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Theilen G. H., Olson C. Hybridization of bovine papilloma virus type 1 and type 2 DNA to DNA from virus-induced hamster tumors and naturally occurring equine tumors. Intervirology. 1979;11(4):227–233. doi: 10.1159/000149038. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Berg L., Weiher H., Botchan M. Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol. 1983 Jun;3(6):1108–1122. doi: 10.1128/mcb.3.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meischke H. R. In vitro transformation by bovine papilloma virus. J Gen Virol. 1979 Jun;43(3):473–487. doi: 10.1099/0022-1317-43-3-473. [DOI] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H., Jarrett W. F. Persistence of non-integrated viral DNA in bovine cells transformed in vitro by bovine papillomavirus type 2. Nature. 1981 Oct 29;293(5835):749–751. doi: 10.1038/293749a0. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O., Stevens J. G. Two colinear and spliced viral transcripts are present in non-virus-producing benign and malignant neoplasms induced by the shope (rabbit) papilloma virus. J Virol. 1982 Oct;44(1):263–268. doi: 10.1128/jvi.44.1.263-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON C., Jr, COOK R. H. Cutaneous sarcoma-like lesions of the horse caused by the agent of bovine papilloma. Proc Soc Exp Biol Med. 1951 Jun;77(2):281–284. doi: 10.3181/00379727-77-18750. [DOI] [PubMed] [Google Scholar]
- Puget A., Favre M., Orth G. Induction de tumeurs fibroblastiques cutanées ou sous-cutanées chez l'Ochotone afghan (Ochotona rufescens rufescens) par inoculation du virus du papillome bovin. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jun 23;280(24):2813–2816. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SMITHIES L. K., OLSON C. Antigen of bovine cutaneous papilloma detected by fluorescent antibodies. Cancer Res. 1961 Dec;21:1557–1559. [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Leung D. W., Gillam S., Astell C. R., Montgomery D. L., Hall B. D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979 Apr;16(4):753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- THOMAS M., BOIRON M., TANZER J., LEVY J. P., BERNARD J. IN VITRO TRANSFORMATION OF MICE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1964 May 16;202:709–710. doi: 10.1038/202709a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Stevens J. G. Transcription of the viral genome in papillomas and carcinomas induced by the Shope virus. Virology. 1981 Mar;109(2):448–451. doi: 10.1016/0042-6822(81)90517-1. [DOI] [PubMed] [Google Scholar]