Abstract
Background
Clinical studies have demonstrated that intracoronary or intramyocardial transplantation of bone marrow mononuclear cells (BMMNCs) into ischemic myocardium improves cardiac function. The objective of the present study was to evaluate the safety and feasibility of intramyocardial BMMNC transplantation into nongraftable areas in combination with off-pump coronary artery bypass grafting in patients with ischemic cardiomyopathy.
Methods
Five male patients with myocardial infarction lasting for more than 1 month and with nongraftable myocardium received autologous mononuclear cell transplantation during off-pump coronary artery bypass grafting. Autologous bone marrow was aspirated from the iliac crest. BMMNCs (mean 1.6, standard error [SE] 0.3 × 109) including CD34-positive cells (mean 6.8, SE 1.1 × 106) and AC133-positive cells (mean 3.1, SE 1.7 × 106) were injected into the nongraftable ischemic myocardium. Heart function was evaluated with the use of echocardiography, and myocardial perfusion was examined with single photon emission computed tomography technetium-99mTc sestamibi scans.
Results
Two months after cell transplantation, the mean ejection fraction had increased by 7.4%, SE 1.9% (p = 0.016) compared with that before cell transplantation and off-pump coronary artery bypass grafting. The increase in ejection fraction was not correlated with the number of transplanted total mononuclear cells, CD34-positive cells and AC133-positive cells. Myocardial perfusion at the cell-transplanted area increased after cell transplantation and off-pump coronary artery bypass grafting. No arrhythmia was observed.
Conclusion
The present clinical study suggests that intramyocardial transplantation of autologous BMMNCs into the ischemic area during off-pump coronary artery bypass grafting is both feasible and safe and has beneficial effects on cardiac function.
Abstract
Contexte
Des études cliniques ont démontré que la transplantation intracoronarienne ou intramyocardique de cellules mononucléaires de moelle osseuse (CMNMO) dans un myocarde ischémique améliore la fonction cardiaque. L'étude visait à évaluer la sécurité et la faisabilité d'une transplantation intramyocardique de CMNMO dans des régions non greffables combinée à un pontage aortocoronarien hors circuit chez des patients atteints de myocardiopathie ischémique.
Méthodes
Cinq patients victimes d'un infarctus du myocarde ayant duré plus d'un mois et dont le myocarde ne permettait pas une greffe ont reçu une transplantation de cellules mononucléaires autologues au cours d'un pontage aortocoronarien pratiqué hors circuit. On a effectué une ponction de moelle osseuse autologue au niveau de la crête iliaque. On a injecté des CMNMO (moyenne de 1,6, erreur type [ET] de 0,3 × 109), y compris des cellules positives pour CD34 (moyenne de 6,8, ET de 1,1 × 106) et des cellules positives pour AC133 (moyenne de 3,1, ET de 1,7 × 106) dans le myocarde ischémique où l'on ne pouvait pratiquer de greffe. On a évalué la fonction cardiaque par échocardiographie et examiné une perfusion du myocarde par imagerie de perfusion myocardique par tomographie d'émission monophonique au technétium-99mTc.
Résultats
Deux mois après la transplantation de cellules, la fraction d'éjection moyenne avait augmenté de 7,4 %, avec ET de 1,9 % (p = 0,016), comparativement à ce qu'elle était avant la transplantation de cellules et le pontage aortocoronarien hors circuit. On n'a pas établi de lien entre l'augmentation de la fraction d'éjection et le nombre de cellules mononucléaires, de cellules positives pour CD34 et de cellules positives pour AC133 transplantées au total. La perfusion du myocarde dans la région de la transplantation de cellules a augmenté après celle-ci et le pontage aortocoronarien hors circuit. On n'a pas observé d'arythmie.
Conclusion
L'étude clinique en cours indique que la transplantation intramyocardique de CMNMO autologues dans la région ischémique au cours d'un pontage aortocoronarien hors circuit est à la fois faisable et sans danger et a des effets bénéfiques sur la fonction cardiaque.
Interventional revascularization and coronary artery bypass grafting are standard treatment methods for patients with ischemic cardiomyopathy. These approaches successfully relieve angina and improve heart function in most patients with ischemic myocardium. However, complete revascularization is not feasible in a large number of patients who have a nongraftable area of ischemic myocardium due to diffuse small vessel disease or multifocal stenotic coronary artery. Without the salvage of stunned or hibernating myocardium, myocardial ischemia rapidly results in necrosis and is followed by heart failure. Patients with end-stage heart failure can only be treated by heart transplantation. Although the outcome of heart transplantation has been excellent, this therapy is limited by the shortage of donor organs and the side effects of long-term immunosuppression.1,2
Cell transplantation has recently received great attention as an alternative strategy in the treatment of patients with failing hearts. Several studies using various animal models have reported that fetal cardiomyocytes,3 skeletal myoblasts,4 smooth muscle cells,5 embryonic stem cells6 and bone marrow cells7–9 survive in the infarcted myocardium after transplantation and improve heart function. The use of autologous stem cells eliminates both ethical concerns inherent in using embryonic or fetal cells and the clinical problem of immunorejection.10 In several clinical studies, transplantation of autologous bone marrow cells11–15 and skeletal myoblasts16,17 has been investigated for angiogenesis and myogenesis in ischemic myocardium. These studies demonstrated that stem cell transplantation enhanced myocardial perfusion and cardiac function in ischemic hearts of patients with acute or chronic myocardial infarction.
In the present study, autologous bone marrow mononuclear cells (BMMNCs) were transplanted by intramuscular injection into nongraftable ischemic myocardium in 5 patients who were not suitable candidates for complete revascularization. Cell transplantation was performed during off-pump coronary artery bypass grafting. We evaluated the clinical safety and feasibility of a combined therapy of bone marrow cell transplantation and off-pump coronary artery bypass grafting. We examined cardiac function and myocardial perfusion of the ischemic hearts 2 months after treatment.
Methods
Patients
The protocol for the clinical study conformed to the principles outlined in the Declaration of Helsinki,18 and the study was approved by the Ethical Committee of the Yonsei University College of Medicine. Written informed consent was obtained from all patients. Five patients were recruited in this study (Table 1). All had a myocardial infarction lasting for more than 1 month as well as unstable angina before admission. On electrocardiography (ECG), all patients were found to have a myocardial infarction with pathological Q waves. They did not have symptoms of congestive heart failure. After admission, the patients' vessel disease, myocardial infarction and cardiac function were examined. The patients had triple vessel coronary disease with distinct areas of akinesia or severe hypokinesia at the left ventricular wall, as identified by coronary angiography and echocardiography. In all the patients, 3 coronary arteries had severe stenosis and calcification, and thus it was not easy to try internal intervention such as coronary stent insertion or balloon dilatation. Because these patients had limited graftable areas in the myocardium, coronary artery bypass grafting alone did not seem an option to improve myocardial function significantly. These patients were not candidates for complete revascularization and had nongraftable but viable ischemic myocardium. Prior to operating, we determined graftable myocardium for coronary artery bypass grafting and viable regions in nongraftable myocardium for cell transplantation, using coronary angiography and magnetic resonance imaging (MRI), respectively. In these patients, improvement of myocardial function was not expected to occur in the nonbypassed region just by improvement of collateral blood flow. We used echocardiography to evaluate cardiac function. A single photon emission computed tomography (SPECT) technetium-99mTc sestamibi (MIBI) scan was used to confirm the angiographic and echocardiographic data and to identify the areas unsuitable for coronary artery bypass grafting or interventional revascularization as candidate regions for cell transplantation. All surgeries were performed under normal as opposed to emergency conditions, without an intra-aortic balloon pump.
Table 1
Cell preparation and identification
With the patients under general anesthesia, we aspirated 300–350 mL of bone marrow from the posterior left iliac crest and collected it in a tube containing heparinized buffered saline. A COBE 2991 Cell Processor (CaridianBCT) was used to isolate BMMNCs and concentrate them in a final volume of 10 mL. The total time for cell processing was less than 1 hour. The BMMNCs were immunocytochemically stained for CD34 and AC133 with the use of FITC-labelled anti-CD34 antibodies (BD Biosciences) and PE-labelled anti-AC133 antibodies (Miltenyi Biotech), respectively. The percentage of CD34- or AC133-positive cells was analyzed by 2-colour flow cytometry (BD FACScan; BD Biosciences) and compared with an immunoglobulin G isotype control.
Cell transplantation
During the isolation of mononuclear cells, we performed off-pump coronary artery bypass grafting on the patients, using composite grafts with a left internal mammary artery or a radial artery. We transplanted BMMNCs into myocardium, excluding graftable regions irrespective of myocardial infarction. BMMNCs were injected into nongraftable and infarcted regions in 3 patients (patients 1, 2 and 3). In noninfarcted myocardium, there can be nongraftable regions that are due to severe calcification or multifocal stenosis, and BMMNCs were injected into nongraftable and noninfarcted regions in 2 patients (patients 4 and 5). After coronary artery bypass grafting, 10 mL of BMMNC suspension was injected into 10–15 areas (0.7–1.0 mL per injection) of nongraftable myocardium with a 26-gauge needle. Finger pressure was applied at the injection sites to prevent cell leakage after cell transplantation. Before cell injection, patients received standard cardiac surgery. After completion of the operation, patients were transferred to the intensive care unit and monitored according to standard procedures.
Safety assessment
We monitored all patients by continuous ECG 2 days after their operation. Until discharge, ECG and auscultation were performed once and 6 times daily, respectively, to detect ventricular arrhythmia. At 2 and 4 months after coronary artery bypass grafting and BMMNC transplantation, we monitored all patients by ECG to detect any unfavourable events such as ventricular arrhythmia, inflammation and neoplasm. Immediately after surgery and 24 hours after surgery, we measured creatine kinase MB (CK-MB) in all patients.
Measurement of myocardial perfusion and cardiac function
Two months after coronary artery bypass grafting and cell transplantation, cardiac function of the patients was evaluated with echocardiography. One patient was followed with MRI to examine myocardial wall motion of the cell-injected region. Between 2 and 4 months after treatment, SPECT MIBI scanning was performed to evaluate myocardial perfusion. Data obtained were analyzed by hospital cardiologists.
Statistical analysis
Quantitative data were expressed as mean and standard error (SE). We compared cardiac ejection fraction (EF) before and after cell transplantation and coronary artery bypass grafting, using the paired t test. We used linear regression analysis to evaluate the correlation between cardiac function improvement and the number of transplanted cells.
Results
Characterization of transplanted BMMNCs
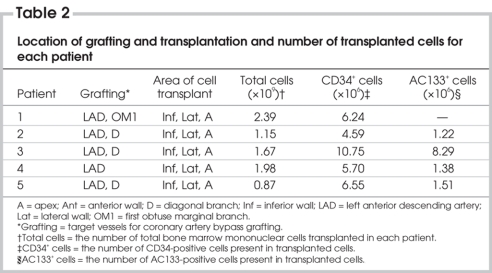
The total number of mononuclear cells (Fig. 1) isolated from 300 to 350 mL of bone marrow was 1.6 (SE 0.3) × 109. Flow cytometric analysis showed that the mononuclear cells contained 6.8 (SE 1.1) × 106 CD34-positive cells and 3.1 (SE 1.7) × 106 AC133-positive cells (Table 2).
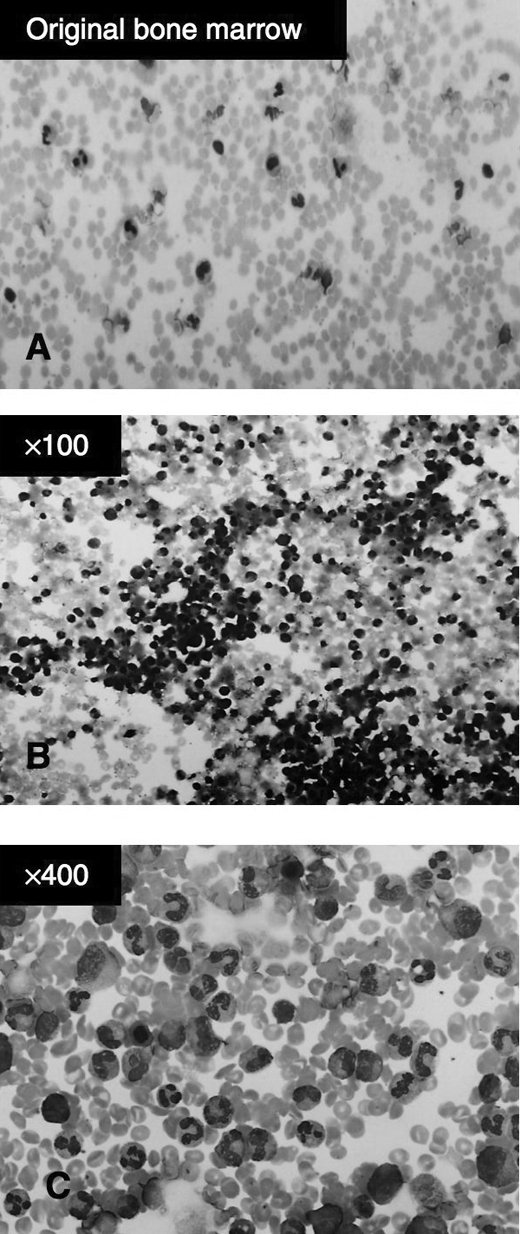
FIG. 1. Wright Giemsa staining of (A) freshly isolated bone marrow cells and (B and C) purified bone marrow mononuclear cells before cell transplantation.
Table 2
General patient information following cell transplantation
The patients received 1–2 bypass grafts. After bypass grafting, we performed multiple site injections of BMMNCs (0.87–2.39 × 109; mean 1.6 [SE 0.3] × 109 cells/patient). Because there was no obvious leakage from the cell injection sites, the injected cells might be directed into the nongraftable territories of myocardium. There was no mortality during or after surgery. Patients were extubated within the first 12 hours (range 4–12 h, mean 6 h) and monitored in the intensive care unit for 2 days. There was no ventricular arrhythmia or hemodynamic abnormality recorded during this period (Table 3). Immediately and 24 hours after surgery, no increase in the levels of CK-MB activity was detected in all patients. This result revealed that there was no myocardial damage in all patients during surgery. The median hospital time after coronary artery bypass grafting and cell transplantation was 9 (range 7–30) days. An improvement in symptoms was observed in all patients 2 months after coronary artery bypass grafting and cell transplantation.
Table 3
Cardiac function
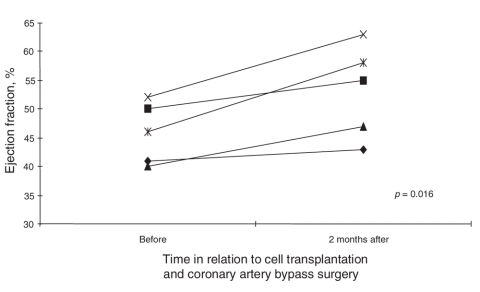
Prior to coronary artery bypass grafting and cell implantation, the mean EF of the 5 patients as evaluated by echocardiography was 45.8% (SE 2.4%) (Fig. 2). Two months after coronary artery bypass grafting and cell transplantation, the mean EF of the 5 patients was 53.2% (SE 3.6%) (Fig. 2). These results show that cell transplantation and coronary artery bypass grafting significantly improved cardiac function (p = 0.016).
FIG. 2. Ejection fraction of each patient before and 2 months after cell transplantation and coronary artery bypass surgery.
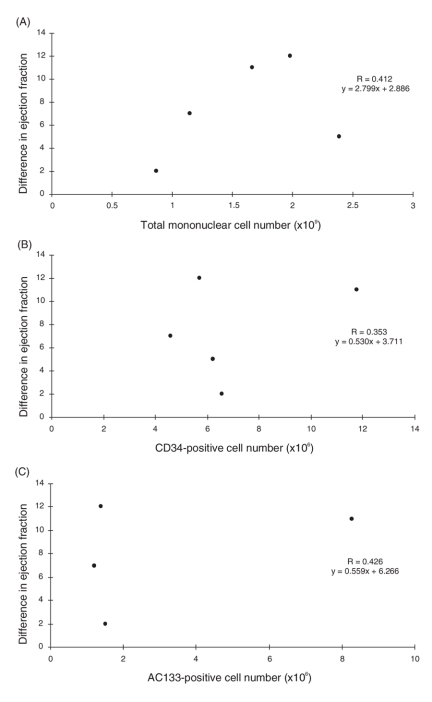
Correlations between the number of total transplanted mononuclear cells, CD34-positive cells and AC133-positive cells and the difference in EF before and after treatment were evaluated with linear regression analysis. No significant correlations were observed between the difference in EF and the number of total mononuclear cells, CD34-positive cells or AC133-positive cells (r = 0.412, 0.353 and 0.426, respectively, Fig. 3A, B and C).
FIG. 3. Correlation between the difference in ejection fraction before and after treatment and the number of transplanted (A) total mononuclear cells, (B) CD34-positive cells and (C) AC133-positive cells.
Myocardial perfusion
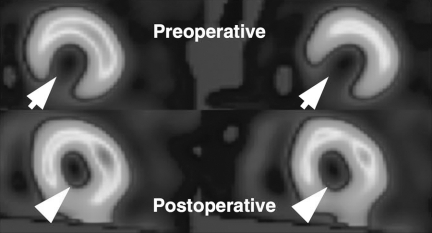
SPECT MIBI scans performed between 2 and 4 months after surgery showed that myocardial perfusion at the infarcted region improved significantly in all patients (Table 3 and Fig. 4). None of the patients had ventricular arrhythmia or malignant neoplasia, and all patients were able to resume normal daily activities.
FIG. 4. Photographs of single photon emission computed tomography technetium-99mTc sestamibi scan of patient 3 before cardiac surgery and cell transplantation (top panel) and after cardiac surgery and cell transplantation (bottome panel). Preoperative ischemic regions are indicated by arrows, and postoperative regions that received cell transplantation are indicated by arrowheads. Improvement in myocardial perfusion was observed in the ischemic region after bypass surgery and cell transplantation.
Discussion
The present study demonstrates that a combined therapy of off-pump coronary artery bypass grafting and intramuscular transplantation of autologous BMMNCs for the treatment of myocardial infarction is safe and feasible and improves perfusion and myocardial contractility. The clinical status of all patients had improved after 2–4 months follow-up (Table 3). Examination with ECG showed that left ventricular wall motion in the cell-transplanted area was improved in all patients. Consistent with improved regional wall motion, EF increased significantly (p = 0.016) after cell transplantation and off-pump coronary artery bypass grafting (Fig. 2).
The present phase I clinical study focused on the safety and efficacy of BMMNC transplantation combined with off-pump coronary artery bypass grafting for the treatment of myocardial infarction. There were no complications such as myocardial damage, malignant neoplasia and cardiac or systemic inflammation immediately or 2–4 months after cell transplantation. In particular, ventricular arrhythmia that would be fatal in patients receiving cell therapy was not observed in any patient. All patients resumed normal daily activity. Thus the combined therapy might be safe in clinical application. This result is consistent with that of a previous study indicating that intramyocardial injection of autologous BMMNCs did not increase the incidence of ventricular arrhythmia and inflammation in humans.19 However, because the follow-up period in the present study was short, the safety of this therapy must be further assessed in longer-term studies with larger numbers of patients. Because transplanted cells are exposed to perfusion immediately after injection in cell transplantation following off-pump coronary artery bypass grafting, the survival and engraftment of the transplanted cells may be enhanced in comparison with on-pump bypass surgery. In hibernating areas, the underlying physiological state allows for restoration of myocardial function if myocardial perfusion is improved.11 Therefore, we carefully selected patients who had viable myocardium with akinesia or severe hypokinesia, as evaluated by echocardiography and MRI. Intramyocardial cell delivery was performed because these patients had had myocardial infarction and unstable angina for more than 1 month, making it likely that homing signalling would be weak and not optimal for cell engraftment.
The present study demonstrated that a combined therapy of BMMNC transplantation and off-pump coronary artery bypass grafting improved cardiac function and myocardial perfusion of infarcted heart. In the present study, all patients were followed by echocardiography to examine myocardial wall motion. With echocardiography, it is difficult to distinguish the wall motion of the cell-injected region from global myocardial wall motion. Therefore, one patient was monitored with MRI, which is a more accurate method of examining regional myocardial wall motion. With MRI, we observed mild improvement of myocardial wall motion in cell-injected regions. However, we did not observe a correlation between the number of injected cells and improvement in EF (Fig. 3). This might be owing to the small number of patients rather than to nonefficacy of combined therapy. In addition, SPECT MIBI scan results (Table 3) showed that perfusion improvement was the most significant in the patient (patient 3) receiving the largest number of CD34- and AC133-positive cells. SPECT MIBI scan results can be influenced by adjacent area revascularization, but the perfusion improvement in the regions with no perfusion might be mainly due to cell transplantation. This correlation should be examined further in a future study with a large number of patients.
Several clinical studies have reported that implantation of skeletal myoblasts16,17 or cells derived from bone marrow11–15 into ischemic heart induced myogenesis and angiogenesis and thereby increased myocardial perfusion and prevented ventricular dilatation and heart failure. Menasche and colleagues16,17 performed the first clinical trial of autologous myoblast transplantation into infarcted myocardium in combination with coronary artery bypass grafting. After cell transplantation, there was evidence of contraction and viability in the cell-transplanted scar area. Although the results are encouraging, transplantation of only myogenic cell types might not induce efficient revascularization of ischemic tissue. Stamm and colleagues12 transplanted autologous AC133-positive bone marrow cells into the infarcted area of patients after myocardial infarction and reported that bone marrow cell transplantation is safe and of benefit to cardiac function. The present study revealed that autologous stem cell therapy could be applied with coronary artery bypass grafting to treat myocardial infarction. The significant finding of the present study is that intramyocardial transplantation of autologous BMMNCs during off-pump coronary artery bypass grafting is feasible and safe and has beneficial effects on myocardial perfusion. BMMNCs have been known to contain various stem and progenitor cells, including mesodermal progenitor cells, hematopoietic progenitor cells and endothelial progenitor cells.13 CD34- and AC133-positive cells in bone marrow are known as endothelial progenitors capable of differentiating into endothelial cells.20 In addition, BMMNCs secrete various cytokines (i.e., vascular endothelial growth factor, insulin-like growth factor and platelet-derived growth factor) that can stimulate regeneration and proliferation in residual normal myocytes and endogenous cardiac stem cells.15 To take advantage of this large and heterogeneous regenerative potential, we decided to use all the mononuclear cells isolated from the bone marrow aspirate rather than a subpopulation. The transplanted cell number of the present study was not significantly different from that of a previous clinical study.12
The major limitations of the present study are that the study design and the small number of patients enrolled limit conclusions about the efficacy and safety of the combined therapy. The type and number of transplanted cells used for the treatment of myocardial infarction should be optimized to enhance therapeutic efficacy in further studies. That said, after cell transplantation, the myocardial perfusion at the cell-transplanted area, which was previously nonperfused or severely hypoperfused, was significantly improved in all patients (Table 3 and Fig. 4). Although coronary artery bypass grafting can contribute to improvement in myocardial perfusion, an increase in perfusion by collaterals originating from the bypassed areas after coronary artery bypass grafting would not be expected to cause functional recovery of areas that are difficult to perfuse.16 Thus the improved perfusion in the cell-transplanted area in the present study is assumed to be mainly due to the engrafted BMMNCs. However, the present study was not designed to compare the therapeutic efficacy of single treatment because this study has no control groups (a BMMNC transplantation–only group or an off-pump coronary artery bypass grafting–only group). The effects of concomitant coronary artery bypass grafting should also be addressed in further studies with control groups.
Conclusion
In summary, the present phase I clinical study demonstrates that intramyocardial implantation of autologous BMMNCs following coronary artery bypass grafting ameliorated myocardial ischemia and improved the cardiac function of ischemic hearts. It was safe and feasible to simultaneously perform coronary artery bypass grafting and cell transplantation in ischemic myocardium of beating hearts. Further studies with a large number of patients and long-term follow-up are required to evaluate the effectiveness of this method.
Acknowledgments
This research was supported by a research grant (No. 02-PJ10-PG8-EC01-0016) of the Korea Health 21 R&D Project, the Ministry of Health and Welfare, Republic of Korea.
Contributors: Dr. Yoo designed the study and collected the data. Drs. Yoo, H.-O. Kim and Cho wrote the article. All authors analyzed the data, reviewed the written article and gave final approval for its publication.
Competing interests: None declared.
Accepted for publication Jan. 17, 2007
Correspondence to: Dr. K.-J. Yoo, Department of Thoracic and Cardiovascular Surgery, Yonsei Cardiovascular Research Institute, Yonsei University College of Medicine, 134 Shinchon-dong, Seodaemun-ku, Seoul 120-752, Korea; fax 82-2-313-2992; kjy@yumc.yonsei.ac.kr; Dr. B.-S. Kim, Department of Bioengineering, Hanyang University, 17 Haengdang-dong, Seongdong-ku, Seoul 133-791, Korea; fax 82-2-2291-0838; bskim@hanyang.ac.kr
References
- 1.Brann WM, Bennett LE, Keck BM, et al. Morbidity, functional status, and immunosuppressive therapy after heart transplantation: an analysis of the Joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. J Heart Lung Transplant 1998;17:374-82. [PubMed]
- 2.Pennington DG, Oaks TE, Lohmann DP. Permanent ventricular assist device support versus cardiac transplantation. Ann Thorac Surg 1999;68:729-33. [DOI] [PubMed]
- 3.Li RK, Mickle DA, Weisel RD, et al. Natural history of fetal rat cardiomyocytes transplanted into adult rat myocardial scar tissue. Circulation 1997;96(9 Suppl):II-179-86; discussion 186-7. [PubMed]
- 4.Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium; improved performance after skeletal myoblast transplantation. Nat Med 1998;4:929-33. [DOI] [PubMed]
- 5.Li RK, Jia ZQ, Weisel RD, et al. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol 1999;31:513-22. [DOI] [PubMed]
- 6.Min JY, Yang Y, Converso KL, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 2002;92:288-96. [DOI] [PubMed]
- 7.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. [DOI] [PubMed]
- 8.Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999;100(19 Suppl):II247-56. [DOI] [PubMed]
- 9.Tomita S, Mickle DA, Weisel RD, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg 2002;123:1132-40. [DOI] [PubMed]
- 10.Schwartz Y, Kornowski R. Progenitor and embryonic stem cell transplantation for myocardial angiogenesis and functional restoration. Eur Heart J 2003;24:404-11. [DOI] [PubMed]
- 11.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003;107:2294-302. [DOI] [PubMed]
- 12.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45-6. [DOI] [PubMed]
- 13.Strauer BE, Brehm M, Zeus T, et al. Repair of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in human. Circulation 2002;106:1913-8. [DOI] [PubMed]
- 14.Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003;361:47-9. [DOI] [PubMed]
- 15.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol 2005;46:1651-8. [DOI] [PubMed]
- 16.Menasche P, Hagege AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet 2001;357:279-80. [DOI] [PubMed]
- 17.Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe left ventricular dysfunction. J Am Coll Cardiol 2003;41:1078-83. [DOI] [PubMed]
- 18.World Medical Association, Inc. World Medical Association Declaration of Helsinki: recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res 1997;35:2-3. [PubMed]
- 19.Tse HF, Thambar S, Kwong YL, et al. Safety of catheter-based intramyocardial autologous bone marrow cells implantation for therapeutic angiogenesis. Am J Cardiol 2006;98:60-2. [DOI] [PubMed]
- 20.Reyes M, Dudek A, Jahagirdar B, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 2002;109:337-46. [DOI] [PMC free article] [PubMed]