Abstract
The myelodysplastic syndromes (MDS) remain challenging to the clinician in terms of diagnosis and management. The diagnosis is essentially one of exclusion in first ruling out other disorders that can also cause peripheral blood/bone marrow cell dysplasia and cytopenias. Recent studies implicate extensive apoptosis as the explanation of the paradoxical observation of marrow hyperplasia, but peripheral blood cytopenia. The clonal nature of MDS places it also at continual risk for transformation to acute leukemia. Predicting overall survival as well as the risk of acute myeloid leukaemia (AML) transformation has been improved by the development of the International Prognostic Scoring System (IPSS). Management of MDS can now be based on the patients respective prognostic subgrouping. Low-risk patients should be considered for hematopoietic growth factor singly or in combination, while high-risk patients should be offered AML-induction therapy or novel therapeutic agents. Common complications are neutropenias with recurrent infections and red cell transfusion dependence. Future advances upon understanding the molecular details of the MDS clone should ultimately improve the care of patients with MDS.
Keywords: myelodysplastic syndromes, apoptosis, prognostic system IPSS, acute myeloid leukaemia, therapeutic strategies
The myelodysplastic syndromes (MDS) are clonal hematologic disorders characterized by ineffective hematopoiesis. The natural history of these syndromes ranges from a chronic course that may span years to a rapid course of leukemic progression. Unfortunately, the nomenclature and classification systems used to describe these conditions are cumbersome and contentious.
The International Myelodysplastic Syndrome Risk Analysis Workshop substantially advanced the characterization of myelodysplasia with its International Prognostic Scoring System (IPSS). The overall score is the sum of the scores for bone marrow blasts, karyotype, and cytopenias. The percentage of blasts is scored as follows: <5 %, 0; 5 to 10 %, 0.5; 11 to 20 %, 1.5; and 21 to 30 %, 2.0. Cytogenetic features associated with a good prognosis (normal karyotype, Y-, 5q-, or 20q-) are scored as 0; those associated with a poor prognosis (abnormal chromosome 7 or three or more abnormalities) are scored as 1.0; and all other cytogenetic abnormalities, which are associated with an intermediate prognosis, are scored as 0.5. A score of 0 is assigned if the patient has no cytopenia or only one type, and a score of 0.5 is assigned if the patient has two or three types of cytopenia. The various types of cytopenia are defined as follows: hemoglobin <10g/dl; absolute neutrophil count <1500/mm3; and platelet count <100,000/mm3. Low risk patients (IPSS 0) have a median survival of 5.7 years, Intermediate-1 risk patients (IPSS 0.5 or 1.0) have a median survival of 3.5 years, Intermediate-2 risk patients (IPSS 1.5 or 2.0) have a median survival of 1.2 years, while High risk patients (IPSS ≥ 2.5) have a median survival of 0.4 months1.
Nonetheless, myelodysplastic syndromes are viewed by most hematologists as stages of neoplastic hematopoiesis associated with cytopenias. In general, myelodysplastic conditions are preleukemic disorders in which the neoplastic clone is established, but not all cases of myelodysplasia terminate in acute myeloid leukemia (AML). The process of apoptosis is implicated in the pathogenesis of MDS. In the present article, we discuss the role of apoptosis in MDS and address the therapeutic challenges facing physicians who care for patients with myelodysplasia1. Therapeutic dilemmas exist in MDS because of the diseases multifactorial pathogenetic features, heterogeneous clinical stages and the patients' generally old ages. Until today, MDS are believed to involve innate stem cell lesions, cellular cytokine mediated stromal defects and immunologic derangements2.
The role of apoptosis in myelodysplastic syndromes (MDS)
Several techniques are used for the study of apoptosis in MDS. The TUNEL technique (nick-end labeling method), the technique of in situ end labeling (ISEL), the flow cytometry and annexin V (for studying the apoptosis of the CD34+ bone marrow cells)3–6. In the early stages of myelodysplastic syndromes, there is excessive apoptosis (excessive ratio of apoptosis in comparison to cell proliferation), causing severe cytopenias. On the contrary, in late stages of the disease, in case of evolution of the disease, leukaemic transformation is observed, after inhibition of the apoptotic process5. The proteins causing apoptosis (pro-apoptotic) are: Bax, Bad, Bak and Bcl-xS, while the proteins Bcl-2 and Bcl-xL are anti-apoptotic. The expression of anti-apoptotic proteins Bcl-2 and Bcl-xL is higher in bone marrow cells of Refractory Anemia with Excess of Blasts (RAEB), Refractory Anemia with Excess of Blasts in Transformation (RAEB-t) and Chronic Myelomonocytic Leukaemia (CMML) patients, in contrast to Refractory Anemia (RA) and Refractory Anemia with Ringed Sideroblasts (RARS) patients6. Consequently, the pro-apoptotic proteins are related to early MDS stages, whereas the increase of the levels of anti-apoptotic proteins is related to late MDS stages. Increased expression of pro-apoptotic proteins is related to increased survival and reduced risk of leukaemic transformation, while the expression of anti-apoptotic proteins in MDS is related to decreased survival6 (Figures 1–5)7.
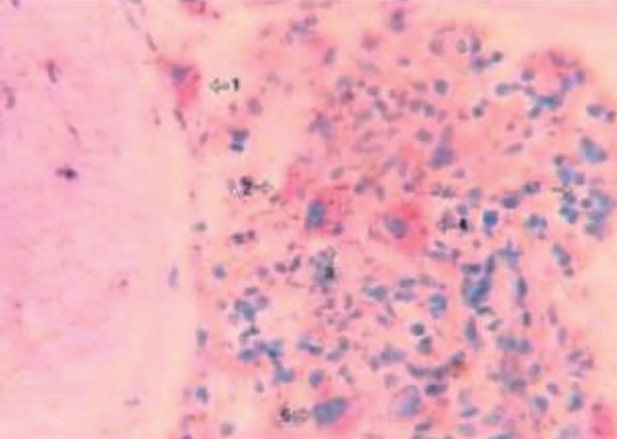
Figure 1. Marrow section from a patient with myelodysplastic syndrome (refractory anemia) showing micro-megakaryocytes (1,2) (CD41+) and apoptotic megakaryocytes (3) (ISEL+) (original magnification x400) (Xiao Li, et al7).
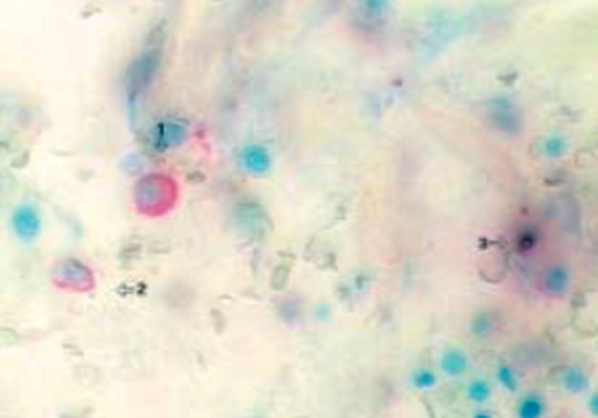
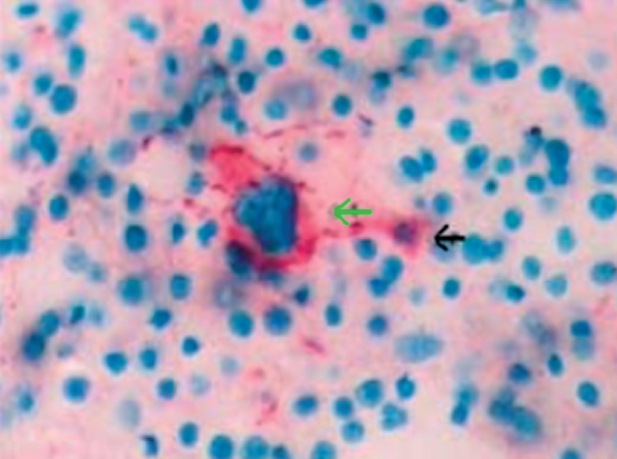
Figure 5. Marrow section from a patient with refractory anemia with excess blasts. The green arrow indicates a morphologically normal CD41-positive megakaryocyte, not showing apoptosis. The black arrow indicates a CD41-positive micro-megakaryocyte undergoing apoptosis (original magnification x40) (Xiao Li, et al7).
MDS patients have much higher percentage of apoptotic cells in the bone marrow in comparison to healthy individuals8. The patients who responded to treatment with erythropoietin plus G-CSF showed a significant decrease to the percentage of apoptotic cells3. There is increasing evidence that the excessive expression of anti-apoptotic proteins and p53, inhibits apoptosis of bone marrow cells and increases the possibility of leukaemic transformation of MDS4. Therefore, during the evolution of MDS syndromes to acute myeloid leukaemia (AML), the apoptotic rate decreases8,9. The oncogenic mechanism seems to differ between de novo acute myeloid leukaemia and secondary leukaemia following MDS syndrome4. Moreover, the expression of Fas and FasL of bone marrow CD34+ cells is significantly increased to MDS patients, in comparison to control groups9. Finally, it has been shown that interleukin-15 stimulates the proliferation and the differentiation of MDS CD34+ cells10.
It has been reported that the activation of apoptosis takes place in MDS, when the expression of human telomerase reverse transcriptase (hTERT) is lacking. This implicates the excessive apoptosis and the non satisfactory hemopoiesis with the absence of high telomerase activity11. It has also been reported that poor prognosis is correlated to excessive apoptosis, low expression of hTERT and higher expression of caspase-3 (pro-apoptotic molecule). Moreover, in MDS, the activity of heterogeneous nuclear ribonucleoprotein B1 (hnRNP B1) is related possibly with leukaemic transformation11.
It has also been shown that the increased expression and release of TNF-Related Apoptosis-Inducing Ligand (TRAIL) in bone marrow harms the erythropoiesis and contributes to the anemia of MDS12.
Researchers from Karolinska Institute of Sweden underline that the apoptotic process of MDS is related to increased activation of caspases and explain the anti-apoptotic mechanism of growth factors in MDS treatment13–15. During the apoptotic process in MDS, the mitochondrial apoptotic axis is activated after the activation of caspases and the release of cytochrome C from mitochondria to cytosol. The amount of cytochrome C in cytosol can be evaluated by a special method of immunofluorescence. The combination treatment of erythropoietin and G-CSF to the patients mentioned above, decreases the number of apoptotic bone marrow progenitors14.
G-CSF acts by a double mechanism to inhibit and eliminate apoptosis. First, G-CSF inhibited the Fas-related activation of caspases (extrinsic pathway of apoptosis) and secondly, G-CSF blocked the release of cytochrome C from mitochondria into cytosol (intrinsic pathway of apoptosis)14,15.
Current therapeutic challenges in myelodysplastic syndromes (MDS)
MDS patients are classified according to the prognostic system IPSS, as low risk patients (low, intermediate-1) and high risk patients (intermediate-2, high)16. The IPSS system classifies MDS patients, determines treatment and predicts the outcome of the disease. It comprises the percentage of blasts in the bone marrow, the number of hematopoietic lineages involved in the cytopenia and the specific cytogenetic abnormalities1.
All MDS patients should receive supporting treatment, which involves antibiotics for infections and transfusions of erythrocytes and platelets (blood support) for the treatment of symptomatic cytopenias17. Iron chelation therapy should be given to MDS patients after 20-40 RBC transfusions, particularly to low risk patients18 (NCCN guidelines). In a Danish study in 1996, a small group of MDS patients was evaluated. The patients had received multiple RBC transfusions (mean of 90 transfusions) and showed increased serum ferritin levels and high liver iron (MRI finding). These individuals received desferrioxamine chelation therapy for 2 years with an improvement in haematologic parameters (increase in their haemoglobin levels, neutrophil and/or platelet counts). This also resulted in the inhibition of the toxic iron overload19. Desferrioxamine is administered as a subcutaneous infusion, for 8 to 12 hours 5 to 7 nights per week20. Novel clinical trials are being conducted at the moment, for other new oral iron chelators, deferiprone and deferasirox, with extremely positive results20.
Other low risk MDS patients with symptomatic anemia should be treated by erythropoietin (or darbepoetin), with or without G-CSF (granulocyte colony stimulating factor)20. The treatment of recombinant human erythropoietin is followed by 16% response, according to meta-analysis, which involves all MDS subtypes21. The co-treatment of recombinant human erythropoietin and G-CSF improves the anemia of MDS syndromes22. Patients with 5q- syndrome should initially receive lenalidomide, a substance which is an inhibitor of angiogenesis and also has immunomodulatory activities20. Additional immunomodulatory substances (cyclosporine, anti-thymocyte globulin) should be supplied to all patients with resistant cytopenias20. Younger patients, positive patients to HLA-DR15 and patients with a small duration of transfusion therapy, are more likely to respond to immunosuppressive therapy23.
For high risk patients, therapeutic choices involve agents who inhibit methylation (azacitidine, decitabine), intensive chemotherapy, and haematopoietic stem cell or bone marrow transplantation, according to the patient's age, his/hers general health condition and the availability of a donor. Intensive chemotherapy treatment to MDS patients is related to high mortality rates, low percentages of remissions and shorter duration of them. Nevertheless, intensive chemotherapy can delay the leukaemic transformation and improve the cytopenias to particular patients. Hypomethylating agents can be used as temporary treatment, while the location of the suitable donor is anticipated or for the decrease in the marrow blasts ratio, so that the patient is more bound to respond to transplantation. Moreover, by supplying azacitidine or decitabine to MDS patients there is reduced frequency to evolution to AML, or prolongation of the interval between the MDS diagnosis and the leukaemic transformation. Side effects of the above agents are the thrombocytopenias and the cytopenias20.
The epigenetic DNA changes cause pathological gene expression. The increased DNA methylation seen in MDS patients leads to the inhibition of the expression of onco-suppresive protective genes, which causes myelodysplasia24,25. Thus, the use of agents which inhibit methylation (DNMT inhibitors) in the treatment of MDS, can be explained. Similarly, Histone Deacetylase Inhibitors (HDAC inhibitors - for example valproic acid or the cyclic peptide FK228) are used in combination with hypomethylating agents for MDS treatment24–26. Despite the clinical activity of the agents, there is not convincing proof, that the activity is attributed to epigenetic modification of the genome in MDS. Clinical trials are being conducted at the moment for all the agents mentioned above24,25. For the patients who do not respond to first line treatment, clinical trials with novel therapeutic agents should be conducted20.
For the treatment of CMML, hydroxyurea is considered as first line treatment and is preferable in comparison to oral supply of etoposide. Moreover, the most commonly used intensive chemotherapy involves cytosine-arabinoside, etoposide, idarubicine, topotecane, daunorubicine, mitoxandrone and/or fludarabine27. The intensive chemotherapy treatment cannot usually be applied, because of the old age of MDS patients and coexistent diseases. In such cases low-dose cytosine-arabinoside is used, causing response to 40% of patients and complete remission to 20% of patients28. Low-dose etoposide is the right therapeutic approach not only for high risk MDS, but for MDS-induced AML as well29.
The question of whether the use of growth factors (GM-CSF, G-CSF) for MDS treatment, causes evolution of the disease to acute leukaemia, came to the surface30. After various clinical trials, it was established that the AML transformation is a physical evolution of the MDS disease in some cases, and not the result of the growth factor use. Moreover, their use in combination with erythropoietin, improves the survival of MDS-induced AML patients30. The combination of cytosine-arabinoside and idarubicine plus GM-CSF in high risk MDS patients and to MDS-induced AML shows high percentages of complete remission and low percentages of toxic deaths. Nevertheless, the duration of complete remission remains short. A relatively satisfactory number of patients shows prolonged survival over 3 years31. Combination treatment with all-trans retinoic acid, a-interferon and G-CSF in low-risk MDS patients is effective in 35% (6/17 patients) and can result in complete remission in isolated cases32.
It has been reported that the comparison between exclusive chemotherapy and chemotherapy plus allogeneic or autologous bone marrow transplantation in MDS high risk patients, shows no significant differences. Both therapeutic approaches are equally supported. The critical question does not seem to be which therapeutical approach is better, but to what extent each one can be conducted for the production of better results33. There is an upper age limit of 55-65 years for conducting a bone marrow transplantation, because of the increased number of complications of the elderly MDS patients and the peculiar nature of the disease20. Nevertheless, the bone marrow transplantation can be conducted with success to MDS patients over 55 years34.
There is disagreement whether high risk MDS patients benefit by intensive chemotherapy, for achieving remission, just before the bone marrow transplantation. Some studies show significantly greater survival ratios to transplanted patients, who earlier had achieved complete remission, but randomized prospective clinical trials to examine the value of chemotherapy before HSCT, have not been conducted20. According to the guidelines of the American Society of Hematology, for IPSS low risk patients (low, intermediate-1), the delayed carrying out of HSCT is related with the highest survival rates. For IPSS high risk patients (intermediate-2, high) the urgent realization of HSCT is necessary35. On the contrary, according to the British guidelines for the treatment of MDS, all patients <65 years old of intermediate-1 IPSS category, should be promoted for allogeneic stem cell transplantation, when their health condition allows it27. Finally, it is proposed that the combination of low-dose fludarabine, busulphan and anti-thymocyte globuline before HSCT has very good results to the patients who do not have the potential of a myeloablative therapy before HSCT36.
Figure 2. Marrow section from a patient with refractory anemia showing micro-megakaryocytes (1-5) adjacent to trabecula with no apoptotic cells (original magnification x400) (Xiao Li, et al7).
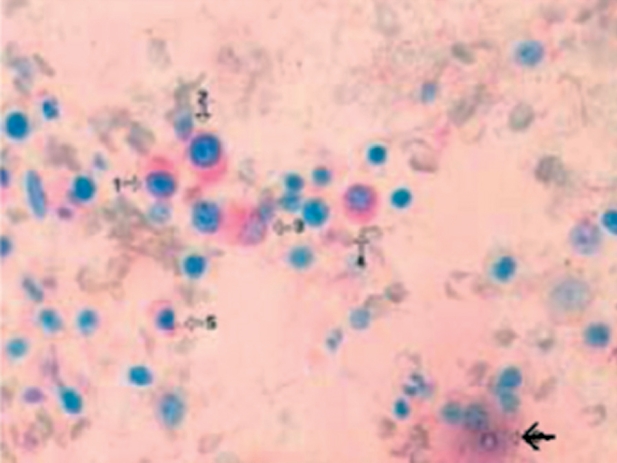
Figure 3. Marrow section from a patient with refractory anemia showing a cluster of non-apoptotic micro-megakaryocytes (1-5). The black arrow indicates an apoptotic CD41- negative cell (original magnification x400) (Xiao Li, et al7).
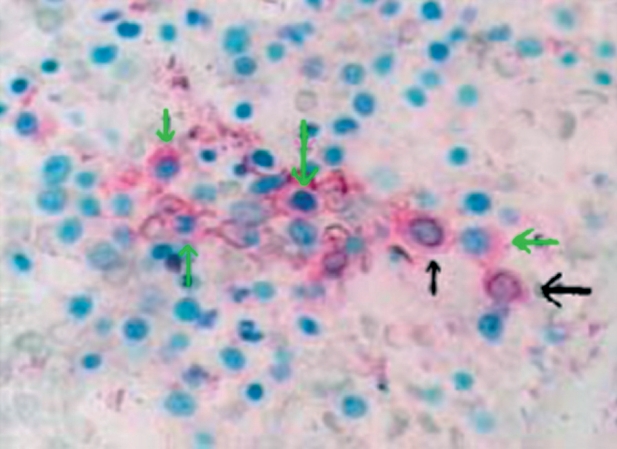
Figure 4. Marrow section from a patient with refractory anemia showing a cluster of micro-megakaryocytes. The black arrows indicate apoptotic CD41-positive cells. The green arrows indicate non-apoptotic CD41-positive cells (original magnification x400) (Xiao Li, et al7).
References
- 1.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649–1660. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PL, Young NS, Gattermann N. Myelodysplastic Syndromes -Controversies and Therapeutic Options in Myelodysplastic Syndrome: Biologically Targeted Approaches -Role of the Immune System in the Pancytopenia of MDS and Immunosuppressive Therapies -Ring Sideroblast Formation and Its Role in MDS Pathophysiology. American Society of Hematology. 2002:136–161. doi: 10.1182/asheducation-2002.1.136. [DOI] [PubMed] [Google Scholar]
- 3.Hellström-Lindberg E, Kanter-Lewensohn L, Øst A. Morphological Changes and Apoptosis in Bone Marrow from Patients with Myelodysplastic Syndromes Treated with Granulocyte-CSF and Erythropoietin. Leuk Res. 1997;21:415–425. doi: 10.1016/s0145-2126(96)00110-5. [DOI] [PubMed] [Google Scholar]
- 4.Hidekachi K, Yaeko T, Kazunori N, Soroku Y. Apoptosis, bcl-2 Expression and p53 Accumulation in Myelodysplastic Syndrome, Myelodysplastic-Syndrome-Derived Acute Myelogenous Leukemia and de novo Acute Myelogenous Leukemia. Acta Haematol. 1999;102:115–123. doi: 10.1159/000040984. [DOI] [PubMed] [Google Scholar]
- 5.Parker JE, Mufti GJ, Rasool F, et al. The Role of Apoptosis, Proliferation and the Bcl-2-related Proteins in the Myelodysplastic Syndromes and Acute Myeloid Leukemia Secondary to MDS. Blood. 2000;96:3932–3938. [PubMed] [Google Scholar]
- 6.Boudard D, Vasselon C, Berthas MF, et al. Expression and Prognostic Significance of Bcl-2 Family Proteins in Myelodysplastic Syndromes. American Journal of Hematology. 2002;70:115–125. doi: 10.1002/ajh.10108. [DOI] [PubMed] [Google Scholar]
- 7.Xiao L, Quan P. Megakaryocytopoiesis and Apoptosis in Patients with Myelodysplastic Syndromes. Leuk Lymph. 2005;46:387–391. doi: 10.1080/10428190400013126. [DOI] [PubMed] [Google Scholar]
- 8.Claessens YE, Bouscary D, Dupont JM, et al. In Vitro Proliferation and Differentiation of Erythroid Progenitors from Patients with Myelodysplastic Syndromes: Evidence for Fas - Dependent Apoptosis. Blood. 2002;99:1594–1601. doi: 10.1182/blood.v99.5.1594. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Xie J. Expression of Fas, FasL and Bcl-2 and Apoptosis of Bone Marrow CD34+ Cells in Patients with Myelodysplastic Syndrome. J Exp Hematol. 2003;11:274–277. [PubMed] [Google Scholar]
- 10.Cheng MZ, Ye ZL, Cai KR, et al. Effect of IL-15 on the Proliferation, Differentiation and Anti-apoptosis of CD34+ Cells in Patients with MDS. J Expl Hematol. 2005;13:620–623. [PubMed] [Google Scholar]
- 11.Ohshima K, Karube K, Shimazaki K, et al. Imbalance Between Apoptosis and Telomerase Activity in Myelodysplastic Syndromes: Possible Role in Ineffective Hemopoiesis. Leuk Lymph. 2003;44:1339–1346. doi: 10.1080/1042819031000083037. [DOI] [PubMed] [Google Scholar]
- 12.Campioni D, Secchiero P, Corallini F, et al. Evidence for a Role of TNF-Related Apoptosis - Inducing Ligand (TRAIL) in the Anemia of Myelodysplastic Syndromes. Am J Pathol. 2005;166:557–563. doi: 10.1016/S0002-9440(10)62277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellström-Lindberg E, Schmidt Mende J, Forsblom AM, et al. Apoptosis in Refractory Anaemia with Ringed Sideroblasts is Initiated at the Stem Cell Level and Associated with Increased Activation of Caspases. Br J Haematol. 2001;112:714–726. doi: 10.1046/j.1365-2141.2001.02581.x. [DOI] [PubMed] [Google Scholar]
- 14.Tehranchi R, Fadeel B, Forsblom AM, et al. Granulocyte Colony - Stimulating Factor Inhibits Spontaneous Cytochrome C Release and Mitochondria - Dependent Apoptosis of Myelodysplastic Syndrome Hematopoietic Progenitors. Blood. 2003;101:1080–1086. doi: 10.1182/blood-2002-06-1774. [DOI] [PubMed] [Google Scholar]
- 15.Tehranchi R, Fadeel B, Schmidt Mende J, et al. Antiapoptotic Role of Growth Factors in the Myelodysplastic Syndromes : Concordance Between in Vitro and in Vivo Observations. Clin Cancer Res. 2005;11:6291–6299. doi: 10.1158/1078-0432.CCR-04-1850. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg P, Cox C, LeBeau MM, et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 17.Kumar P, Clark M, editors. Kumar & Clark clinical medicine. 6th ed. Edinburgh; New York: Elsevier Saunders; 2005. pp. 455–456. [Google Scholar]
- 18.Greenberg PL, Baer MR, Bennett JM, et al. Myelodysplastic Syndromes Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2006;4:58–77. [PubMed] [Google Scholar]
- 19.Jensen PD, Heickendorff L, Pedersen B, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94:288–299. doi: 10.1046/j.1365-2141.1996.d01-1795.x. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg P. New therapies and the role of haematopoietic stem cell transplantation in myelodysplastic syndromes. Haema. 2005;8:S136–S144. 16th Hellenic Congress of Hematology. [Google Scholar]
- 21.Hellström-Lindberg E. Efficacy of erythropoietin in the myelodysplastic syndromes: A meta-analysis of 205 patients from 17 studies. Br J Haematol. 1995;89:67–71. doi: 10.1111/j.1365-2141.1995.tb08909.x. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani L, Lentini G, Hentschel B, et al. Treatment of Anaemia in Myelodysplastic Syndromes with Prolonged Administration of Recombinant Human Granulocyte Colony Stimulating Factor and Erythropoietin. Br J Haematol. 2000;109:367–375. doi: 10.1046/j.1365-2141.2000.02016.x. [DOI] [PubMed] [Google Scholar]
- 23.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A Simple Method to Predict Response to Immunosuppressive Therapy in Patients with Myelodysplastic Syndrome. Blood. 2003;102:3025–3027. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 24.Fenaux P. Inhibitors of DNA Methylation: Beyond Myelodysplastic Syndromes. Nat Cl Pr Onc. 2005;2:S36–S44. doi: 10.1038/ncponc0351. [DOI] [PubMed] [Google Scholar]
- 25.Gore SD. Six (or more) Drugs in Search of a Mechanism: DNA Methyltransferase and Histone Deacetylase Inhibitors in the Treatment of Myelodysplastic Syndromes. J Natl Compr Canc Netw. 2006;4:83–90. doi: 10.6004/jnccn.2006.0009. [DOI] [PubMed] [Google Scholar]
- 26.Issa JP. Optimizing Therapy with Methylation Inhibitors in Myelodysplastic Syndromes: Dose, Duration and Patient Selection. Nat Cl Pr Onc. 2005;2:S24–S29. doi: 10.1038/ncponc0355. [DOI] [PubMed] [Google Scholar]
- 27.Bowen D. Guidelines for the Diagnosis and Therapy of Adult Myelodysplastic Syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 28.Aul C, Gattermann N. The Role of low-dose Chemotherapy in Myelodysplastic Syndromes. Leuk Res. 1992;16:207–215. doi: 10.1016/0145-2126(92)90058-f. [DOI] [PubMed] [Google Scholar]
- 29.Ogata K, Yamada T, Ito T, et al. Low dose Etoposide: a Potential Therapy for Myelodysplastic Syndromes. Br J Haematol. 1992;82:354–357. doi: 10.1111/j.1365-2141.1992.tb06429.x. [DOI] [PubMed] [Google Scholar]
- 30.Saba HI. Myelodysplastic Syndromes in the Elderly: The Role of Growth Factors in Management. Leuk Res. 1996;20:203–219. doi: 10.1016/0145-2126(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 31.Papageorgiou E, Economopoulos T, Papageorgiou S, et al. Treatment of High Risk Myelodysplastic Syndromes and Acute Myeloid Leukaemia Following MDS with Idarubicin and Cytocine Arabinoside Supported by Granulocyte - Macrophage Colony Stimulating Factor: A Single Center Experience. Haema. 2005;8:262–268. [Google Scholar]
- 32.Hofmann WK, Ganser A, Seipelt G, et al. Treatment of Patients with Low Risk Myelodysplastic Syndromes Using a Combination of All- Trans Retinoic Acid, Interferon Alpha and Granulocyte - Colony Stimulating Factor. Ann Hematol. 1999;78:125–130. doi: 10.1007/s002770050488. [DOI] [PubMed] [Google Scholar]
- 33.Oesterveld M, Muus P, Suciu S, et al. Chemotherapy Only Compared to Chemotherapy Followed by Transplantation in High Risk Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia; Two Parallel Studies Adjusted for Various Prognostic Factors. Leukemia. 2002;16:1615–1621. doi: 10.1038/sj.leu.2402591. [DOI] [PubMed] [Google Scholar]
- 34.Deeg HJ, Shulman HM, Anderson JE, et al. Allogeneic and Syngeneic Marrow Transplantation for Myelodysplastic Syndrome in Patients 55 to 66 Years of Age. Blood. 2000;95:1188–1194. [PubMed] [Google Scholar]
- 35.Cutler CS, Lee SJ, Greenberg P, et al. A Decision Analysis of Allogeneic Bone Marrow Transplantation for the Myelodysplastic Syndromes: Delayed Transplantation for Low - Risk Myelodysplasia is Associated with Improved Outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 36.Kröger N, Bornhäuser M, Ehninger G, et al. Allogeneic Stem Cell Transplantation after a Fludarabine/Busulfan-based Reduced-Intensity Conditioning in Patients with Myelodysplastic Syndrome or Secondary Acute Myeloid Leukemia. Ann Hematol. 2003;82:336–342. doi: 10.1007/s00277-003-0654-9. [DOI] [PubMed] [Google Scholar]