Abstract
Backgroung: Although intravenous drug users (IVDUs) comprise the majority of patients with chronic hepatitis C, most of them are excluded from treatment because of concerns about adherence to treatment and side effects.
Material and methods: In this study we retrospectively evaluated safety, compliance to treatment and efficacy of treatment in IVDUs with HCV infection in 163 former IVDUs with chronic hepatitis C, who were not in methadone substitution and were attending our clinics the period 1997-2004. All subjects were HCVRNA (+), had ALT levels >x1.5 UNL and were treated for their HCV infection. Treatment consisted of three different regimens: IFN-α monotherapy (39.8%), IFN-α/ribavirin combination therapy (30.1%) and pegylated IFN-α/ribavirin combination therapy.
Results: Eighty seven over 163 patients (53.3%) discontinued treatment early due to drug abuse relapse (62%), side effects (32.1%, 10% psychiatric) and 5,7% for other reasons. Eighty precent of those who discontinued treatment had pre-treatment drug abstinence ≤ 9 months. Seventy over 76 patients who completed therapy had an end-of-treatment virologic response (ETR, 92%). Fifty four over 76 patients showed sustained virologic response (SVR, 71.05%). ETR and SVR were significantly higher in both combination therapies compared to IFN-α monotherapy. The most prevalent HCV genotype was 3 (65%) and mild histological lesions were detected in the majority of subjects.
Conclusions: Our findings show that treatment for chronic hepatitis C was reasonably safe and sufficiently effective in our group of non methadone-substituted IVDUs, despite the fact that more than half of them discontinued treatment early and many relapsed to drug abuse. We suggest that the optimal duration of pretreatment abstinence from drug abuse should be ≥ 9 months.
Keywords: HCV infection, intravenous drug users, IFNα / IFNα ribavirin combination treatment
During the last few years, there has been a shift of risk factors for infection with HCV. While transmission of HCV through contaminated blood products decreased dramatically due to the implementation of anti-HCV screening tests, intravenous drug users (IVDUs) became the primary source of new HCV infections. It has been calculated that almost 60% of new cases of HCV infection in Western countries concern IVDUs. The prevalence of hepatitis C in this group of patients is extremely high. According to a recent study up to 88% of IVDU's in North America is anti-HCV positive1. In a previous study conducted in our department we found that 75% of large cohort of IVDUs were anti-HCV (+)2.
These patients are in danger of severe consequences of their infection if they are left untreated, given the fact that most of them are young adults. Furthermore they constitute a serious source of transmission to others.
Although IVDUs represent the majority of incident and prevalent cases of HCV infection, many are excluded from treatment because of concerns about adherence to treatment, the postulated high risk of reinfection and an increased risk of IFN-mediated neuropsychiatric side effects. However the arguments for this exclusion are often not based on suitable prospective and controlled clinical studies, including large numbers of HCV infected current or former IVDUs. Furthermore most published studies concerning treatment of HCV infected IVDUs, were conducted in methadone-substituted patients, while systemic data assessing treatment of hepatitis C in IVDUs without any substitution therapy are rare.
The aim of this retrospective study was to evaluate safety, compliance to treatment and efficacy of treatment in IVDUs with HCV infection, while they were attending a detoxification program without substitution therapy in a therapeutic community and moreover to estimate the optimal duration of pretreatment abstinence of drug use in this population.
Patients and Methods
One hundred sixty three former IVDUs (141 men- 22 women, mean age 31 years) with chronic hepatitis C, who were members of a detoxification program without methadone substitution and were attending our clinics during 1997-2004, were retrospectively studied.
All subjects were anti-HCV (+), had ALT>x1.5 ULN in at least two measurements within 2 month interval, were HCV-RNA (+) by PCR (COBAS AMPLICOR HCV, v 2.0), were HBsAg(-), HIV (-). HCV genotype was determined in all subjects using the LIPA Assay (Versant HCV genotype Assay). Liver histology was studied in 134 patients who consented to undergo liver biopsy, using the modified Knodell system scoring the Histological Activity Index (HAI) 0-18 and stages of fibrosis 1-4.
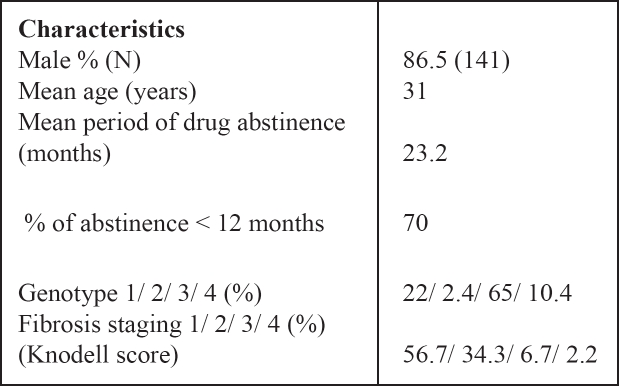
Baseline characteristics of all subjects studied are shown in Table 1.
Table 1. Baseline descriptive statistics.
All our patients were treatment naive. Three different regimens were applied to them according to international guidelines of each time period:
Standard interferon-α monotherapy (65 patients, 39.8 %)
Standard interferon-α/ribavirin combination therapy (49 patients, 30.1%)
Pegylated interferon-α/ribavirin combination therapy (49 patients, 30.1%)
Compliance to treatment, side effects and end of treatment (ETR) and sustained virological response (SVR) were analyzed.
Informed consent was obtained from each subject and the study was approved by both the Ethics Committee of Hippokration Hospital and Medical School of the Aristotle University of Thessaloniki.
Results
The duration of abstinence from illicit drug use was 23.2 months (< 12 months in 70% of subjects). No patient reported current alcohol ingestion because this is strictly forbidden by the rules of the therapeutic community.
HCV genotype 3 was the most prevalent and was detected in 65% of patients, genotype 1 was detected in 22% of patients, genotype 2 in 2.4% and genotype 4 in 10.4% of patients. As far as liver histology is concerned, a mild histological activity and mild fibrosis was detected in the majority of subjects.
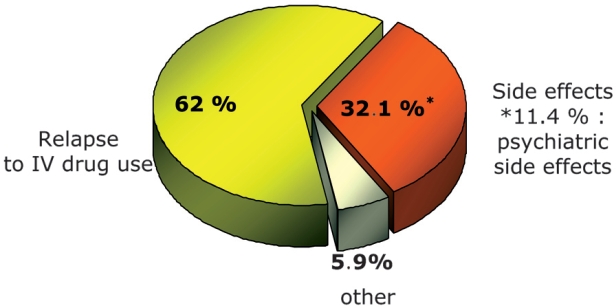
Eighty-seven out of 163 patients (53.3%) discontinued treatment early. The main reason for treatment discontinuation (in 62% of the patients) was relapse to drug abuse. Eighty percent of those who relapsed to drug abuse had pretreatment drug abstinence ≤ 9 months, irrespective of treatment regimen. Second most common reason for early treatment discontinuation was side effects (32.1%). Psychiatric side effects such as anguish, irritability and depression were predominant (9 patients). Finally, 5,7% of patients discontinued treatment because of personal reasons (Figure 1.).
Figure 1. Reasons for premature discontinuation of treatment.
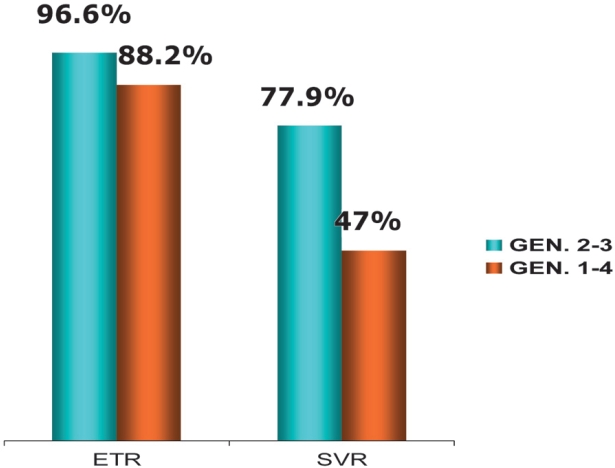
As far as efficacy of treatment is concerned, 70/76 patients (92%) who completed therapy had virological response at end of treatment (ETR). Sustained virological response (SVR) was achieved in 54 out of 76 patients (71.05%). ETR and SVR were significantly higher in both combination therapies compared to IFN-α monotherapy (p=0.0006 and p=0.0001 respectively). Figure 2 shows treatment response according to different HCV genotypes.
Figure 2. Response to treatment (ETR and SVR) according to HCV genotype.
Discussion
Until recently a significant proportion of hepatitis C patients with past or current drug abuse were excluded from treatment, because of concerns about adherence to treatment and side effects. It has been reported that up to 50% of these patients remain untreated, especially in case of a concomitant psychiatric disorder3. However, large prospective and controlled clinical studies concerning drug users infected with HCV are missing. In fact only a small number of published studies have focused on hepatitis C treatment in this special group of patients. In a recent review of more than 150 clinical trials about IVDUs with hepatitis C, published between 1987 - 2003, only seven trials focusing on treatment were found4, while the majority was about epidemiology of HCV infection in IVDUs.
In the above mentioned trials adherence to treatment ranged from 46% to 94%, whereas relapse to drug abuse (wherever mentioned) ranged from 0% (in a trial, where methadone substituted former IVDUs were included) to 50% (in another trial, where active IVDUs were included).
Furthermore it was shown that combination therapy with pegylated interferon plus ribavirin can be effective for HCV infected patients with former or current drug abuse5–7. Response rates are reported to be similar to those of patients with no drug dependence8–10. All authors conclude that this therapy can be successfully given to IVDUs in an outpatient setting, even in the presence of preexisting psychiatric disorders, intervening drug use or short duration of pretreatment drug abstinence.
Our data clearly show that former IVDUs with chronic hepatitis C should not be excluded from antiviral treatment, and treatment should not be limited to methadone-substituted patients. About half of our patients discontinued treatment early, and this was mainly due to relapse to drug abuse. However those patients who managed to complete treatment had response rates similar to chronic hepatitis C patients with no drug dependence, as referred in other related articles. According to our results the probability of relapse to drug abuse is diminished when the time interval between discontinuation of drug abuse and initiation of therapy is > 9 months. This is probably because the use of needles for interferon injection may cause relapse to illicit drugs injection. However this long period of pretreatment abstinence probably applies to our selected population and it could be shorter when highly motivated patients are treated.
In conclusion, treatment for chronic hepatitis C seems reasonably safe and sufficiently effective in non methadone- substituted IVDUs, despite the diminished ability of these patients to adhere to treatment, the relatively high rate of adverse events of treatment and the probability of relapse to drug use. Close monitoring and psychological support in cooperation with substance abuse treatment services could possibly augment adherence to therapy and improve response rates in this difficult to treat and continuously growing patient population.
Acknowledgments
This research was not supported by grants
References
- 1.Wood E, Kerr T, Stoltz J, et al. Prevalence and correlates of hepatitis C infection among users of North Americas first medically supervised safer injection facility. Public Health. 2005;119:1111–1115. doi: 10.1016/j.puhe.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Raptopoulou M, Lalla E, Vakolas J, et al. Prevalence of HCV infection and liver damage in ex-drug addicts. J Hepatol. 1997;26(Suppl 1):203. [Google Scholar]
- 3.National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36(Suppl 1):S1–S52. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alpha and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer M, Heinz A, Backmund M. Treatment of chronic hepatitis C in patients with drug dependence: time to change the rules? Addiction. 2004;99:1167–1175. doi: 10.1111/j.1360-0443.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- 6.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 7.Sylvestre DL. Approaching treatment for hepatitis C virus in substance users. Clin Infect Dis. 2005;41(Suppl 1):S79–S82. doi: 10.1086/429501. [DOI] [PubMed] [Google Scholar]
- 8.Grebely J, Duncan F, Viljoen M, et al. Directly Observed Therapy (DOT) for the treatment of hepatitis C virus (HCV) infection in injection drug users (IDUS) An interim analysis. Hepatology. 2005;42(Suppl 1):686A. [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]