Abstract
Performance of high repetition tasks with or without force is associated with peripheral tissue inflammation, decreased nerve function and motor dysfunction. Here, we examined whether a low repetition task with negligible force (LRNF) produces fewer tissue and behavioral pathologies than previously observed with high repetition tasks using our rat model of repetitive motion injury (RMI). Thirty-seven rats were randomized into control or LRNF groups, the latter reaching and grasping a 45 mg food pellet at a rate of 3 reaches/min. This task was performed in 4, 0.5 hr sessions with 1.5 hrs rest periods for 3 days/week for up to 12 weeks. Examination of distal median nerve, forelimb flexor tendons and bones for ED1-positive cells (macrophages and osteoclasts) revealed increases in nerve and bone in week 12. The nerve also contained increased TNF-α expressing cells in week 12. Examination of spinal cord dorsal horns revealed increased immunoexpression of Substance P in week 8 and neurokinin-1 in weeks 8 and 12 in the superficial lamina. Motor behavioral analyses showed no changes in reach rate across weeks, slightly reduced task duration (a measurement of voluntary task participation) in week 12, but significantly increased extra arm movement reversals during reaching in week 8. These extra movement reversals were corrections for missed food pellets during a reach. Thus, performance of even a low repetition, negligible force upper extremity task for 3 months can induce mild peripheral tissue inflammation, neurochemical increases in spinal cord dorsal horns, and declines in fine motor control.
1. Introduction
Performance of repetitive, forceful, or awkward movements over time may lead to repetitive motion injuries (RMIs), also known as overuse injuries and work-related musculoskeletal disorders. These disorders include sprains and strains, back pain, carpel tunnel syndrome, and diseases/disorders of the musculoskeletal, neural or connective tissues that develop in response to bending, reaching, overexertion or repetitive movements (Bureau of Labor Statistics, 2005). More than 5 hours a week of productive time are lost in workers due to common pain conditions such as back pain, arthritis, and musculoskeletal pain, costing an estimated $61.2 billion annually (Stewart et al., 2003). Epidemiological evidence confirms the close relationship between exposures to repetitive and/or forceful motion and the prevalence and incidence of these disorders (Bernard et al., 1997; National Research Council, 2001). Several researchers have attempted to establish criteria for maximum acceptable forces and movements for work tasks based on psychosocial outcomes (see Barr and Barbe, 2002 for review). Silverstein et al. (1986) performed job analyses of industrial workers and defined high repetition rate as less than 30s/cycle and low repetition rate as greater than 30s/cycle. Using these criteria, several animal models have been developed to simulate RMI (Soslowsky et al., 1996; Nakama et al., 2005; Williams and Stauber, 1999; Sommerich et al, 2007).
Our laboratory has developed a rat model of RMI that leads to exposure-dependent pathophysiological and behavioral changes similar to chronic median nerve compression or carpel tunnel syndrome after performance of a voluntary, highly repetitive upper extremity task with negligible or high force (Clark et al 2003, 2004). Nerve fibrosis and motor dysfunction resulting from performing high repetition, high force tasks were clearly greater than responses to a high repetition task with negligible force (Clark et al, 2004). In addition, our model induces inflammatory responses in forearm nerve and musculoskeletal tissues (Barbe et al., 2003; Barr et al., 2004; Clark et al., 2003, 2004; Al-Shatti et al., 2005; Barbe et al., in press). For example, performance of a high repetition, negligible force task (HRNF) resulted in increased ED1 positive macrophages and cells immunopositive for pro-inflammatory cytokines (IL1-α, IL1-β, TNF-α, and IL6) in the median nerve (Clark et al., 2003; Al-Shatti et al., 2005). We have yet to study the effects of performing a low repetition, negligible force (LRNF) task on peripheral nerves using our model. A number of other investigations have found histopathological changes following repetitive motion tasks, including necrotic muscle fibers with inflammatory cell infiltrates and tendinopathy (Baker et al., 2007; Geronilla et al., 2003; Nakama et al., 2005; Perry et al, 2005; Stauber and Willems, 2002). For example, using a 16-week overuse injury model, Soslowsky and colleagues showed temporal fluctuations of inflammatory markers in tendon (Perry et al., 2005). At 8 weeks of intensive treadmill running by rats, there was a significant but transient increase in tendon cyclooxygenase-2 (Perry et al., 2005). However, there are no studies of RMIs examining whether these peripheral tissue injury and inflammatory changes are associated with altered neurochemical changes in the spinal cord.
Numerous studies have found that Substance P (SP) and its receptor, neurokinin-1 (NK-1), are significantly elevated in the spinal cord dorsal horns after peripheral nerve injuries, such as nerve ligation and chronic compression, and in models of peripheral inflammatory pain (Delander et al., 1997; Rothman et al., 2005; Honore, 1999, 2000; McCarson, 1999; Abbadie et al., 1996; Allen et al., 1999). Since the neurochemical response of the spinal cord to peripheral neuroinflammation has not been studied in a model of RMI, we have begun our investigation using a low repetition task with negligible force (LRNF) performed for 3 months. We hypothesize that this low demand task will produce low grade, but significant, inflammatory changes in peripheral tissues towards the end of this time period, changes that will correlate with increases in spinal cord neurochemical production. Furthermore, we hypothesize that these peripheral and central changes will be associated with a small decline in motor function. To explore these hypotheses, we examined the effects of performing the LRNF task on macrophage infiltration of forearm nerve, tendon and bone, and immunoexpression of SP and NK-1 in the dorsal horns of the cervical spinal cord. We also examined three variables of motor performance: reach rate (ability or willingness to maintain task pace), task duration (ability or willingness to participate), and number of extra forearm movement reversals during a reach in order to successfully retrieve a food pellet (examines accuracy of fine motor skills).
2. Results
There were no significant differences (p>0.05) between the number of ED1-macrophages in the median nerves of normal control rats compared to trained control rats, therefore these data were combined for subsequent comparisons with rats performing a LRNF task. Also, there were no significant differences (p>0.05) between the percent area immunoexpression of SP or NK-1 in the normal control rats compared to trained control rats, therefore these data were also combined for subsequent comparisons with LRNF rats.
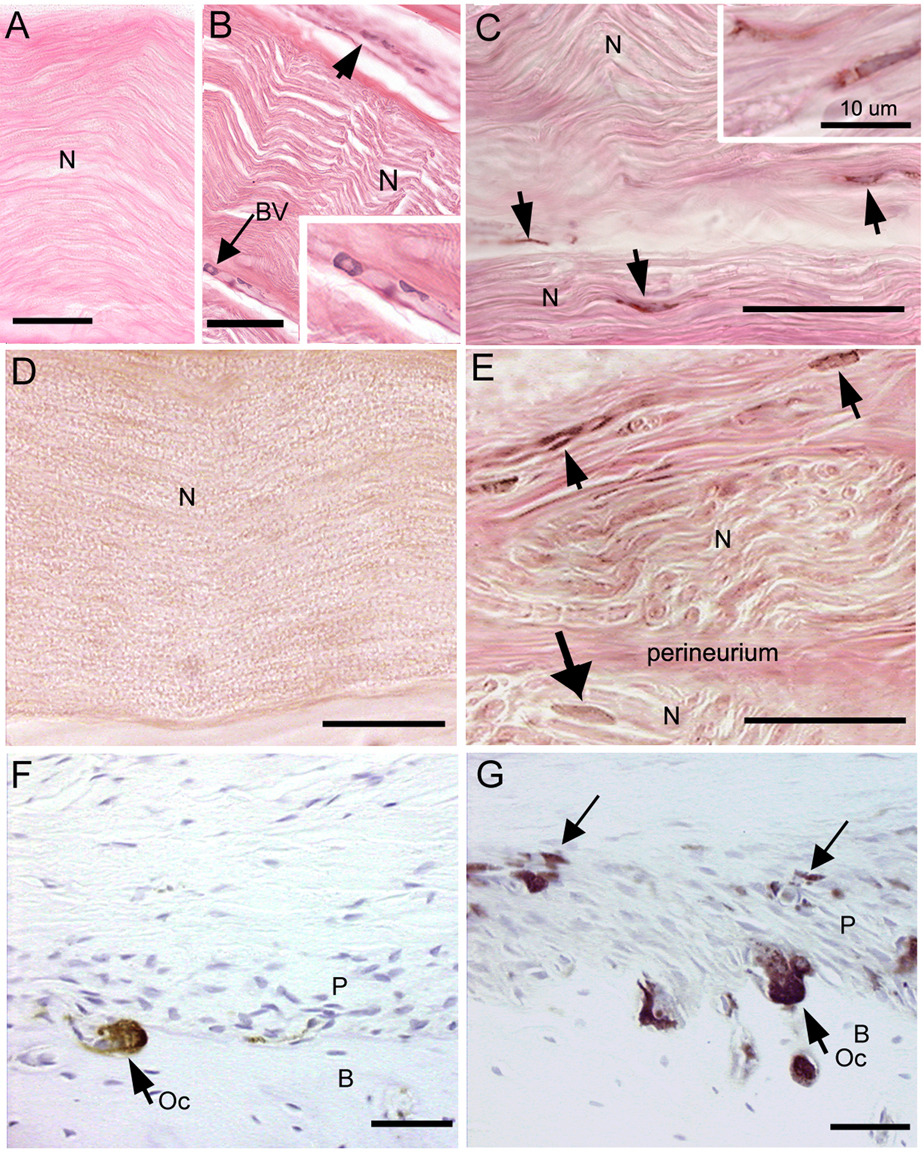
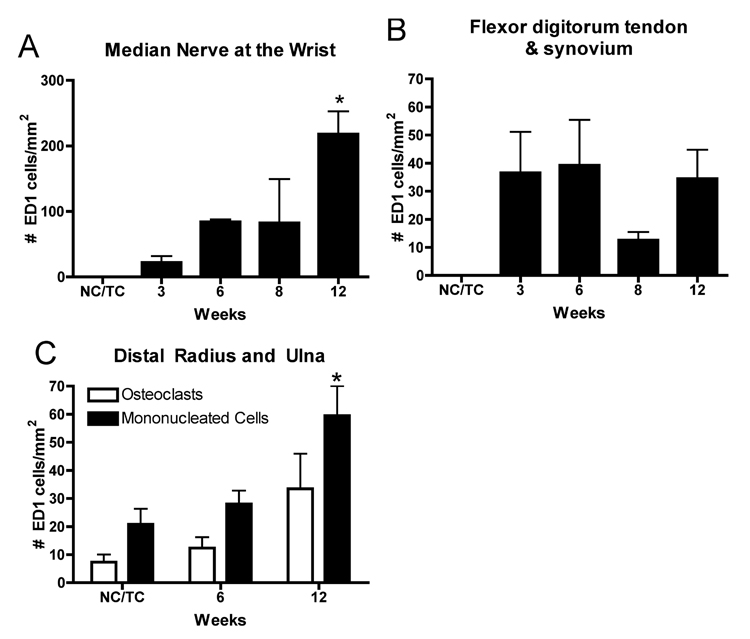
ED1 positive macrophages increased in the median nerves of the reach limbs in weeks 8 and 12 of the LRNF task (Fig. 1B,C and insets), compared to trained control rat nerves (Fig. 1A). ED1 macrophages were visible in the epineurium and within nerve associated blood vessels in week 8 (Fig. 1B and inset). By week 12, ED1 macrophages were also associated directly with nerve axons (Fig. 1C and inset). Similar increases of ED1 positive osteoclasts and mononucleated cells were seen at the periosteal-bone interface of the distal radius and ulna bones of 12 week LRNF rats (Fig. 1G), compared to control rats (Fig. 1F). ANOVA showed significant differences in ED1 positive macrophages in the median nerve (p=0.04) and ED1 positive mononucleated cells in the distal radius and ulna (p= 0.02) with task performance. Bonferroni post-hoc analysis indicated that there were more ED1 positive mononucleated cells in nerve and bone in week 12 LRNF rats than in control rats (p<0.05 each; Fig. 2A,C). There were no significant increases in ED1 macrophages in the flexor digitorum tendon or sheath with task performance (p=0.16; Fig. 2B), or in ED1 positive osteoclasts (which are multinucleated) in forelimb bones (p=0.13; Fig. 2C)
FIG.1.
Micrographs of ED1 and TNF-α immunoreactive cells (stained brown with diaminobenzidene (DAB) in forelimb nerve, tendon sheaths and bone. (A) No ED1 macrophages are visible in a median nerve (N) of a trained control rat. (B) The median nerve (N) from the reach limb of an 8 week LRNF rat showing infiltrating ED1 positive macrophages in the epineurium (short arrow) and blood vessels (BV, long arrow). (C) Several ED1 macrophages (arrows) are visible within the median nerve of a 12 week LRNF rat. (D) No TNF-α cells are visible in the median nerve of a trained control rat. (E) Several TNF-α positive cells (arrows) are localized to the epineurium and within the median nerve (N) of a 12 week LRNF rat. (F) The distal radius of a trained control rat contains a single ED1 positive osteoclast (Oc) at the periosteal (P) and cortical bone (B) interface. (G) The distal radius of a 12 week LRNF rat contains several ED1 positive osteoclasts (Oc) and small mononucleated cells (long arrows). Bar = 50 µm, except in insert of C in which bar = 10 µm. Eosin counterstain for A–D, hematoxylin counterstain for E and F.
FIG.2.
Quantification of the number of ED1-DAB-positive cells in forearm nerve, tendon and bones in normal and trained controls rats (NC/TC) and in rats performing a LRNF task for up to 12 weeks. (A) ED1 positive macrophages in the median nerve at the level of the wrist are significantly increased in 12 week LRNF rats compared to NC/TC rats. (B) There were no significant increases in ED1 positive macrophages in the distal tendon and associated synovial sheaths of the flexor digitorum in LRNF rats compared to control rats. (C) ED1 positive mononucleated cells (progenitors of osteoclasts and macrophages), but not osteoclasts, increased significantly in the distal radius and ulna in 12 week LRNF rats compared to NC/TC rats. *: p <0.05. Mean number of cells per mm2 + SEM is shown. The number of animals quantified per group: Controls (n=9), 3 week (n=3), 6 week (n=4), 8 week (n-4), and 12 week (n=3).
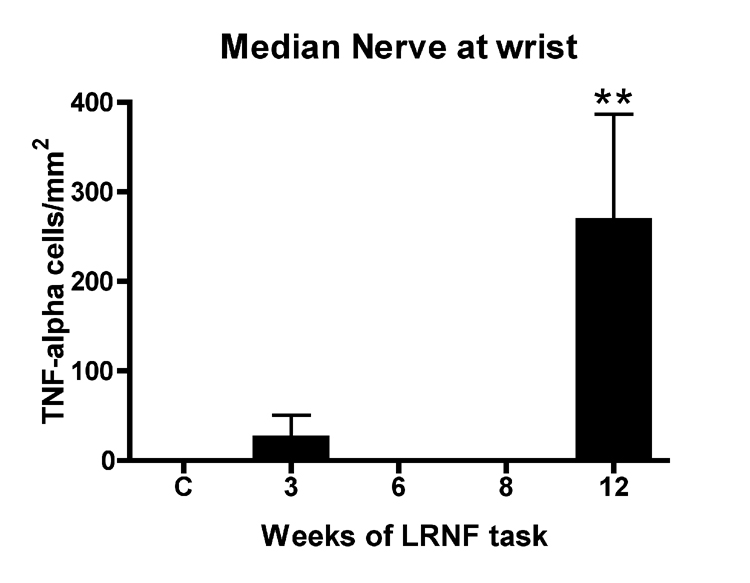
No significant increase of TNF-α was found in median nerves of rats performing the LRNF task for 3, 6 or 8 weeks compared to control rats using ELISA despite pooling of this small nerve by week of task performance in an effort to increase protein levels in the nerve lysates (data not shown). However, immunohistochemical probing of nerve sections showed an increase in TNF-α immunoreactive cells in the epineurium (Fig 1E, short arrows) and intra-neurally (Fig. 1E, long arrow) in week 12 LRNF rats, but an absence of TNF-α cells in control rat nerves (Fig. 1D). ANOVA showed a significant difference in TNF-α immunoreactive cells in the median nerve with task performance (p=0.001). Bonferroni post-hoc analysis indicated that the greatest increase was in week 12 compared to control nerves (p<0.001; Fig. 3).
FIG 3.
Quantification of TNF-α DAB positive cells in the median nerve at the level of the wrist. TNF-α immunopositive cells increased in rats performing a LRNF task for 12 weeks compared to normal control rats (C). **: p <0.01. Mean number of cells per mm2 + SEM is shown. The number of animals quantified per group: Controls (n=9), 3 week (n=3), 6 week (n=4), 8 week (n-4), and 12 week (n=3).
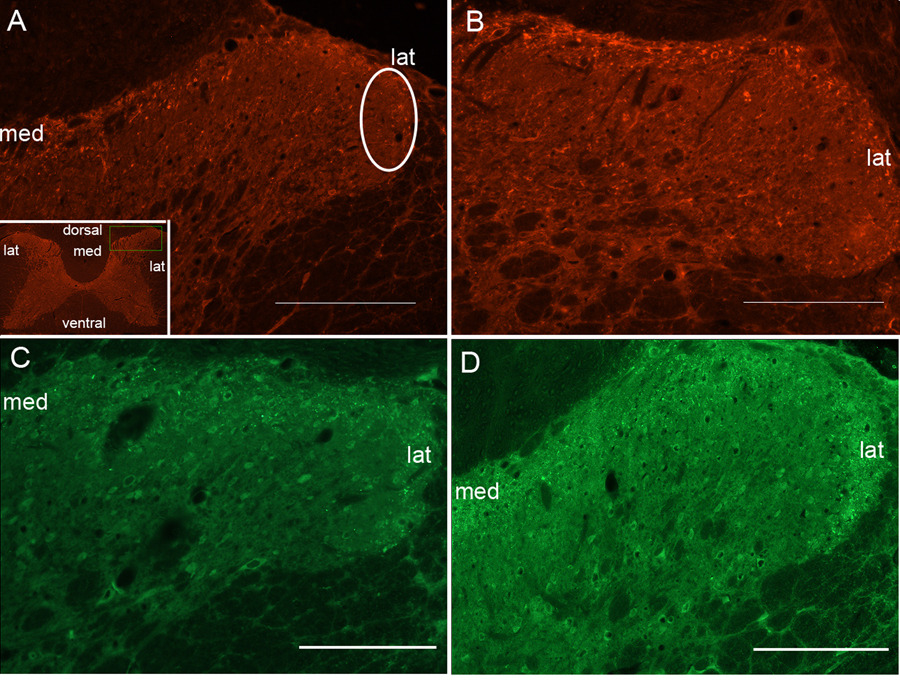
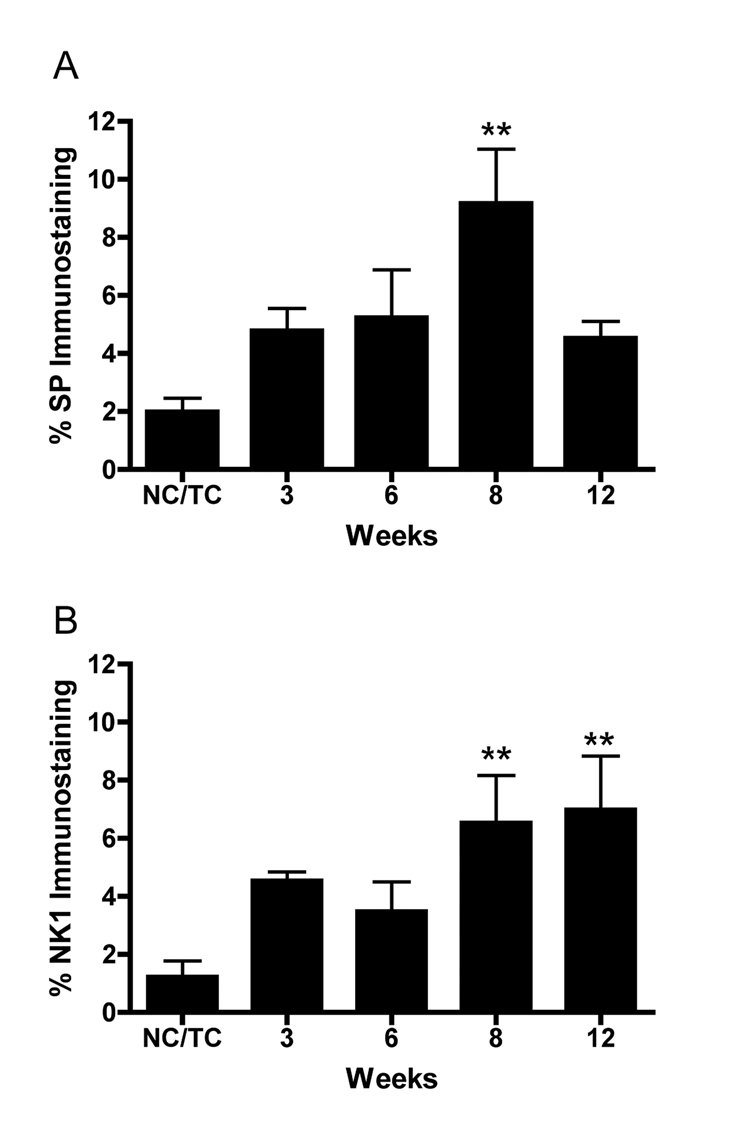
Examination of the dorsal horns of the spinal cord revealed increased SP and NK-1 immunoexpression in the superficial lamina of mid to lower cervical segments (C4–C8) after the LRNF (Fig. 4B,D), compared to trained controls (Fig. 4A,C). ANOVA showed a significant difference in percent immunoexpression of SP and NK-1 with task performance (p=0.002 and p=0.02, respectively). Bonferroni post-hoc analysis indicated that by week 8 LRNF, both SP and NK-1 immunoexpression were significantly increased, p<0.001 and p<0.01, respectively, compared to control rat levels (Fig. 5). NK-1 immunoexpression remained significantly elevated in week 12 (p<0.01; Fig. 5B), while SP levels returned to baseline levels (Fig. 5A).
FIG. 4.
FIG. 4. Micrographs of rat spinal cord dorsal horns showing Substance P and NK-1 immunostaining in the superficial lamina. (A) Dorsal horns of control rats have low levels of NK-1 immunofluorescence staining observed in the dorsal horn superficial lamina. One region of interest (white eclipse) is shown. Inset in panel A shows a C7 spinal cord cross-section at low power. Medial (med) and lateral (lat) regions of the superficial lamina are indicated. (B) Dorsal horns of week 8 LRNF rats have NK-1 immunofluorescence (red) staining on plasma membranes and dendrites with some endosome swellings that spans the entire zone of the superficial lamina in which increased expression is observed more medially. (C) Dorsal horns of control rats have low levels of SP immunofluorescence staining in the superficial lamina. (D) Dorsal horns of week 8 LRNF rats have SP visualized as punctuate immunofluorescence (green) staining that is consistently distributed across the entire zone, medial to lateral, of the superficial lamina. Bar = 50 µm.
FIG. 5.
Quantification of Substance P (SP) and neurokinin-1 (NK-1) immunofluorescence in superficial lamina of the spinal cord dorsal horns. (A) SP expression is significantly increased in week 8 compared to normal control and trained control rats (NC/TC). **: p<0.001. (B) NK-1 expression is significantly increased in weeks 8 and 12 compared to normal control and trained control rats (NC/TC).**: p<0.01. Mean percent immunostaining + SEM is shown. The number of animals quantified per group: Controls (n=9), 3 week (n=3), 6 week (n=4), 8 week (n-4), and 12 week (n=3).
NK-1 immunoexpression levels in the cervical spinal cord were positively correlated with the number of ED1+ cells in the median nerve (r = 0.54, p= 0.03), as well as the number of TNF-α cells in the nerve (r= 0.65, p= 0.005). SP in the spinal cord did not correlate with nerve TNF-α cells (r= 0.17, p= 0.45), but had a trend towards significant correlation with nerve ED-1 cells (r = 0.42, p= 0.07). SP immunoexpression levels in the spinal cord were positively correlated with ED1+ mononucleated cells in the radius and ulna periosteal-bone interface (r = 0.53, p= 0.03), as was NK-1 immunoexpression (r = 0.55, p= 0.04).
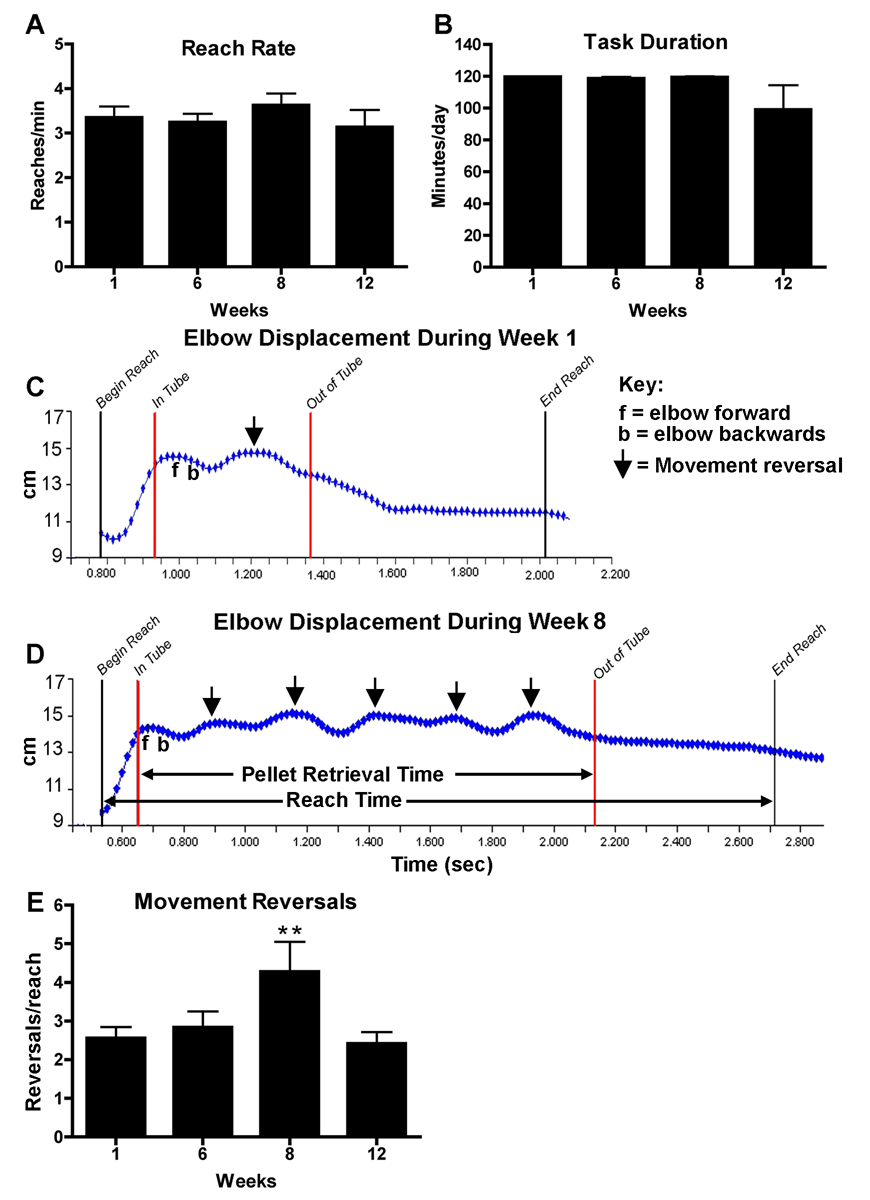
Reach rate was unchanged for the entire 12 week duration of LRNF task (Fig. 6A). Mean reach rates ± SEM for 1, 6, 8 and 12 weeks were 3.3 ± 0.3, 3.3 ± 0.2, 3.6 ± 0.2, and 3.2 ± 0.4 reaches per minute, making this a fairly low reach rate task based on definitions by Silverstein et al. (1986) that low repetition is greater than 30s/cycle. Task duration was slightly decreased at 12 weeks although statistical significance was not reached in this repeated measures ANOVA possibly due to a smaller number of animals at the 12 week end point (Fig. 6B). Total reaches, an index of the cumulative work performed by each group, measured from the start of the LRNF task regimen until the day of euthanasia was 2263 ± 385.4 for week 6 rats, 3178.8 ± 78.1 for 8 week rats, and 4543.2 ± 377.7 for 12 week rats.
FIG. 6.
Motor performance data in rats performing the LRNF task from baseline (week 1) to week 12. (A) Reach rate and (B) task duration show no change. (C, D) Representative reach sequence of a LRNF rat analyzed from video tape segments collected in week 1(C) and week 8 (D). The black lines indicate the beginning and the end time points of one entire reach, at which point a foot pellet is eaten. The red lines indicate the point at which the elbow enters the tube (In Tube) and the point at which it is withdrawn (Out of Tube); a food pellet is retrieved during this time period. Key: f= forward movement of the elbow; b= backward movement of the elbow; arrows indicate an extra movement reversal during the reach sequence in which the rat was retrieving a food pellet. (E) The mean number of extra movement reversals per reach is significantly increased in week 8 of LRNF task indicating more errors and thus a decline in fine motor abilities. **:p < 0.01. Mean per week + SEM is shown. The number of animals quantified per group: Controls (n=9), 3 week (n=7), 6 week (n=7), 8 week (n-7), and 12 week (n=3).
Using video motion analysis, we examined the number of extra movement reversals made by rats in an effort to successfully retrieve a food pellet during a reach (Fig. 6C–E). A normal reach will include just one movement reversal, whereas, extra movement reversals are considered abnormal. Figure 6C shows a representative reach movement sequence in a rat performing the LRNF task in week 1. The entire reach sequence took approximately 1.2 seconds, and the food retrieval period was only 0.4 seconds. The rat made one extra elbow movement reversal during the food retrieval period of this reach sequence. Figure 6D shows a representative reach of the same rat performing the task in week 8. This reach sequence took approximately 2 seconds to complete, with the food pellet retrieval period taking approximately 1.4 seconds (1 second longer than in week 1). In week 8, this rat made 5 extra movement reversal corrections before retrieving the food pellet (Fig 6D, arrows). ANOVA showed a significant increase in the number of extra movement reversals per reach in week 8 (p<0.01; Fig. 6E).
3. Discussion
Results from the present study show that continued performance of a low demand repetitive task produced a mild peripheral nerve and bone inflammatory response, increased immunoexpression of spinal cord neurochemicals involved in nociception, and slight declines in motor function. Collectively, these changes occurred in the later weeks of task performance. The increase in nerve and distal bone inflammation correlated with increased NK-1 expression in the superficial lamina of spinal cord dorsal horns, as did TNF-α positive cells in the median nerve. SP increases in the dorsal horn superficial lamina correlated only with bone inflammatory cell increases. The timing of both SP and NK-1 in the spinal cord was also associated with the ability of rats to accurately and efficiently retrieve food pellets during a reach.
Compared to previous studies from our laboratory examining the effects of performing a high repetition negligible force (HRNF) task in which rats had a mean reach rate of 8 reaches/minute, the LRNF rats in this study had a lower mean reach rate, by experimental design, of only 3.3 reaches/minute. As a result of this lower reach rate, the LRNF rats examined here have 17% fewer inflammatory cells in the median nerve in week 12 than rats performing a HRNF task (see Clark et al., 2003), and a 2 week delay of macrophage infiltration into the nerve, which peaked in week 8 in HRNF rats. The number of TNF-α positive cells in LRNF rat median nerves was also fewer and later than in HRNF rats (see Al-Shatti et al., 2005). The LRNF task did not induce macrophage infiltration of tendons, in contrast to our previous findings in HRNF rats (see Barbe et al, 2003). In that study, ED1 macrophage infiltration of tendon and tendon sheaths increased significantly after 3 weeks of the HRNF task (1.4 fold more than in this study), an increase that persisted for the length of that study (8 weeks). Lastly, the increase in ED1 positive mononucleated cells was lower and later in distal forelimb bones with the LRNF task than the HRNF task (see Barr et al, 2003). Thus, these findings show a cellular inflammatory response in peripheral tissues of LRNF rats that is lower overall than that induced by a HRNF task, and at a later time point, supporting our hypothesis of exposure dependent tissue changes. Because the bone cell increases resolved by 12 weeks in HRNF rats, we presume that the increase in bone ED1 cells in these LRNF rats will also resolve, although longer duration studies must be performed to confirm this hypothesis.
In agreement with our findings of increased macrophages in the peripheral tissues, a number of other investigations found inflammatory cell infiltrates in muscle and tendon following repetitive motion injuries (Baker et al, 2007; Geronila et al., 2003; Nakama et al., 2005; Perry et al., 2005; Stauber and Willems, 2002). Wagner et al. (1998) has also reported increased ED1 positive macrophage infiltration and TNF-α expression in the sciatic nerve following chronic nerve constriction injury. We have previously shown in HRNF rats that potential cellular sources of increased production of several pro-inflammatory cytokines include intraneural ED1 positive macrophages, nerve fibroblasts and Schwann cells (Al-Shatti et al., 2005). While we did not use co-localization techniques in this study to determine if the intraneural TNF-α expressing cells are macrophages, we observed associated increases of both in week 12.
Significant findings from this study of repetitive motion injury are that spinal cord neurochemical changes were associated with increased peripheral nerve and bone phagocytic cell and inflammatory cytokine increases. Our findings support the collective works by Woolf, which have shown that peripheral inflammation leads to increased membrane excitability and synaptic efficiency in the spinal cord dorsal horn (Woolf and Salter, 2000). In agreement, several other investigators have shown that spinal cord levels of SP and NK-1 are significantly increased using various models of peripheral inflammation (Honore, 1999, 2000; McCarson, 1999; Allen et al., 1999; Abbadie et al., 1996). For example, Honore et al. (2000) found significantly increased SP and SP receptors in the spinal cord combined with hindpaw edema, the latter indicative of a peripheral inflammatory response after injection of Freund’s adjuvant. However, the present study is the first to show an association between peripheral nerve inflammatory markers and neurchemical changes in the spinal cord in a model of repetitive motion injury. In this current study, the increase of SP in the spinal cord superficial lamina in week 8 preceded significant increases in inflammatory/phagocytic cells and TNF-α in nerve and bone in week 12. There may be other inflammatory markers or neuromodulators that are associated with the week 8 increase in SP. However, our observed peripheral inflammatory increases matched temporally with elevated spinal cord superficial laminae levels of NK-1. The prolonged increase in NK-1 is suggestive of a long-lasting neuronal plasticity in the spinal cord that persists despite the decrease in SP by week 12. Substance P and its receptor, NK-1, have been proposed to contribute to central sensitization (Ji et al., 2003; Abbadie et al., 1996). Past research on central sensitization has shown changes to occur in the spinal cord and brain following noxious stimuli, peripheral inflammation, and nerve injury in which changes in neurotransmitter release and action are an integral part of this phenomenon (Woolf, 2007).
Lastly, our observed increase in movement reversals during a reach sequence suggests a decline in fine motor control, because these extra movement reversals occurring during a reach sequence are corrections for missed food pellets. These increases in arm movement reversals may be due to discomfort, changes in afferent or efferent nerve function, or both. The small decline in voluntary task participation, measured as task duration, in week 12 is also suggestive of discomfort. Using a voluntary repetitive pinching task in a monkey (Sommerich et al., 2007), Sommerich and colleagues reported decreased performance as force level and pinch hold time increased. In one monkey, there was a substantial reduction from a previously high work rate, as well as complete task cessation that lasted for days. Sommerich et al. suggest that the animal was self-limiting the work rate. In our current study, because these experiments did not extend past 12 weeks, a time when the inflammatory, neurochemical, and behavioral changes are just initiating, it is uncertain whether longer periods of this LRNF task would induce greater motor deficits, increased tissue inflammation and chronic pain.
In conclusion, we found that persistent repetitive motion even under low force and repetition demands induced a low-grade local inflammatory cell invasion of peripheral nerve and distal bone that correlated with nociceptive neurochemical increases in the spinal cord, and that was associated temporally with a transient decline in motor accuracy. Furthermore, the increased NK-1 expression persisted even after the increased SP immunoexpression had resolved, suggestive of a central sensitization of spinal cord neurons. Although peripheral inflammatory responses were apparent, they were much lower in this low demand task than in our previous studies examining the effects of high repetition tasks. This in combination with the preservation of reach rate suggests that this low task exposure level may allow for adequate tissue recovery and prevention of functional losses. Understanding the role of inflammation as a mechanism of nociceptive sensitization is an important step toward developing treatment interventions (pharmacological and ergonomic) for chronic pain and reduced motor control experienced by some patients with RMI, as well as the development of ergonomic preventive strategies.
4. Experimental Procedure
Adult female Sprague-Dawley rats (3.5 months of age at onset of experiments) were obtained from ACE, PA. The animals were housed in the Central Animal Facility on the Health Sciences Campus at Temple University. Animal care and use was monitored by the University Animal Care and Use Committee to assure compliance with the provisions of Federal Regulations and the NIH "Guide for the Care and Use of Laboratory Animals".
4.1 Repetitive Movement Task
Thirty-seven rats were randomized into one of three groups: a low repetition negligible force group (LRNF; n=24), a normal control group (NC; n=9) or a trained only control group (TC; n=4). The LRNF rats and the trained control rats, which underwent the initial training (shaping) only, learned to reach for a food pellet during an initial 12–14 day shaping period as described earlier in Barbe et al., 2003 and Clark et al., 2003, although the target reach rate in this study was reduced to half that of high repetition negligible force rats examined in those earlier studies (see below). Rats were allowed to use their preferred limb to reach, hereafter referred to as the "reach limb". The side used to reach was recorded in each session using a video camera. Estrous cycles were not controlled in the female rats in order to maintain the random selection process for designated groups by weeks.
Rats were placed in operant test chambers for rodents (Med. Associates, VT) with a portal located in one end, a testing chamber described in our previous studies (Barbe et al., 2003; Clark et al., 2003; Barr et al., 2003). The task rats performed a low demand, repetitive reaching and grasping task for a food pellet for 2 hours/day, 3 days/week for 3 (n=7), 6 (n=7), 8 (n=7) or 12 (n=3) weeks. The defined target rate of this LRNF task was 2 reaches/minute, although the rats reached above this target maintaining an average, actual reach rate of 3.3 reaches/minute. This target reach rate was half that for HRNF rats (target of 4 reaches/min; actual 8 reaches/min) used in our previously described high repetition task studies (see Barbe et al., 2003 and Clark et al., 2003). The daily task was divided into 4, 0.5-hour training sessions separated by 1.5 hours.
4.2 Behavioral Analysis
The effects of the task on motor performance were evaluated using variables that included reach rate (reaches/min) and task duration (hours/day) as described previously (Barbe et al., 2003; Clark et al., 2003). Total reaches were also reported to clarify the differences in cumulative workload for 6, 8 and 12 week groups. Total reaches were defined as the sum of the number of reaches per week (product of reach rate, task duration, and number of days/week) across weeks of task performance.
We also used quantitative movement pattern analysis to examine for extra arm movement reversals made during reaching in an effort to successfully retrieve a food pellet. A JVC CCD video camera (Model TK-C1380, 60 Hz) was used to record the reach limb from a lateral view. A landmark on the forelimb was digitized and then tracked at 60Hz (X and Y coordinates) and a graphic display of this landmark coordinate was visually assessed to determine if (and how many) additional arm movement reversals occurred during a reach sequence (See Fig 6C,D). Rats were video taped for 15 minutes at the end of weeks 1, 6, 9 and 12. Each 15 minute videotape was divided into 5 equal (3 minute) segments, and the first reach sequence in each 3 minute segment that satisfied the criteria described below was digitized using video motion analysis (VMA) (Vicon Motus v.8.0, Vicon, Centennial, CO). The five representative videotaped reaches were selected for each weekly endpoint using a systematic procedure. First, a representative reach was defined as a reach sequence beginning with the reach paw in a fixed position and the snout in the tube opening and ending with the consumption of a food pellet. The start of the reach sequence was the first frame in which the forepaw began its ascent toward the tube opening (called Begin Reach in Fig 6C,D). The end of the reach sequence was the first frame in which the head moved away from the forepaws following consumption of the food pellet (called End Reach in Fig 6C,D). The pellet retrieval period was defined as the first frame in which the reach forepaw entered the tube opening (called In Tube in Fig 6C,D), until the first frame in which the reach forepaw exited the tube opening with the food pellet (called Out of Tube in Fig 6C,D). The point on the forearm chosen for digitization was on the proximal forearm segment just below the elbow joint, as this was easily visualized on the videotape throughout the reach sequence. The number of extra elbow movement reversals (fore-aft movements of the forelimb) made during the digitized reaches was used as an indicator of motor dysfunction (e.g. diminished fine motor coordination) and/or discomfort.
4.3 Collection of tissues for immunohistochemical analyses
All animals were euthanized by lethal overdose (Nembutal, 120 mg/kg body weight) and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). These methods are consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Rats performing the LRNF task were euthanized at 3 (n=3), 6 (n=4), 8 (n=4) or 12 (n=3) weeks, as were normal control (NC; n=5) and trained only control (TC; n=4) rats. Forelimbs from the preferred reach limbs were collected. Tissues were then postfixed “en bloc” by immersion overnight. The flexor forelimb mass with the median nerve and flexor digitorum tendon was dissected out, incubated for 3 days in 30% sucrose, cryosectioned en bloc into 16 µm longitudinal slices, and mounted onto coated slides (Ultrastick; Corning). Forearm radius and ulna bones were also collected, paraffin embedded, and sectioned as described in Barr et al., 2003. Cervical and upper thoracic spinal cord segments were removed and the dorsal roots marked with an indelible marker for segmental identification later (in combination with cresyl violet morphological differences to distinguish lower cervical from upper cervical and thoracic spinal cord segments). The spinal cord was then incubated for 3 days in 30% sucrose (at 4°C) before being cryosectioned into 16 µm cross sections.
4.4 Immunohistochemistry and Quantification
For the flexor forelimb mass, every seventh section through the median nerve, the nerve being approximately 1.5 mm in height, was stained immunohistochemically for ED1 using a HRP tagged secondary antibody and DAB for visualization. For bone, every fourth section through the longitudinal center of the radius and ulna was stained for ED1 using a HRP tagged secondary antibody and DAB for visualization. Sections on slides were treated with 3% H2O2 in methanol for 30 minutes, washed, treated with 0.05% pepsin in 0.01 N HCl for 20 min at room temperature, and then blocked with goat serum (4%) for 30 minutes at room temperature. Sections were incubated for 48 hours at 4° C with an antibody directed against ED1 (activated macrophage marker; 1:250, catalog no. MAB1435, Chemicon) diluted with 4% goat serum/PBS. Sections with nerves were also probed with an antibody directed against TNF-α (Chemicon, catalog no. AB1837P, 1:300) diluted with 4% goat serum/PBS for 48 hours at 4° C. After washing, sections were incubated with an appropriate secondary antibody (Jackson Immuno) conjugated to HRP, diluted 1:100 for 2 hours at room temperature. HRP was visualized using enzymatic FAST Sigma diaminobenzidene (DAB). DAB treated sections were counterstained with eosin (nerve and tendon) or hematoxylin (bone), dehydrated, coverslipped with DPX, and then examined using bright field microscopy. Negative control staining for DAB-stained sections was performed by omitting the primary antibodies or by omitting the secondary antibody.
To quantify ED1 staining in nerves, tendons, and bones sections were chosen using a random start method, and were then systematically measured thereafter. The number of ED1 positive macrophages or TNF-α positive cells (each visualized with a HRP tagged secondary antibody and DAB) in median nerves was quantified bilaterally using a microscope interfaced with an image analysis system (Bioquant II) using methods previously described in Clark et al, 2003 and 2004. The region of the median nerve chosen for quantification was the level of the carpal tunnel as well as immediately distal or proximal to the carpal ligament. ED1 cells in the flexor digitorum tendon and distal radius and ulna was quantified (also after visualization with HRP-DAB) using methods previously described in Barbe et al, 2003 and Barr et al, 2003. Three sections were measured using a 40X objective, at a magnification factor of 700, per nerve, tendon or bone for a total of 3 field counts per tissue; inter-section interval was 112 microns. For the nerve and flexor digitorum tendon, the irregular region of interest tool in Bioquant was used so that only the nerve or tendon was measured in the image field, as described in Al-Shatti et al, 2005. Group means and standard error of the mean (n=3–9/group) were plotted against week of task performance and are expressed as the mean number of ED1 positive cells/mm2.
For the spinal cord, every tenth section of spinal cord segments C5 through C7 was utilized for SP and NK-1 immunochemistry using fluorescent tags for visualization and double-labeling techniques. Sections were incubated for 24 hours at 4° C with antibodies directed against SP (1:250, Chemicon, catalog no. AB1566) and NK-1 (1:1000, Chemicon, catalog no. AB5060) diluted with 4% goat serum/PBS. Sections were washed, then incubated with appropriate secondary antibodies (Jackson Immuno) conjugated to either Cy2 or Cy3, diluted 1:100 for 2 hours at room temperature. Slides of sections treated with fluorescent antibodies were coverslipped with 70% glycerol/PBS and examined using an epifluorescent microscope. Negative control staining for fluorescent stained sections was performed by omitting the primary antibodies or by omitting the secondary antibody.
To quantify changes in SP and NK-1 immunoexpression in the spinal cord with task performance, immunofluorescent stained slides were analyzed using an image analysis system (Bioquant II) using similar videocount thresholding methods as described previously (Al-Shatti et al, 2005). Briefly, video count area is the number of pixels in an image field that meet a user defined criterion multiplied by the area of a pixel at the selected magnification (20X objective and 350 magnification factor for this analysis). The mean area fraction of thresholded immunofluorescent reactive product in a selected region of interest, which included the superficial lamina of the dorsal horns only, was determined by dividing the videocount area of pixels above background thresholds by the total number of pixels in the entire chosen image field. An EXFO X-Cite 120 Fluorescent illuminator (EXFO America, Richardson, TX) and a Retiga EXI cooled camera (QImaging, Surrey, BC) were utilized to maintain consistent illumination throughout the project. Gain and exposure were standardized to allow for the cleanest image with fast, short exposure times and remained constant for the entire fluorescent quantification. We used a fixed size eclipse-shaped field (40 × 60 pixels on the computer screen using a 20 X objective) for each measurement. Three to 6 fields were measured in 3 spinal cord sections per rat (inter-section interval was 150 microns). Spinal cord segments (C5–C7) were analyzed bilaterally because previous studies from our lab showed both limbs were affected by the task (Barbe et al., 2003; Barr et al., 2004). Group means and standard error of the mean (n=3–9/group) were plotted against week of task performance and are expressed as mean percent area immunoreactivity. All assessments and image analyses were carried out in a blinded fashion.
4.5 ELISA analysis of median nerves
Rats were euthanized with an overdose of sodium pentobarbital (Nembutol; 120 mg/kg body weight) and the mid-forearm to palmar section of the median nerve was collected from the reach limbs. Reach limb median nerves were pooled since previous attempts to analyze individual median nerves using ELISA failed to give detectable levels of cytokines due to the minute size of rat median nerves (unpublished data). Thus median nerves were pooled by week of task performance as follows: 4 reach limb nerves for week 3, 3 reach limb nerves for week 6 and 3 reach limb nerves for week 8. Eight median nerves from 4 control rats were also pooled into two batches (n=4 nerves for each pool of control nerves). The pooled nerves were then flash-frozen, stored at −80°C, homogenized and tested for TNF-α according to manufacturer’s protocol for ELISA kits (BioSource International, CA). Each sample was run in duplicate. ELISA assay data (pg cytokine protein) was normalized to micrograms of total protein (determined by bicinchoninic acid assay according to manufacturer’s directions; Pierce).
4.6 Statistical analyses
Mixed model univariate ANOVAs (Prism) were used to determine whether week of task performance had any effect on the percent area of immunoreactivity for SP and NK-1 in the spinal cord. A p value of <0.05 was considered significant for all analyses. Microscopic field (3–6 observations/dorsal horn) was used as a blocking factor in the analyses. Post-hoc analyses were carried out using the Bonferroni method for multiple comparisons, and adjusted p values reported. Post hoc analyses compared the percent area of immunostaining for SP and NK-1 in control rat tissues to those in subsequent weeks (3, 6, 8 and 12). The number of ED1 and TNF-α positive cells was analyzed similarly with 3 microscope fields per tissue used as a blocking factor. A two-tailed Spearman rank correlation analysis with Gaussian approximation was used to assess the relationship between ED1 positive macrophages in the median nerve and percent area immunoexpression of SP or NK-1 in the spinal cord. A mixed model ANOVA with repeated measures was used to analyze differences in reach rate, task duration, and movement reversals by week.
Acknowledgements
The authors would like to thank Shreya Amin for her assistance with the cryosectioning. This project was supported by Grant Number OH 03970 from CDC-NIOSH to M.F.B. and Grant Number AR051212 from NIH-NIAMS to A.E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melanie B. Elliott, Email: melanie.dion@temple.edu.
Ann E. Barr, Email: ann.barr@jefferson.edu.
David M. Kietrys, Email: kietrydm@umdnj.edu.
Talal Al-Shatti, Email: tshatti@hsc.edu.kw.
Mamta Amin, Email: mamta@temple.edu.
Mary F. Barbe, Email: mary.barbe@temple.edu.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord Substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J. Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. Journal of Neuroimmunology. 2005;167:13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Impact of repetition number on muscle performance and histological response. Med. Sci. Sports and Exerc. 2007;39:1275–1281. doi: 10.1249/mss.0b013e3180686dc7. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. Journal of Orthopaedic Research. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Elliott ME, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. Journal of Orthopaedic Research. doi: 10.1002/jor.20674. In Press. [DOI] [PubMed] [Google Scholar]
- Barr AE, Barbe MF, Clark BD. Systemic inflammatory mediators contribute to widespread effects in work-related musculoseletal disorders. Exerc. Sport Sci. Rev. 2004;32:135–142. doi: 10.1097/00003677-200410000-00003. [DOI] [PubMed] [Google Scholar]
- Barr AE, Barbe MF, Clark BD. Pathological tissue changes associated with repetitive movement: a review of the evidence. Phys. Ther. 2002;82(2):173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard BP, editor. Musculoskeletal Disorders (MSDs) and Workplace factors: A Critical Review of Epidemiological Evidence for Work-related Musculoskeletal Disorders of the Neck, Upper Extremity, and Low Back. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; 1997. Publication no. 97–141. [Google Scholar]
- Bureau of Labor Statistics. United States Department of Labor News USDL 05-521; Lost-work time injuries and illnesses: characteristics and resulting days away from work. 2005 March 30;
- Clark BD, Al-Shatti TA, Barr AE, Amin M, Barbe MF. Performance of a high-repetition, high-force task induces carpel tunnel syndrome in rats. J Orthop Sports Phys Ther. 2004;34:244–253. doi: 10.2519/jospt.2004.34.5.244. [DOI] [PubMed] [Google Scholar]
- Clark BD, Barr AE, Safadi FF, Beitman L, Al-Shatti T, Amin M, Gaughan JP, Barbe MF. Median nerve trauma in a rat model of work-related musculoskeletal disorder. Journal of Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delander GE, Schott E, Brodin E, Fredholm BB. Temporal changes in spinal cord expression of mRNA for substance P, dynorphin and enkephalin in a model of chronic pain. Acta Physiol Scand. 1997;161:509–516. doi: 10.1046/j.1365-201X.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Geronilla KB, Miller GR, Mowrey KF, Wu JZ, Kashon ML, Brumbaugh K, Reynolds J, Hubbs A, Cutlip RG. Dynamic force responses of skeletal muscle during stretch-shorting cycles. Eur J Appl Physiol. 2003;90:144–153. doi: 10.1007/s00421-003-0849-8. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning P, Rogers SD, Nicholas ML, Basbaum AI, Besson J, Mantyh PW. Spinal Substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. The Journal of Neuroscience. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Ji R, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in Neurosciences. 26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- McCarson KE. Central and peripheral expression of neurokinin-1 and neurokinin-3 receptor and Substance P-encoding messenger RNAS: peripheral regulation during formalin- induced inflammation and lack of neurokinin receptor expression in primary afferent sensory neurons. Neuroscience. 1999;93:361–370. doi: 10.1016/s0306-4522(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamasson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. Journal of Orthopaedic Research. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Perry S, Mclhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- Silverstein BA, Fine LJ, Armstrong TJ. Hand wrist cumulative trauma disorders in industry. Br. J. Ind. Med. 1986;42:7790784. doi: 10.1136/oem.43.11.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerich CM, Lavender SA, Buford JA, Banks J, Korkmaz SV, Pease WS. Towards development of a nonhuman primate model of carpal tunnel syndrome: performance of a voluntary, repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J Orthop Res. 2007;25:713–724. doi: 10.1002/jor.20363. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR, Arbor A. Development and use of an animal model for investigations on rotator cuff disease. Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- Stauber W, Willems M. Prevention of histopathologic changes from 30 repeated stretches of active rat skeletal muscles by long inter-stretch rest times. Eur j Appl Physiol. 2002;88:94–99. doi: 10.1007/s00421-002-0672-7. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the us workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-α expression. Pain. 1998;74:3542. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Williems M, Stauber WT. Isometric and concentric performance of electrically stimulated ankle plantar flexor muscles in intact rat. Experimental Physiology. 1999;84:379–389. [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]