Abstract
Brain-derived neurotrophic factor (BDNF) is implicated in regulation of adult hippocampal neurogenesis, presumably via its primary receptor, TrkB, but controversy exists about how BDNF affects neurogenesis (e.g. proliferation vs. survival/differentiation). This controversy arises, in part, due to the lack of information about if and when TrkB is expressed on adult neural precursors in vivo. Using multiple methods to analyze proliferating and maturing cells in the adult mouse subgranular zone (SGZ), we find that the proportion of proliferating cells that are TrkB-IR is low and it remains low for at least one week following BrdU labeling, but increases as neuroblasts mature. Use of the nestin-GFP transgenic mouse revealed the likelihood of being TrkB-IR increased with presumed maturity of the cell type. Stem-like cells, which rarely divide, were likely to express TrkB. However, early progenitors and late progenitors, which are still in the cell cycle had rare TrkB expression. Immature neuroblasts, however, were more likely to express TrkB, especially as their morphology became more mature. Taken together, these findings emphasize that expression of TrkB protein is closely linked to progression towards neuronal maturity. This provides evidence that maturing cells but not proliferating cells in the adult mouse SGZ have the molecular machinery necessary to respond directly to BDNF. Furthermore, these findings lay critical groundwork for further exploration of the role of BDNF-TrkB signaling in regulation of adult hippocampal neurogenesis.
Keywords: BDNF, neurogenesis, BrdU, nestin, doublecortin
Introduction
A tremendous amount of correlative data suggests that BDNF is critical for regulation of mammalian adult neurogenesis in the SGZ of the hippocampus (e.g. Russo-Neustadt et al., 2004; Xu et al., 2004). Recent work has moved beyond correlative evidence by showing that intra-hippocampal infusion of BDNF increases neurogenesis (Scharfman et al., 2005). While BDNF binds several receptors including p75 and truncated TrkB, decreasing either full-length TrkB activity or BDNF protein levels causes reductions in neurogenesis (Sairanen et al., 2005). These studies strengthen the link between BDNF-TrkB signaling and adult hippocampal neurogenesis, but questions remain about how BDNF actually affects neurogenesis. For example, it is not known whether BDNF acts directly on TrkB-expressing precursor cells or indirectly through nearby cells. It is also unclear whether the neurogenic action of BDNF is due primarily to effects on rates of cell division versus on survival and differentiation of newborn cells. It is even controversial whether BDNF-TrkB signaling increases or decreases cell division in the SGZ (Duman and Monteggia, 2006; Lee et al., 2002; Sairanen et al., 2005). A critical step in addressing these questions is to first understand when TrkB is expressed during division and maturation of neural precursors in the adult SGZ, and therefore when these cells might be able to respond directly to BDNF.
It is striking that progenitor cells in the adult SGZ have not been examined for the presence of TrkB. Embryonic and postnatally-derived neural precursors express TrkB protein in vitro (e.g. Gascon et al., 2005; Lachyankar et al., 1997). However, TrkB expression in cultured cells may not accurately reflect in vivo conditions (Genc et al., 2005), and direct support for in vivo expression of TrkB on neural precursors is notably absent from the literature (Giuliani et al., 2004). Indirect support for TrkB protein expression on neural precursors comes from expression of TrkB protein and mRNA in neurogenic regions of the adult brain (e.g. Linnarsson et al., 2000; Yan et al., 1997) and the altered proliferation or differentiation seen in BDNF or TrkB transgenic mice (Lee et al., 2002; Sairanen et al., 2005). However, no publications have examined hippocampal neural precursors in vivo for expression of TrkB protein.
The lack of evidence regarding TrkB protein in adult hippocampal neural precursors in vivo has been a major obstacle specifically to more sophisticated analysis of how BDNF regulates adult hippocampal neurogenesis, and more generally to greater appreciation of how neural stem cells respond to their environment. Here we provide the first direct evidence that hippocampal progenitor cells in vivo contain TrkB protein. Our study lays the critical groundwork for further investigation of BDNF-TrkB regulation of adult hippocampal neurogenesis, particularly in regards to the endogenous microenvironment so central to adult neurogenesis (e.g. Palmer et al., 2000).
Materials and Methods
Bromodeoxyuridine (BrdU) injections and tissue preparation
C57Bl/6 mice (8 weeks old, Jackson Laboratories) were given one i.p. injection of BrdU (150mg/kg; Boehringer Mannheim, Mannheim, Germany; in 0.007N NaOH/saline at a concentration of 10mg/ml). Four mice were perfused at each of five timepoints after BrdU (2 hours, 24 hours, 6 days, 12 days, or 32 days). To examine neural stem cell maturation, four homozygous nestin-GFP (green fluorescent protein) transgenic mice (8 weeks old; Yamaguchi et al., 2000) were also perfused. Mice were perfused (10 minutes) and postfixed (45 minutes) with 2% paraformaldehyde in 0.1M PBS. Coronal sections (40 μm) through the entire hippocampus were cut on a freezing microtome and stored in 0.1% NaN3/PBS.
Immunohistochemistry (IHC)
For all double- and triple- IHC, free-floating sections were first stained for TrkB and then mounted on slides prior to additional slide-mounted IHC. Free-floating sections were exposed to: 0.3%H2O2 (30 minutes), 3% normal donkey serum (NDS; 30 minutes), rabbit polyclonal anti-TrkB (1:3000; sc-12; Santa Cruz, Santa Cruz, CA; in 3% NDS/PBS; overnight at 4°C), biotinylated secondary (donkey anti-rabbit, 1:200; Vector; Burlingame, CA; 1.5% NDS/PBS; 1 hour) and HRP linking agent (ABC Elite; Vector; 1 hour). Sections were then floated onto uncharged slides, excess liquid was removed, and CY3-TSA solution (Perkin-Elmer, Norton, Ohio; 15 minutes) was applied. Sections were floated off slides, fixed in 4% paraformaldehyde (1 hour), and mounted onto charged slides before slide-mounted IHC (described below). For the BrdU-timecourse, slides were coded, and the code was only broken after data collection.
For GFP/Dcx and NeuN IHC, sections underwent antigen unmasking (0.01M citric acid, pH 6.0, 95°C, 10 min) and were incubated overnight at room temperature in rabbit anti-green fluorescent protein (GFP; 1:3000; ab290; Abcam; Cambridge, UK) and goat anti-doublecortin (Dcx; 1:1000; sc-8066; Santa Cruz) or mouse anti-Neuronal Nuclei (NeuN; 1:50; MAB377; Chemicon, Temecula, CA). Visualization for GFP and Dcx was accomplished sequentially: biotinylated donkey anti-rabbit (1:200; Vector), ABC and fluorescein-TSA (Perkin-Elmer) to visualize GFP; 0.3% H2O2, and subsequent incubation in biotinylated horse anti-goat (1:200; Vector), ABC, and CY5-TSA (Perkin-Elmer) to visualize Dcx. Visualization for NeuN utilized CY3 donkey anti-mouse (1:200; Jackson ImmunoResearch, West Grove, PA).
For BrdU IHC, sections underwent antigen unmasking, membrane permeabilization (0.1% trypsin in 0.1M Tris and 0.1% CaCl2, 10 min), and DNA denaturation (2M HCl in 1X PBS, 30 min) and were incubated overnight at room temperature in rat anti-BrdU (1:500; OBT0030; Accurate, Westbury, NY). Visualization for BrdU utilized CY2 donkey anti-rat (1:200; Jackson). Slides were counterstained with DAPI (1:5000; Roche, Basel, Switzerland).
Verification of TrkB antibody specificity
Previously, antisense knock-down of TrkB in the developing retina has been shown to substantially reduce TrkB-IHC using this antibody (Rickman and Bowes Rickman, 1996). To test the specificity of TrkB staining in this tissue, we preincubated the antibody with the TrkB peptide against which it was raised (sc-12p; Santa Cruz). Primary antibody was diluted 1:160 in 3% NDS/ 0.1% NaN3/PBS, and incubated with the blocking peptide (160-fold excess by weight) overnight at 4°C. Primary antibody incubated in solution lacking peptide served as positive control. Preincubation solutions were diluted 1:40 in 3% NDS/PBS for application to sections. IHC followed the protocol described above.
Quantification and Confocal Imaging
In order to determine the extent of colocalization of TrkB with other antibodies, sections were examined with a confocal microscope (Zeiss Axiovert 200M and LSM510-META; emission wavelengths 488, 543, and 633; Eisch et al., 2000). Colocalization was evaluated only in areas with consistent TrkB staining (strong IR in the entire granule cell layer (GCL), visible GC processes extending to the molecular layer) and only in IR cells >100 um or more inside a region of strong immunoreactivity. Evaluation of colocalization of TrkB with cytoplasmic proteins GFP and Dcx required more detailed analysis, which involved importing stacks of Z images into a 3D reconstruction program, Volocity (Improvision, Lexington, MA). Three-dimensional renderings were rotated and colocalization was examined from all perspectives. Staining was evaluated in every ninth section of the hippocampus bilaterally. The Z plane and orthogonal analyses, three-dimensional reconstruction, and SGZ definition have been previously described (Donovan et al., 2006; Lagace et al., 2006; Mandyam et al., 2004).
Statistical Analyses and Presentation
Data are represented as mean±SEM. A p-value<0.05 was required for significance. Statistical analyses employed GraphPad Prism (v3.00, GraphPad, San Diego, CA). Data were collected as percentages, which are not normally distributed. Therefore, data were transformed using the formula Y’=ARCSIN(SQRT(Y/100)) prior to statistical analyses (Motulsky, 1999). The BrdU-timecourse and GFP/Dcx experiment were analyzed via ANOVA with Student-Newman Keuls (SNK) post-hoc tests to account for the large number of comparisons made (e.g. timecourse: 6 groups, 15 comparisons; Motulsky, 1999).
Double and triple-labeled confocal images presented here were taken from a single 0.5-0.6 μm optical slice. Single, double, and triple labeled images were imported into Photoshop CS2 for Windows (Adobe Systems, San Jose, CA) for composition purposes, and only gamma adjustments in the Levels function were altered.
Results
TrkB-IR staining in the hippocampus
Low magnification examination of full-length TrkB staining was as previously reported (Fig 1a; Drake et al., 1999; Yan et al., 1997). The pyramidal neurons of CA1 and their apical dendrites were prominently stained. The dentate gyrus exhibited a uniform staining of the GCL, as well as large, brightly stained cells in the hilus, presumably GABAergic interneurons. Cells with processes extending through the GCL also appeared in the SGZ. Preincubation of the primary antibody with the TrkB peptide against which it was raised abolished staining in the GCL (Fig 1d vs.1e) and throughout the brain, confirming the specificity of this staining for the TrkB protein in this tissue.
Figure 1.
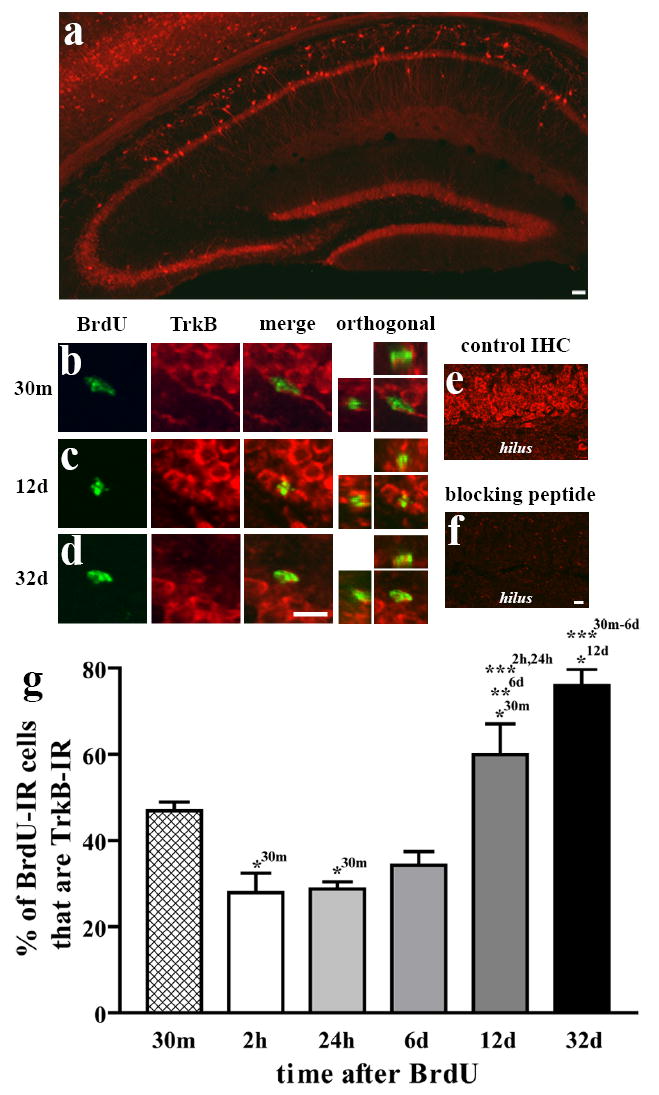
The proportion of BrdU-IR cells that are TrkB-IR changes with survival time after BrdU. Sections from mice injected with BrdU were double-labeled for BrdU and TrkB, and were examined for colocalization. (a) TrkB staining is in pyramidal cells (CA1, CA3) as well as granule cells (dentate gyrus). (b-c) BrdU+/TrkB+ cells from the SGZ at two different timepoints. (d-e) Compared to preincubation of primary antibody with control solution, preincubation with the TrkB peptide against which it was raised completely abolished staining in the GCL. (f) Quantification of the percentage of BrdU-IR cells at each timepoint that colocalized with TrkB (mean±SEM). The likelihood of being TrkB-IR significantly increased after 6 days. *p<0.05, **p<0.01, ***p<0.001 (superscripts denote level of significance between timepoints indicated). Scale bar in (a) =100μm. Scale bar in (c) =10μm and applies to b-c (except orthogonal). Scale bar in (e) =10μm and applies to d-e.
TrkB in BrdU-IR cells varies with survival time after BrdU
Adult mice were given BrdU in order to “birthdate” cells in S phase and were sacrificed at several timepoints later in order to examine cells of a particular “age”. 106±20 BrdU-IR SGZ cells per mouse (4 mice/timepoint) were evaluated for TrkB immunoreactivity (Fig 1b,c). The proportion of BrdU-labeled cells that were TrkB-IR varied with the age of the cells (time after BrdU; Fig 1f). In animals sacrificed 2 hours after BrdU, only 13% of BrdU-IR cells were TrkB-IR. In the 24-hr and 6-day groups, 20% and 23% were TrkB-IR, respectively. However by 12 days after BrdU, 61% of BrdU-IR cells were TrkB-IR, and this proportion increased to 85% at 32 days after BrdU. An ANOVA showed that the TrkB-IR proportion varied significantly among groups (p<0.001), as detailed in Figure 1f. From this experiment, it is clear that the proportion of BrdU-IR cells that are TrkB-IR increased with the age of the cell.
TrkB immunoreactivity increases with the presumed maturity of different cell types
In order to characterize TrkB in SGZ cells with varying degrees of maturity independent of BrdU-labeling, we employed a transgenic mouse that expresses GFP driven by the stem cell gene nestin (Yamaguchi et al., 2000). GFP can be combined with the immature neuron marker, Dcx, to define a lineage of distinct cell types (Fig 2a-b): stem-like cells and early progenitors (type 2a; GFP+/Dcx-) give rise to late progenitors (GFP+/Dcx+), which, in turn give rise to maturing neurons (GFP-/Dcx+; Kronenberg et al., 2003). 265±32 SGZ cells per mouse (4 mice) were evaluated for TrkB immunoreactivity (Fig 2b). The percentage of each cell type that was TrkB-IR is shown in Fig 2c. ANOVA showed that TrkB colocalization varied significantly between cell types (p<0.01), as detailed in Fig 2. In agreement with the BrdU data (Fig 1f), the likelihood of being TrkB-IR in GFP+/Dcx- and GFP+/Dcx+ cells was low (25% and 24% respectively), while GFP-/Dcx+ cells were much more likely to be TrkB-IR (50%). Further subdivision of cell types based on morphology revealed interesting differences. In the minority of GFP+/Dcx- cells which clearly had a stem-like morphology (large, triangular cell body, long process radiating towards the molecular layer), the likelihood of being TrkB-IR was much higher (77%) than in the more common small, compact GFP+/Dcx- cells (21%). This indicates that stem-like (type 1 cells), which rarely divide (Kempermann et al., 2004) may have high TrkB expression, while proliferating progenitors (type 2a and 2b) have low TrkB expression, reflective of short survival BrdU timecourse data. Additionally, in those GFP-/Dcx+ cells which clearly had a long process characteristic of relatively mature young neurons, TrkB-IR was present in every cell examined. It is clear from the results and previous publications (Fig 1a; Drake et al., 1999; Yan et al., 1997) that TrkB protein is present in most mature granule cells. We confirmed this by examining tissue double-labeled for TrkB and NeuN, where all NeuN+ cells appear to be immunoreactive for TrkB (Fig 2d). Taken together, Figures 1 and 2 show that young, undifferentiated cells are unlikely to be TrkB-IR, but that as newborn cells mature and differentiate, their likelihood of expressing TrkB increases dramatically.
Figure 2.
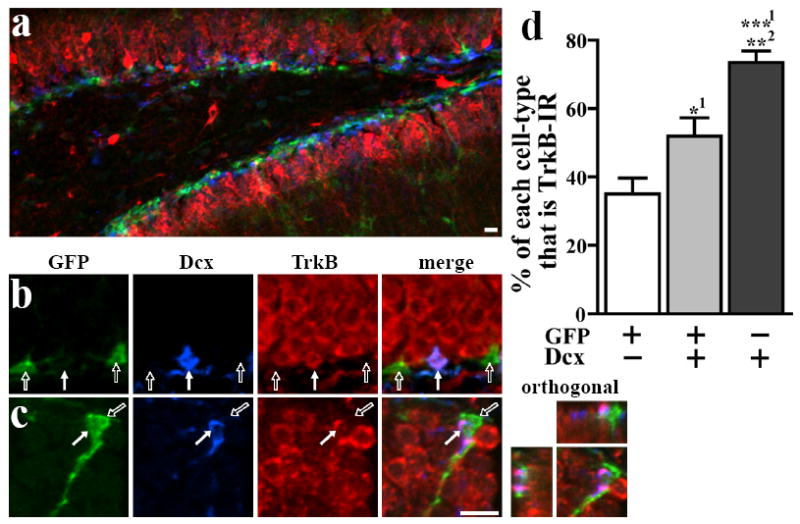
The proportion of cells that are TrkB-IR increases with the presumed maturity of different cell types. (a) Sections from nestin-GFP transgenic mice were triple-labeled for GFP, the immature neuronal marker doublecortin (Dcx), and TrkB. Cells immunoreactive for GFP, Dcx, or both markers were classified into cell types and were examined for TrkB colocalization. (b) Two GFP+/Dcx-/TrkB- cells (open arrows) and one GFP-/Dcx+/TrkB+ cell (closed arrow) are clearly visible. (c) The percentage of each cell type that colocalized with TrkB (mean±SEM) varied significantly among the different cell types. (d) Sections stained for TrkB and NeuN, a marker for mature neurons, reveal that virtually all mature granule cells are TrkB-IR. *p<0.05, **p<0.01 (superscripts denote level of significance between that cell type and another cell type). Scale bars=10μm.
Discussion
BDNF-TrkB signaling has been strongly linked to adult hippocampal neurogenesis, but controversy exists as to how BDNF impacts neurogenesis, and it was unknown whether and when adult SGZ precursors express TrkB. Here we show that TrkB protein – a receptor for BDNF implicated in regulation of adult neurogenesis – is not expressed in most proliferating SGZ cells. TrkB protein remains low during the first week of survival after mitosis. As newborn cells mature, though, their likelihood of being TrkB-IR increases. This study is the first to directly show TrkB protein in adult hippocampal precursors, and opens avenues for further examination of the role of BDNF-TrkB signaling in regulation of adult hippocampal neurogenesis.
These results suggest that TrkB signaling can directly influence survival, but is less likely to influence proliferation in adult precursors. Our finding that dividing cells do not contain TrkB receptors is consistent with studies showing that BDNF-TrkB signaling impacts survival rather than proliferation (Lowenstein and Arsenault, 1996; Sairanen et al., 2005). The increasing proportion of BrdU-IR SGZ cells that are TrkB-IR after the first week of maturation (Fig 1f) may reflect increasing sensitivity to target-derived BDNF, coincident with making synaptic connections with other neurons (e.g. Hastings and Gould, 1999). Therefore, while controversy exists about how BDNF influences proliferation versus survival in neural precursor cells (Lee et al., 2002; Sairanen et al., 2005), the present data suggest that TrkB is positioned to mainly influence maturation of new hippocampal cells. The differences seen in TrkB expression between in vitro and in vivo experiments (Genc et al., 2005) underscore the importance of the present in vivo analysis examining TrkB in multiple stages of hippocampal neurogenesis. For instance, we observed that stem-like cells (sometimes referred to as “type 1”) may actually be much more likely to contain TrkB receptors than other nestin-GFP+ cells. Although these cells rarely divide, they are thought to play an important role in replenishing the population of rapidly proliferating precursors in the SGZ (Kempermann et al., 2004). Future studies can examine how manipulations that alter BDNF levels (e.g. antidepressants) specifically alter the populations of cells that express TrkB, providing mechanistic clues into regulation of adult neurogenesis.
Our findings suggest that activation of TrkB receptors on proliferating neural precursor cells does not allow BDNF to influence neurogenesis. These findings, however, do not exclude indirect influence of BDNF on neurogenesis, such as via enhanced activity of mature granule cells, which highly express TrkB (Fig 2d). Alternatively, given their close juxtaposition to proliferating precursors, prominent process extending through the GCL, and high TrkB expression (Fig 2c), type 1 cells are perfectly positioned to sense external BDNF concentration and influence proliferation by some secondary mechanism. The dynamic expression of TrkB protein as cells mature underscores the complexity of the potential influence of BDNF on adult neurogenesis. This work encourages, as well as informs, future studies on the consequence of removing or stimulating TrkB specifically on hippocampal progenitors in vivo. Such work will be essential for understanding how BDNF signaling functionally impacts adult hippocampal neurogenesis.
Acknowledgments
The authors are grateful to Eric Nestler, Chris Cowan, Scott Russo, and Deanna Wallace for critical reading of the manuscript.
Grants: Grant sponsor: NIDA; Grant number: (to AJE); Grant sponsor: NARSAD; Grant number: (to AJE); Grant sponsor: NIMH; Grant number: MH07547 (to MHD).
References
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19(18):8009–26. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Zhang H, Barral-Moran MJ, Kiss PJ, Mas C, Kiss JZ. Sequential activation of p75 and TrkB is involved in dendritic development of subventricular zone-derived neuronal progenitors in vitro. Eur J Neurosci. 2005;21(1):69–80. doi: 10.1111/j.1460-9568.2004.03849.x. [DOI] [PubMed] [Google Scholar]
- Genc B, Ulupinar E, Erzurumlu RS. Differential Trk expression in explant and dissociated trigeminal ganglion cell cultures. J Neurobiol. 2005;64(2):145–56. doi: 10.1002/neu.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A, D’Intino G, Paradisi M, Giardino L, Calza L. p75(NTR)-immunoreactivity in the subventricular zone of adult male rats: expression by cycling cells. J Mol Histol. 2004;35(89):749–58. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413(1):146–54. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467(4):455–63. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH. Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neurol. 1997;144(2):350–60. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile Administration of Methylphenidate Attenuates Adult Hippocampal Neurogenesis. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Willson CA, Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Brain Res Mol Brain Res. 2000;75(1):61–9. doi: 10.1016/s0169-328x(99)00295-8. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Arsenault L. The effects of growth factors on the survival and differentiation of cultured dentate gyrus neurons. J Neurosci. 1996;16(5):1759–69. doi: 10.1523/JNEUROSCI.16-05-01759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76(6):783–94. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ. Analyzing data with GraphPad Prism. San Diego, CA: GraphPad Software Inc.; 1999. [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rickman DW, Bowes Rickman C. Suppression of trkB expression by antisense oligonucleotides alters a neuronal phenotype in the rod pathway of the developing rat retina. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12564–9. doi: 10.1073/pnas.93.22.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29(12):2189–99. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25(5):1089–94. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Xu H, Luo C, Richardson JS, Li XM. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J. 2004;4(5):322–31. doi: 10.1038/sj.tpj.6500265. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11(9):1991–6. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378(1):135–57. [PubMed] [Google Scholar]


