Abstract
The meta-halo-3-methylbenzonitrile derivatives (-F, -Cl, -Br, -I) were synthesized as model compounds to study reactivity towards aromatic nucleophilic substitution. A single-mode microwave system was incorporated into a commercial radiochemical synthetic module for F-18 labeling. Labeling yields of 64% for fluoro-, 13% for bromo- and 9% for chloro-precursors were achieved in DMSO in < 3 min. The observed order of reactivity of the leaving groups toward aromatic nucleophilic substitution was F>>Br>Cl>>>I.
Keywords: fluorine-18, radiolabeling, microwave, PET tracer, organic synthesis, substituent effect
1. Introduction
Molecular imaging with positron emission tomography (PET) can provide a non-invasive approach to measure biological or biochemical processes and has become a powerful method in clinical diagnosis and therapy, especially in oncologic diseases (Imam, 2005; Herzog, 2007). Fluorine-18 labeling has been widely used in the radiosynthesis of PET imaging agents owing to its attractive physical and chemical properties. Fluorine-18 (β+, t1/2 110 min) has a favorable half-life suited to imaging biological processes and a short positron range in tissue, resulting in clear images with high-resolution PET. Carbon-fluorine bonds are strong (C–F bond energy on the order of 130 kcal/mol) which translates to good in vitro and in vivo stability (Couturier et al., 2004; Karramkam et al., 2003; Wüst et al., 2003). Fluorine-18 labeled radiopharmaceuticals can be synthesized either by nucleophilic substitution with [18F]F− or electrophilic substitution using [18F]F2. Nucleophilic aromatic substitution is an important reaction for synthesis of 18F aryl fluorides because of the widespread occurrence of aromatic rings in bioorganic molecules. One of the requirements for practical application of 18F tracers is a rapid radiolabeling process with an acceptable radiochemical yield (RCY). However, due to the weak nucleophilic properties of fluoride on a homoaromatic ring, F-18 labeling of aromatic rings requires harsh reaction conditions and long reaction times, which often generates poor RCY and undesired by-products.
Microwave-promoted synthesis is an area of increasing research interest in chemistry as evidenced by a number of papers and recent reviews appearing in the literature (Elander et al., 2000; Stone-Elander et al., 2002). As well as being energy efficient, microwaves can enhance the rate of reactions and improve product yield in many cases. Microwave-induced reaction as a method for radiolabeling provides advantages such as short reaction time, high RCY and high selectivity (Le et al., 2006). With the advent of dedicated chemistry accelerated microwave reactors, many radiosynthetic processes that were previously low yielding or required extended reaction times at elevated temperatures can been transformed into protocols typically requiring only minutes to achieve comparable or superior results (Kappe, et al., 2004).
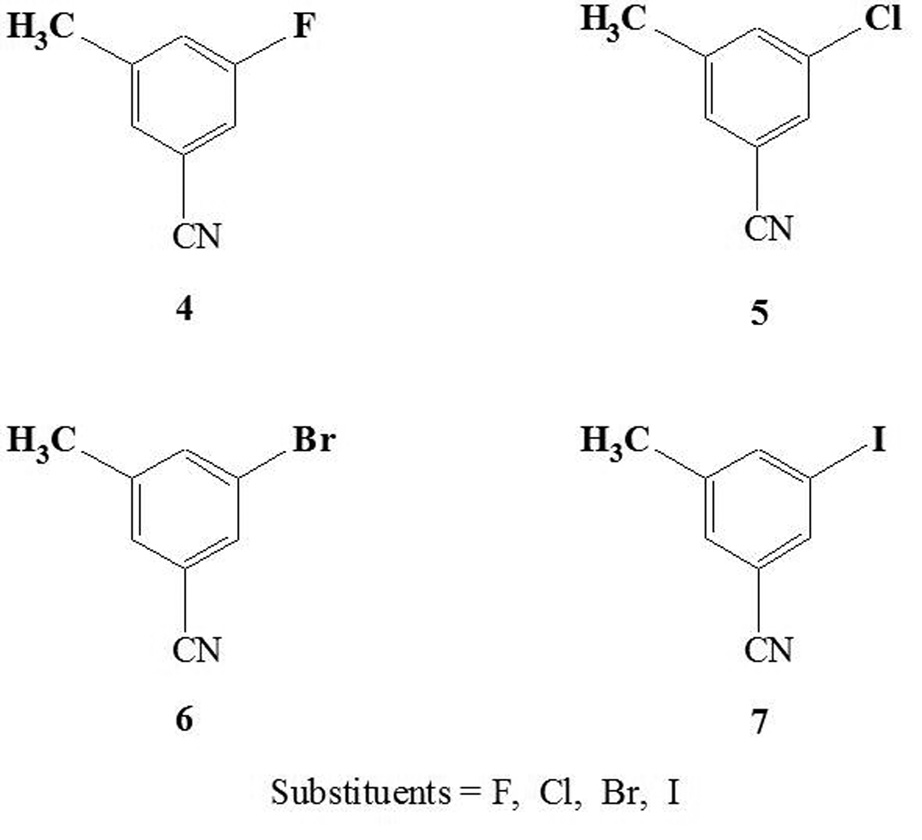
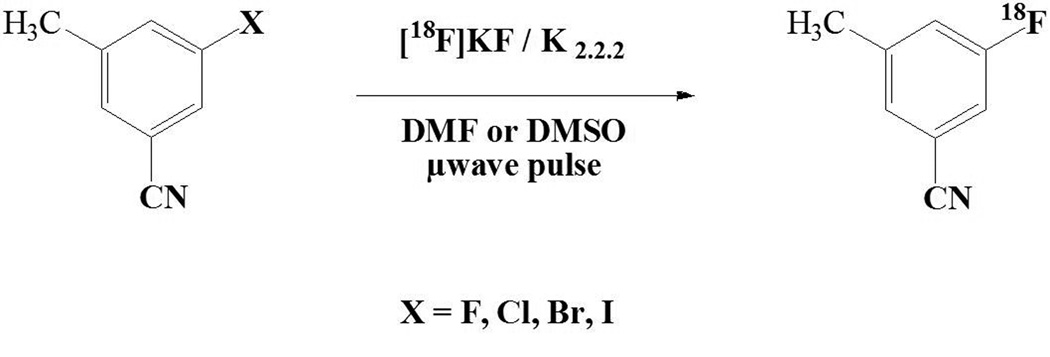
The course and rate of nucleophilic [18F]fluorination reactions depend largely upon the choice of the leaving groups. The most successful leaving groups used in fluorine-18 nucleophilic aromatic substitution are nitro- as in [18F]altanserin (Monclus, et al., 1992; Tan, et al., 1997) and [18F]setoperone (Maziere, et al., 1988), quaternary ammonium (Wilson, et al., 1991), and some halides (Guenther et al., 2006; Willis et al., 2005; de Vries et al., 2003; Zhang et al., 2004; Fei et al., 2004; Roger et al., 2003). Normally, labeling reactions require an electron-withdrawing group ortho or para to the leaving group for reaction to occur, both by reducing electron density at the target carbon and by resonance stabilization of the putative sigma complex in an addition-elimination mechanism. Suitable activating groups include nitro, carbonyl, and cyano substituents. Only a few halogenated compounds in highly activated aromatic rings with electron-withdrawing substituents have been studied for [18F]labeling (Karramkam et al., 2003; Zhang et al., 2004; Dolci et al., 1999; Dischino et al., 2003; Al-Labadi et al., 2006). The application of microwave fluorodehalogenation to less reactive homoaromatic compounds has not been studied in detail, although Hamill et al recently reported labeling the meta-cyano-fluoro compound [18F]FMTEB in low yield by microwave-induced [18F]fluoride displacement of the chloro precursor (Hamill, et al., 2005). We therefore investigated the [18F]fluorodehalogenation reactions of 3-halo-5-methylbenzonitrile derivatives (Figure 1) by microwave acceleration using a dedicated single-mode microwave reactor. To develop a fast and efficient method of remote semiautomatic microwave-induced radiosynthesis, a commercial radiofluorination chemistry module was adapted to work with a microwave apparatus having a detached resonance cavity.,
Fig. 1.
Structure of meta-halo (F, Cl, Br, I)-benzonitrile model compounds
2. Materials and Methods
2.1 Materials
4-Amino-3-methylbenzonitrile was obtained from Lancaster Synthesis Inc. 3-Fluoro-5-methylbenzonitrile was purchased from SynQuest laboratories. QMA® anion exchange Sep-Pak cartridges for F-18 elution was obtained from Waters (Milford MA). Unless specifically indicated, all other reagents and solvents were purchased from Sigma-Aldrich (St. Louis MO) and were of analytical grade or better.
2.2 General Methods
1H and 13C NMR spectra were recorded on a Bruker DPX-300 (300 MHz) spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) downfield from an internal tetramethylsilane standard. Mass spectra were obtained on a TSQ 7000 spectrometer using electrospray ionization (ESI). Accelerated Microwave Heater Model 521 was purchased from Resonance Instruments Inc. (Skokie IL). TRACERlab® FX-FN nucleophilic fluorination module was purchased from GE Medical Systems (Milwaukee WI). Thin layer chromatography (TLC) on silica gel plate containing 254 nm phosphor (2.5 × 10 cm) was used to determine the product yield of the labeling step and was scanned using a Bioscan model AR-2000 TLC Scanner (Washington DC). No-carrier-added [18F]F was produced on a CTI/Siemens RDS-112 cyclotron (proton beam energy 11 MeV) using a Ta target of 99.99% enriched H2[18O]O (2.8 or 3.8 mL) at 40 µA.
2.3 Organic synthesis
4-Amino-3-chloro-5-methylbenzonitrile (2)
4-Amino-3-methyl-benzonitrile (1) (1.0 g, 7.6 × 10−3 mol) was dissolved in 40 mL of 2 M aqueous HCl in a flask in a water bath maintained at or below 30 °C. Chlorine gas was bubbled into the reaction mixture very slowly with rapid stirring until no more precipitate formed. The reaction mixture was filtered by gravity and washed with H2O (3 × 100 mL), then dried at room temperature. The brown crude product was recrystallized from acetic acid/H2O to give 0.82 g (48%) of a white solid. 1H NMR (300 MHz, CDCl3) δ 7.45 (1H, s) 7.24 (1H, s), 4.60_(NH2, s), 2.21 (3H, s).
4-Amino-3-bromo-5-methylbenzonitrile (3)
4-Amino-3-methyl-benzonitrile (1) (1.0 g, 7.6 × 10−3 mol) was dissolved in 40 mL of 2 M aqueous HCl. A solution of bromine (1.5 g, 9.1 × 10−3 mol) was added very slowly with rapid stirring. Following the addition, the reaction mixture was stirred at room temperature for 10 min. The solid was filtered and washed with H2O (3 × 100 mL), then dried in vacuum at room temperature. A bright yellow solid (1.5 g, 95%) was obtained by recrystallization in a solution of 80% acetic acid/H2O. 1H NMR (300 MHz, CDCl3) δ 7.60 (1H, s), 7.27 (1H, s), 4.59_(−NH2, s), 2.22 (3H, s).
3-Chloro-5-methylbenzonitrile (5)
4-Amino-3-chloro-5-methylbenzonitrile (2) (1.0 g, 6.0 × 10−3 mol) dissolved in 40 mL of ethanol in a 100 mL three-neck flask in a water bath. Concentrated sulfuric acid (10 mL) was added dropwise while maintaining the bath temperature below 10° C. Aqueous solution of sodium nitrite (0.50 g, 7.2 × 10−3 mol/10 mL) was added dropwise over a period of 20 min at 5–10°C and maintained at that temperature for 30 min. The temperature of reaction mixture was increased to 40 °C and the stirring continued for 3 h until no more foaming was observed. The product mixture was cooled to room temperature and poured into 200 mL of ice water. The precipitate that formed was filtered and the crude product was purified by flash chromatography using CHCl3. After evaporation of the solvent, 91 mg (14%) of a solid was obtained, mp 61–63°C. 1H NMR (300 MHz, CDCl3) δ 7.45 (1H, s), 7.41 (1H, s), 7.36 (1H, s), 2.39 (3H, s); literature (Murphy, et al., 2007) 7.44 (1H, s), 7.40 (1H, s), 7.35 (1H, s), 2.38 (3H, s); 13C NMR (75 MHz, CDCl3) δ 141.53, 135.24, 134.25, 131.18, 129.34, 118.01, 113.95, 21.34. HR-MS (EI) m/z calcd for, C8H6NClH+: 152.03, C8H6NClNa+: 174.01, Found: 152.0 (100%), 154.0 (35%), 174.0.
3-Bromo-5-methylbenzonitrile (6)
4-Amino-3-bromo-5-methylbenzonitrile (3) (1.0 g, 4.7 × 10−3 mol) was reacted as described above for product 4, pouring the reaction mixture into 400 mL of ice water. The collected precipitate was recrystallized from 95% aqueous EtOH to obtain 0.79 g (90%) of white crystals, mp 67–69°C; literature mp 67–68.5°C (Fisher, et al., 1990); 62°C (Jones, et al., 1955). 1H NMR (300 MHz, CDCl3) δ 7.60 (1H, s), 7.57 (1H, s), 7.40 (1H, s), 2.39 (3H, s); 13C NMR (75 MHz, CDCl3) δ 141.63, 137.12, 132.13, 131.59, 122.95, 117.83 114.16, 21.28. HR-MS (EI) m/z calcd for C8H6NBrH+: 195.98, C8H6NBrNa+: 217.96. Found: 196.0 (100%), 198.0 (90%), 217.8.
3-Iodo-5-methylbenzonitrile (7)
3-Bromo-5-methylbenzonitrile (6) (300 mg, 1.5 × 10−3 mol) dissolved in 25 mL of N2-purged DMF in a 50 mL three-neck flask in an oil bath. Nickel power (200 mg, 3.4 × 10−3 mol), KI (500 mg, 3.0 × 10−3 mol) and I2 (200 mg, 0.8 × 10−3 mol) were added and the reaction mixture was refluxed overnight. After cooling to room temperature, 20 mL of 3% aqueous HCl was added and the mixture was extracted with pentane (3 × 20 mL). The organic layer was washed successively with 10% aqueous NaHSO3 (2×15 mL) and then H2O (2×15 mL), dried over anhydrous Na2SO4, and evaporated to dryness on a rotary evaporator. The residue was purified by flash chromatography using CHCl3/MeOH, 99:1, to obtain 138 mg (37%) of a solid; mp 54–56 °C literature mp 55–56°C (Gore, et al., 1973). 1H NMR (300 MHz, CDCl3) δ 7.60 (1H, s), 7.57 (1H, s), 7.40 (1H, s), 2.39 (3H, s); HR-MS (EI) m/z calcd for C8H6NINa+: 265.94, Found: 266.12.
2.4 [18F]Fluorination Reactions
Apparatus
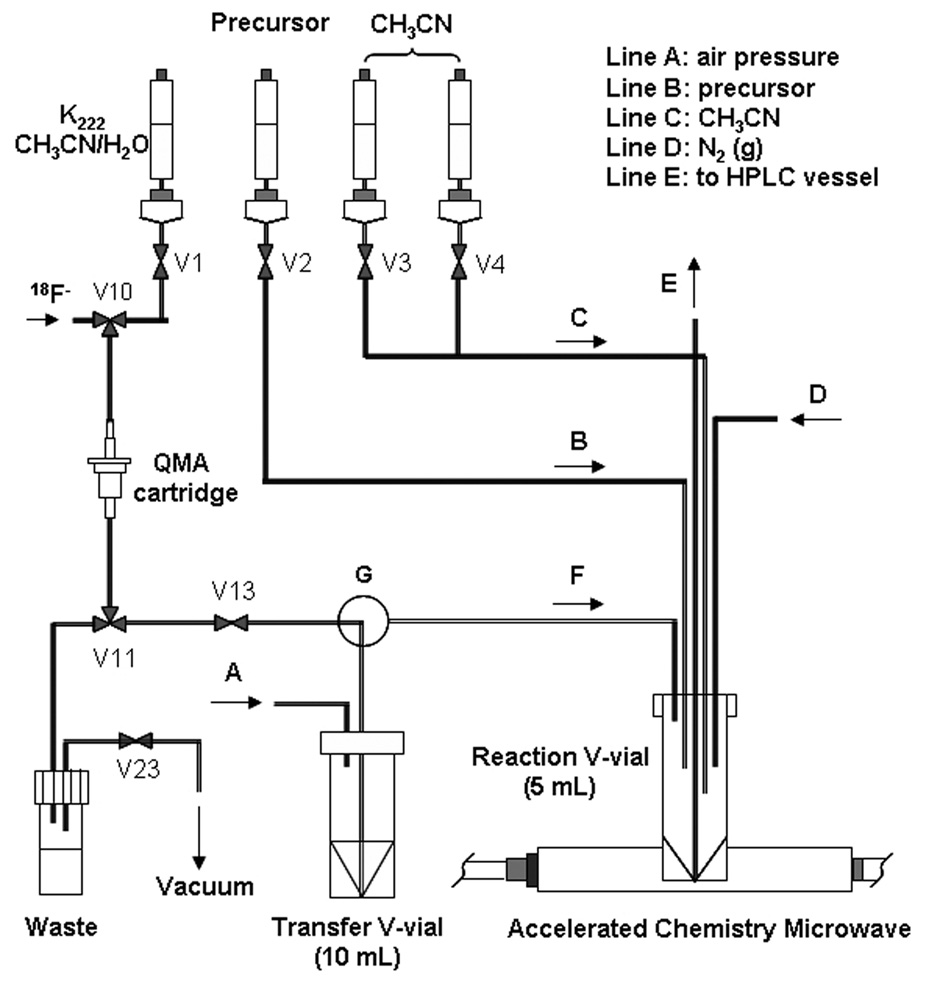
A GE Medical Systems TRACERlab® FX-FN chemistry module was modified as shown in Figure 2. The lines going into the reactor vessel were redirected to an external collar holding lines B to F going into a 5-mL V-vial contained in the cavity of the microwave accelerator. The [18F]fluoride elution line from the QMA anion exchange cartridge (V13) was connected through an extra 3-way valve ‘G’ to a 10-mL V-vial (‘Transfer V-vial’), allowing dispensing aliquots of [18F]F− to the reaction V-vial via controlled pressure on line ‘A’ with an external syringe. Line E was used to transfer aliquots from the reaction vial for chromatographic analysis. In practice, it is connected to the module’s standard collection vessel for HPLC purification and isolation, though not used in these experiments.
Fig. 2.
A design for incorporation of the microwave reactor into the TRACERlab
[18F]Fluoride Solution
Using the manufacturer’s control software, 18F]fluoride in target water was transferred through a QMA® anion exchange cartridge (pre-conditioned with aqueous KHCO3 and H2O), collecting the H2[18O]O in the recovery vial (Figure 2). The cartridge was then eluted with 0.6 mL of a 50:50 mixture of 20 mg/mL Kryptofix-222 in CH3CN and 10 mg/mL K2CO3 in H2O from vessel V1 into the transfer V-vial. Aliquots of the K+(K222)[18F]F− were then transferred into the reaction V-vial by pressure from ‘A’. The mixture was evaporated to dryness with N2(g) flow (line ‘D’) and microwave heating using a pulse sequence of 60 sec at 80W, 110°C max temperature. Acetonitrile (1 mL) was added from vessel V3 by controlled time opening of valve V3, followed by evaporation to dryness under the same conditions; this was repeated twice more.
[18F]Radiofluorination Reactions
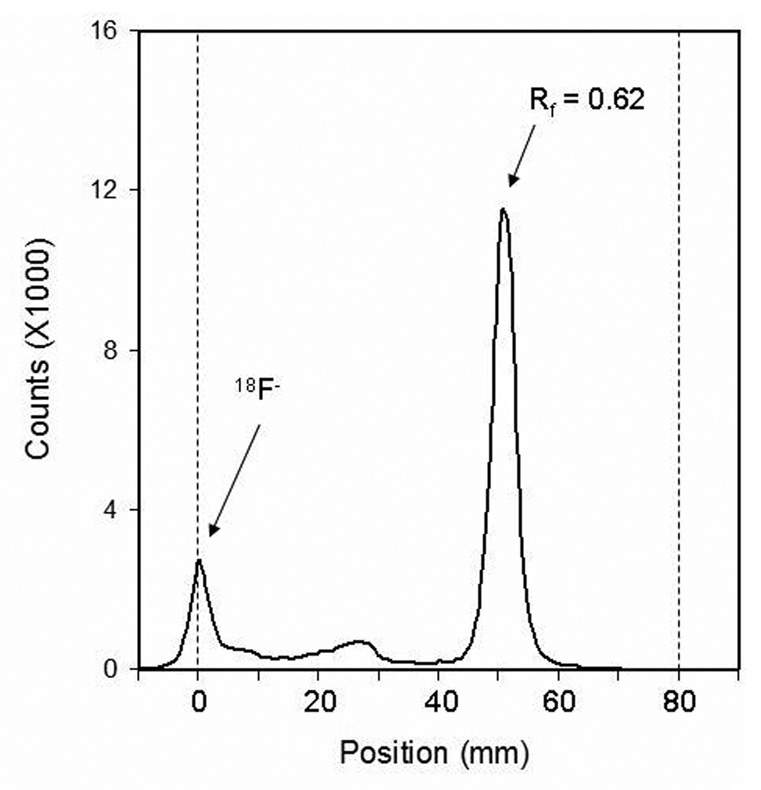
Precursor compound (37 µmol) was dissolved in DMF or DMSO and then added from vessel V2 through line B into the reaction vial containing dried [18F]fluoride. Microwave power was then applied in a sequence of 3 × 20 sec at 150W, maintaining a 145°C maximum temperature, with ca 30 sec pause between pulses, which allowed time to remove a 5 µL aliquot for TLC analysis. The sample was spotted on the TLC strip along with a sample of authentic 3-fluoro-5-methylbenzonitrile in the adjacent lane. After development with 100% CHCl3, the position of the standard was measured by quenching of 254 nm UV light and the distribution of radioactivity was measured on the Bioscan AR2000 scanner.
3. Results and Discussion
3.1 Synthesis of Precursor Compounds
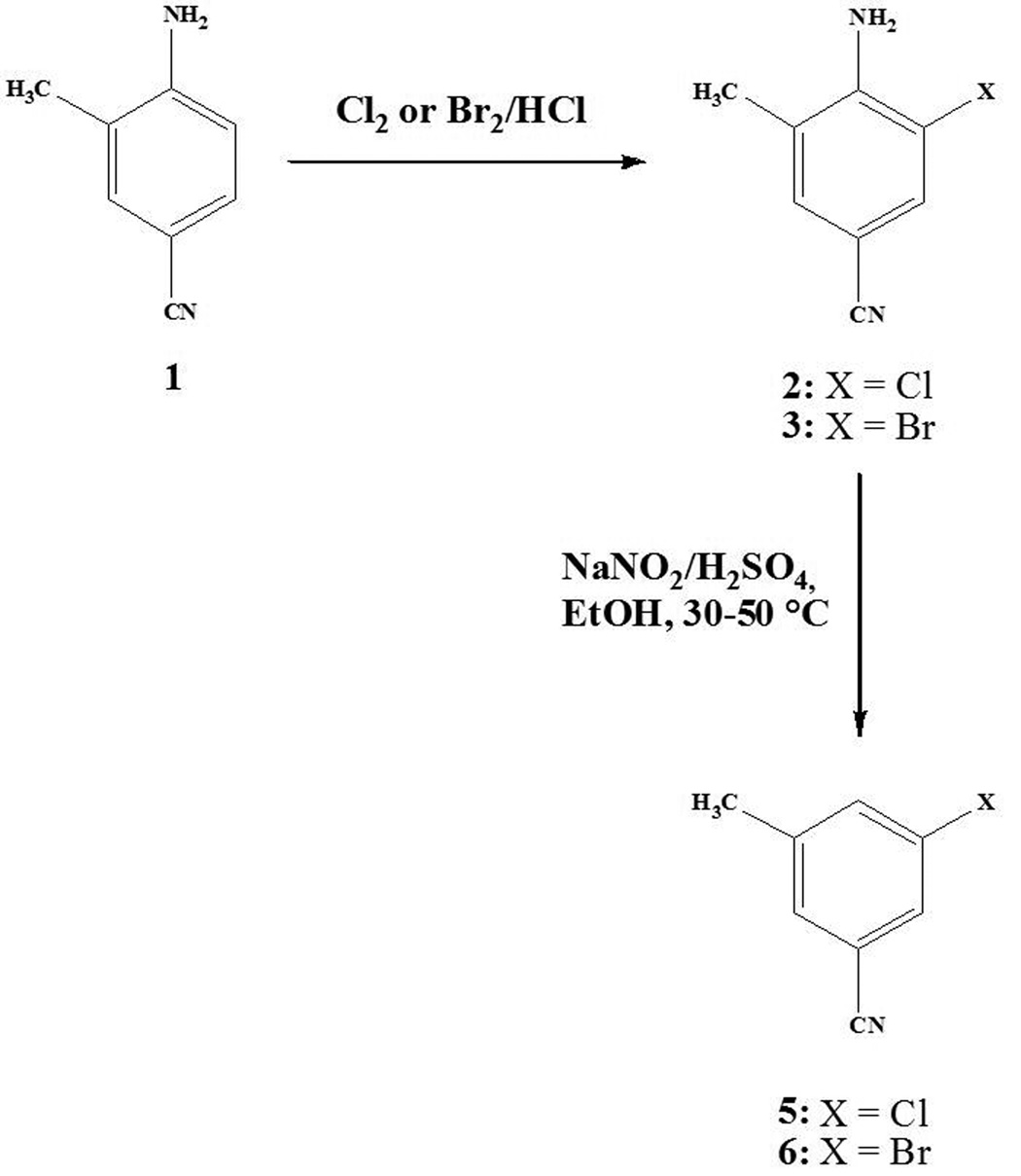
The title compounds were synthesized from 4-amino-3-methylbenzonitrile by electrophilic halogenation followed by reductive deamination. The iodo analog was made by Ni-catalyzed halogen exchange of the bromo compound. There are many methods for the introduction of halogen onto an aromatic ring containing electron-donating groups. However, halogenation of aromatic rings containing electron withdrawing groups such as CN is more difficult, often requiring indirect methods. Although the synthesis of 3-bromo-5-methylbenzonitrile has been reported (Fisher et al., 1990) in several steps, we synthesized 3-chloro- and 3-bromo-5- methylbenzonitrile by a two-step method (Scheme 1) by halogenation of 4-amino-3-methylbenzonitrile followed by deamination via reduction of the diazonium salt. 4-Amino-3-chloro-5-methylbenzonitrile 2 and 4-amino-3-bromo-5-methylbenzonitrile 3 were first prepared in 50–95% yield by reaction with elemental Cl2 or Br2 in 2 M aqueous hydrochloric acid. Generally, deamination reactions can be performed in 50% hypophosphorous acid/H2SO4 with addition of sodium nitrite at low temperature. However, we could only obtain 3-bromo-5-methylbenzonitrile in very low yield and the deamination reaction of 4-amino-3-chloro-5-methylbenzonitrile was not successful. However, changing to ethanol in the presence of NaNO2/H2SO4 was more successful. A good yield (90%) was obtained from the bromo-substituted compound 3 at 40° C stirring for 3 h; a moderate yield (15–20%) was obtained from the chloro compound 3. However, the iodo compound could not be made by this route. Iodination of 4-amino-3-methylbenzonitrile with ICl proceeded smoothly, but the iodoaniline derivative could not be deaminated under any conditions tried, perhaps because of the ready coupling of the intermediate diazonium salt with the iodoaromatic moiety.
Scheme I.
Synthesis of 3-chloro-5-methylbenzonitrile and 3-bromo-5-methylbenzonitrile
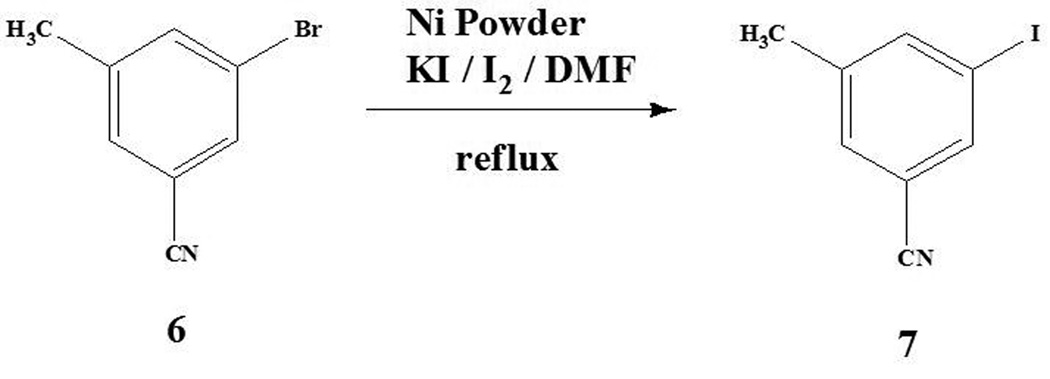
Transition metal-catalyzed halogen-exchange reactions between aryl halides and alkali halides have been used to prepare aryl halides (Yang et al., 1987; Klapars et al., 2003; Arvela et al., 2003). Therefore, 3-iodo-5-methylbenzonitrile 7 was synthesized (in 37% yield) from the bromo compound 6 by reaction with KI/I2 in the presence of nickel powder in refluxing DMF (Scheme 2). All of the compounds were characterized by 1H and 13C NMR and ESI mass spectroscopy.
Scheme II.
Synthesis of 3-iodo-5-methylbenzonitrile
3.2 [18F]Radiofluorination Reactions
A commercial nucleophilic radiofluorination module (GEMS TRACERlab® FX-FN) was modified to combine the [18F]fluoride collection and reagent addition components of the module with a reaction vial contained in the cavity of a dedicated single-mode microwave accelerator to allow semi-automated remote radiofluorination experiments (Figure 2). Aqueous [18F]fluoride was activated by combining with the cryptand Kryptofix-222 and potassium carbonate and dried by azeotropic distillation with acetonitrile by microwave heating and gas flow, followed by reaction with the precursor compounds. Considering a general need of a series of experiments for the testing of labeling reaction conditions during [18F] radiosynthesis, a transfer V-vial (10 mL) was adapted between the QMA cartridge and microwave reaction V-vial (5 mL). By control of the three-way valve G, [18F] fluoride solution eluted from QMA cartridge can be transferred either directly to the microwave reaction V-vial for one experiment or delivered to the transfer V-vials for a further transferring. With a dilution of acetonitrile, it can be transferred to several different microwave reactors for several different experiments in the hot cell. Several factors may affect radiofluorination efficiency, including the drying process, reaction temperature and microwave power, and stability of the precursor, in addition to the experimental variables of leaving group and solvent being studied. To isolate the effect of the target properties, a standardized protocol of 18F isolation and drying was employed for all reactions, and the concentration was fixed at 37 µmol in 0.3 mL of solvent. Two polar non-protic solvents, DMF and DMSO, were tested as media for the fluorination reaction.
Microwave accelerators are capable of rapidly depositing high energy in a small volume. To optimize the microwave reaction conditions while avoiding decomposition of the precursor, pulse heating was applied during the drying process and the labeling reaction. The nucleophilic [18F]fluorination reaction is outlined in Scheme III. Three pulses of 20 sec, 150W, (maximum temperature setting was 145 °C) were applied, with a 30 sec pause between each pulse.
Scheme III.
[18F]Fluorine labeling reaction
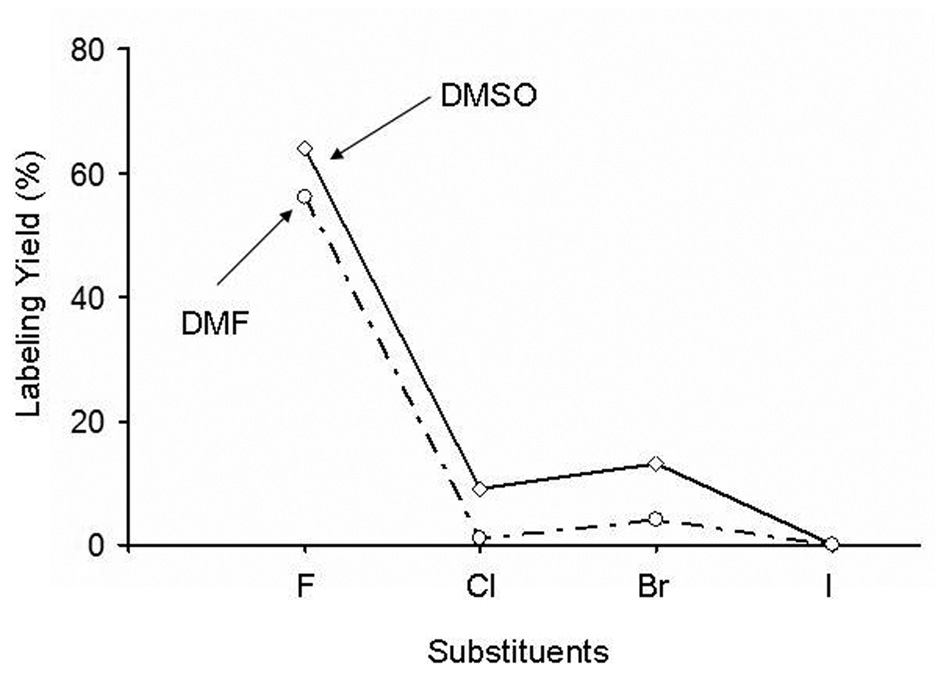
A comparison of labeling reactivity with different halogen substituents in both DMF and DMSO solvents is presented in Table 1. In DMF, the labeling yield of the fluoro-substituted compound [18F]1 increased with the number of the microwave pulses from 4% to 19% to 56% yield after the third pulse. For the bromo compound the labeling yield increased from 6% after one pulse to 10% after two, but decreased to 4% after the third pulse. Likewise, the chloro precursor gave 1%, 2%, and 1% after each pulse, whereas for the iodo precursor, no labeled product was seen even after three pulses. The reaction proceeded more readily in DMSO than in DMF. Highest 18F incorporation was observed to be 64% for fluoro, 13% for bromo and 9% for chloro precursor after three pulses in DMSO. No 18F product was obtained with the iodo precursor in either DMF or DMSO under any conditions.
Compared with DMF, DMSO both increased the labeling yield and stabilized the labeled product, especially for the chloro and bromo precursors (Figure 4). The greater efficacy of DMSO may be related to its higher dielectric constant/heating efficiency on microwave irradiation (dielectric quotient of 0.82 versus 0.16 for DMF) (Kappe, et al 2004). As shown in Figure 4, the order of halogen reactivity toward nucleophilic aromatic [18F]fluorination in both DMF and DMSO is F>>Br>Cl>>>I, which is consistent with the results from heteroaromatic [18F]substitution (Karramkam et al 2003) and favors fluorine as the leaving group, in contrast to aliphatic radiolabeling, in which reactivity is I>Br>Cl>>F.
Fig. 4.
Effect of leaving group on the microwave labeling yield on 3rd irradiation
A fast microwave-induced [18F] labeling could be achieved in less than 3 min and less than 15 min overall (starting at the delivery of aqueous [18F]fluoride). However, continued heating appeared to induce decomposition of the labeled product, as seen by extra peaks on the TLC scan. Thus, selection of the pulse scheme (time, power, temperature) is a key issue for the microwave [18F]fluorination of aromatic rings. The stability of the precursor is another important factor which must be considered.
Generally, radiolabeling by 18F exchange reaction using chloride or bromide as leaving groups requires activation by an ortho or para electron-withdrawing group. However, microwave-induced reactions facilitate F-18 labeling even for less reactive homoaromatic compounds. This opens new windows for obtaining F-18 labeled products, since chloro and bromo substituents can be easily introduced by conventional synthetic organic chemistry methods.
4. Conclusion
In summary, a new synthetic route has been successfully established for the synthesis of 3-methyl-halogenated (Cl, Br, I) benzonitrile derivatives using a two step reaction, halogenation and reductive deamination. A fast and efficient [18F]radiolabeling method involving a microwave-induced nucleophilic substitution of haloaromatic rings has been developed incorporating a semi-automatic system combining a commercial fluorination module with a single-mode microwave accelerator. The order of reactivity of halogens toward homoaromatic nucleophilic substitution is F>>Br>Cl>>>I in both DMF and DMSO, which favors fluorine as the leaving group in contrast to aliphatic substitution. The solvent also influences the radiolabeling activity, with DMSO being a favored choice, especially for less reactive substrates.
Fig. 3.
Radio-TLC of labeling product: 3-[18F]fluoro-5-methylbenzonitrile. TLC was developed in CHCl3
Acknowledgments
This research was supported by the National Institutes of Health (NCI R25T-CA092043) and Vanderbilt University, Department of Radiology and Radiological Sciences. The authors thank Jeff Clanton, Jarrod Driskill and the cyclotron crew for production and delivery of [18F]fluoride, and M. Sib Ansari for help with the apparatus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Labadi A, Zeller K-P, Machulla H-J. Synthesis of 6-[18F]fluoroveratraldehyde by nucleophilic halogen exchange at electron-rich precursors. J. Radioanal. Nuc. Chem. 2006;270(2):313–318. [Google Scholar]
- Arvela RK, Leadbeater NE. Fast and easy halide exchange in aryl halides. Synlett. 2003;8:1145–1148. [Google Scholar]
- Couturier O, Luxen A, Chatal J-F, Vuillez J-P, Rigo P, Hustinx R. Fluorinated tracers for imaging cancer with positron emission tomography. Eur. J. Nucl. Med. & Mol. Imaging. 2004;31(8):1182–1206. doi: 10.1007/s00259-004-1607-9. [DOI] [PubMed] [Google Scholar]
- de Vries EFJ, Waarde AV, Buursma AR, Vaalburg W. Synthesis and in vivo evaluation of 18F-desbromo-DuP-697 as a PET tracer for cyclooxygenase-2 expression. J. Nuc. Med. 2003;44(10):1700–1706. [PubMed] [Google Scholar]
- Ding Y-S. 18F-Labeled biomolecules for PET studies in the neurosciences. J. Fluorine Chem. 2000;101:291–295. [Google Scholar]
- Dischino DD, Dulac HA, Gillman KW, Keller LS, Kozlowski ES, Marcin LR, Mongillo JJ, Starrett JE., Jr Microwave-assisted synthesis and chiral HPLC separation of 18F-labeled MaxiPost™, an agent for post-stroke neuroprotection. J. Label. Compd. Radiopharm. 2003;46:1161–1171. [Google Scholar]
- Dolci L, Dolle F, Valette H, Vaufrey F, Fuseau C, Bottlaender M, Crouzel C. Synthesis of a fluorine-18 labeled derivative of epibatidine for in vivo nicotinic acetylcholine receptor PET imaging. Bioorg. Med. Chem. 1999;7:467–479. doi: 10.1016/s0968-0896(98)00261-2. [DOI] [PubMed] [Google Scholar]
- Elander N, Jones JR, Lu S-Y, Stone-Elander S. Microwave-enhanced radiochemistry. Chem. Soc. Rev. 2000;29:239–249. [Google Scholar]
- Fei X, Zheng Q-H, Wang J-Q, Stone KL, Martinez TD, Miller KD, Sledge GW, Hutchins GD. Synthesis, biodistribution and micro-PET imaging of radiolabeled antimitotic agent T138067 analogues. Bioorg. Med. Chem. Lett. 2004;14:1247–1251. doi: 10.1016/j.bmcl.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Fisher TH, Dershem SM, Prewitt ML. Meta-substituent effects on benzyl free-radical stability. J. Org. Chem. 1990;55:1040–1043. [Google Scholar]
- Getvoldsen G, Fredriksson A, Elander N, Stone-Elander S, Pharmacy K. Microwave-assisted cyclocondensation of 1,2-diaminobenzene with [4-18F]fluorobenzoic acid: microwave synthesis of 2-([4-18F]fluorophenyl) benzimidazole. J. Label. Compd. Radiopharm. 2004;47:139–145. [Google Scholar]
- Gore PH, Thorburn S, Weyell DJ. Friedel–Crafts reactions. Part XXV. Acetylation and benzoylation of iodobenzene and of o-, m-, and p-iodotoluenes. J. Chem. Soc., Perkin Trans.1. 1973;1:2940–2978. [Google Scholar]
- Guenther KJ, Yoganathan S, Garofalo R, Kawabata T, Strack T, Labiris R, Dolovich M, Chirakal R, Valliant JF. Synthesis and in vitro evaluation of 18F- and 19Flabeled insulin: A new radiotracer for PET-based molecular imaging studies. J. Med. Chem. 2006;49(4):1466–1474. doi: 10.1021/jm0509344. [DOI] [PubMed] [Google Scholar]
- Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford NDP, Roppe JR, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O’Malley SS, Hargreaves R, Burns HD. The synthesis, characterization and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- Herzog H. Methods and applications of positron-based medical imaging. Radiat. Phys. Chem. 2007;76:337–342. [Google Scholar]
- Imam SK. Molecular nuclear imaging: the radiopharmaceuticals. Cancer Biotherapy Radiopharm. 2005;20:163–172. doi: 10.1089/cbr.2005.20.163. [DOI] [PubMed] [Google Scholar]
- Jacobson O, Bechor Y, Icar A, Novak N, Birman A, Marom H, Fadeeva L, Golan E, Leibovitch I, Gutman M, Even-Sapir E, Chisin R, Gozin M, Mishani E. Prostate cancer PET bioprobes: synthesis of [18F]-radiolabeled hydroxyflutamide derivatives. Bioorg. Med. Chem. 2005;13:6195–6205. doi: 10.1016/j.bmc.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Jones B, Robinson J. The alkaline hydrolysis of substituted ethyl benzoates. The additive effects of substituents. J. Chem. Soc. 1955:3845–3850. [Google Scholar]
- Kappe CO. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004;43(46):6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- Karramkam M, Hinnen F, Berrehouma M, Hlavacek C, Vaufrey F, Halldin C, McCarron JA, Pike VW, Dollé F. Synthesis of a [6-pyridinyl-18F]-labelled fluoro derivative of WAY-100635 as a candidate radioligand for brain 5-HT1A receptor imaging with PET. Bioorg. Med. Chem. 2003;11:2769–2782. doi: 10.1016/s0968-0896(03)00225-6. [DOI] [PubMed] [Google Scholar]
- Klapars A, Buchwald SL. Copper-catalyzed halogen exchange in aryl halides: an aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002;124:14844–14845. doi: 10.1021/ja028865v. [DOI] [PubMed] [Google Scholar]
- Le HP, Müller CE. Rapid microwave-assisted fluorination yielding novel 5′-deoxy-5′-fluorouridine derivatives. Bioorg. Med. Chem. Lett. 2006;16:6139–6142. doi: 10.1016/j.bmcl.2006.08.093. [DOI] [PubMed] [Google Scholar]
- Maziere B, Crouzel C, Venet M, Stulzaft O, Sanz G, Ottaviani M, Sejourne C, Pascal O, Bisserbe JC. Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors. Nucl. Med. Biol. 1988;15(4):463–468. doi: 10.1016/0883-2897(88)90018-9. [DOI] [PubMed] [Google Scholar]
- Monclus M, Biver F, Goldman S, Luxen A. [18F]Altanserin: a short synthesis of the nitro precursor and preliminary metabolic studies in rat. J. Labelled Compounds Radiopharm. 1992;31:523–524. [Google Scholar]
- Murphy JM, Liao X, Hartwig JF. Meta halogenation of 1,3-disubstituted arenes via iridium-catalyzed arene borylation. J. Am. Chem. Soc. 2007;129:15434–15435. doi: 10.1021/ja076498n. [DOI] [PubMed] [Google Scholar]
- Patel S, Ndubizu O, Hamill T, Chaudhary A, Burns HD, Hargreaves R, Gibson RE. Screening cascade and development of potential positron emission tomography radiotracers for mGluR5: in vitro and in vivo characterization. Mol. Imaging Biol. 2005;7:314–323. doi: 10.1007/s11307-005-0005-4. [DOI] [PubMed] [Google Scholar]
- Roger G, Lagnel B, Rouden J, Besret L, Valette H, Demphel S, Gopisetti J, Coulon C, Ottaviani M, Wrenn LA, Letchworth SR, Bohme GA, Benavides J, Lasne M-C, Bottlaender M, Dollé F. Synthesis of a [2-pyridinyl-18F]-labelled fluoro derivative of (−)-cytisine as a candidate radioligand for brain nicotinic 4_2 receptor imaging with PET. Bioorg. Med. Chem. 2003;11:5333–5343. doi: 10.1016/j.bmc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Ryzhikov NN, Seneca N, Krasikova RN, Gomzina NA, Shchukin E, Fedorova OS, Vassiliev DA, Gulyás B, Hall H, Savic I, Halldin C. Preparation of high specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nuc. Med. Biol. 2005;32:109–116. doi: 10.1016/j.nucmedbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Stone-Elander S, Elander N. Microwave Application in Radiolabelling with Short-Lived Positron-Emmiting Radionuclides. J. Label. Compd. Radiopharm. 2002;45:715–746. [Google Scholar]
- Tan P, Baldwin RM, Soufer R, Garg PK, Charney DS, Innis RB. 12th Int. Symp. Radiopharm. Chem. Sweden: Uppsala; 1997. Jun, A complete remote control system for reliable routine production of [18F]altanserin; pp. 15–19. 1997. [Google Scholar]
- Willis PG, Pavlova OA, Chefer SI, Vaupel DB, Mukhin AG, Horti AG. Synthesis and Structure-Activity Relationship of a novel series of aminoalkylindoles with potential for imaging the neuronal cannabinoid receptor by positron emission tomography. J. Med. Chem. 2005;48:5813–5822. doi: 10.1021/jm0502743. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Dannals RF, Ravert HT, Sonders MS, Weber E, Wagner HN., Jr Radiosynthesis of σ receptor ligands for positron emission tomography: 11C- and 18F labeled guanidines. J. Med. Chem. 1991;34:1867–1870. doi: 10.1021/jm00110a017. [DOI] [PubMed] [Google Scholar]
- Wüst F, Hultsch C, Bergmann R, Johannsen B, Henle T. Radiolabelling of isopeptide Ne-(g-glutamyl)-l-lysine by conjugation with N-succinimidyl-4-[18F]fluorobenzoate. Appl. Radiat. Isot. 2003;59:43–48. doi: 10.1016/s0969-8043(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Yang SH, Li CS, Cheng CH. Halide Exchange Reactions between Aryl Halides and Alkali Halides Catalyzed by Nickel Metal. J. Org. Chem. 1987;52:691–694. [Google Scholar]
- Zhang Y, Pavlova OA, Chefer SI, Hall AW, Kurian V, Brown LL, Kimes A, Kimes S, Mukhin AG, Horti AG. 5-Substituted derivatives of 6-halogeno-3-((2-(S)-azetidinyl)methoxy)pyridine and 6-halogeno-3-((2-(S)-pyrrolidinyl)methoxy)pyridine with low picomolar affinity for α4β2 nicotinic acetylcholine receptor and wide range of lipophilicity: potential probes for imaging with positron emission tomography. J. Med. Chem. 2004;47:2453–2465. doi: 10.1021/jm030432v. [DOI] [PubMed] [Google Scholar]