Abstract
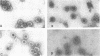
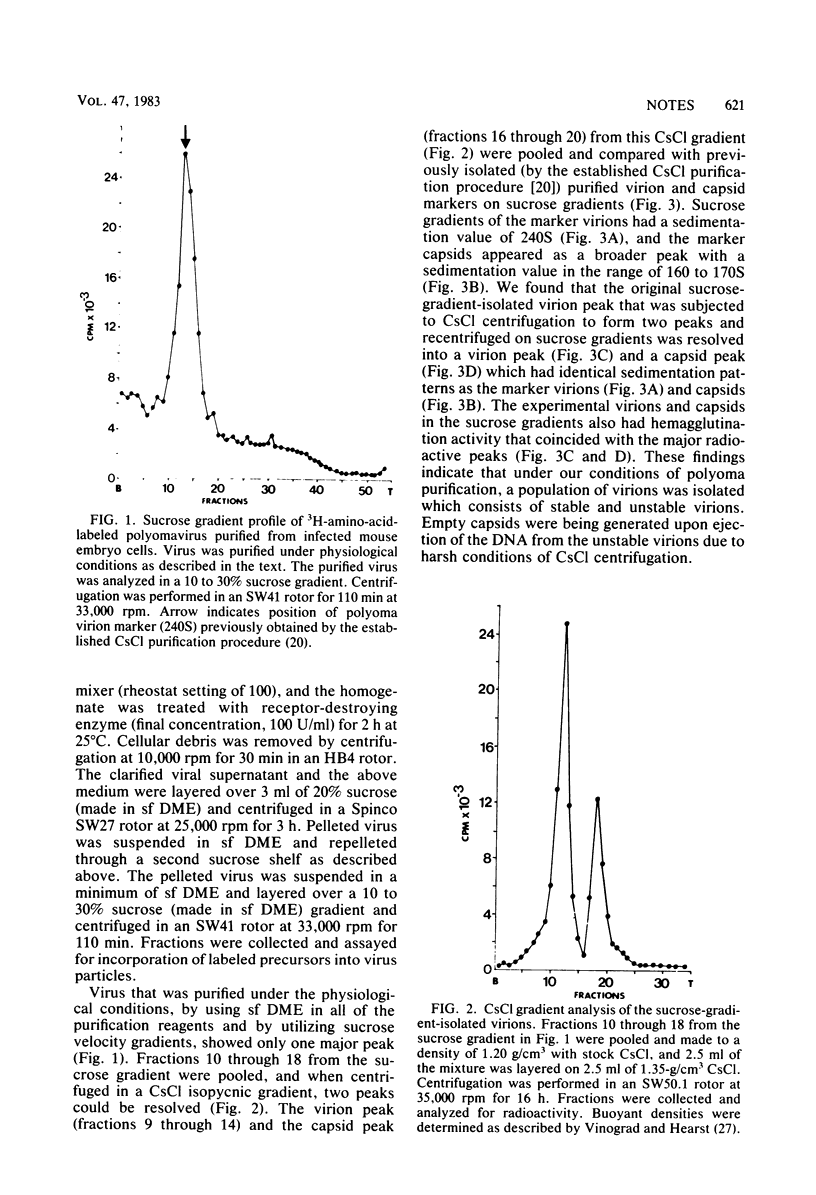
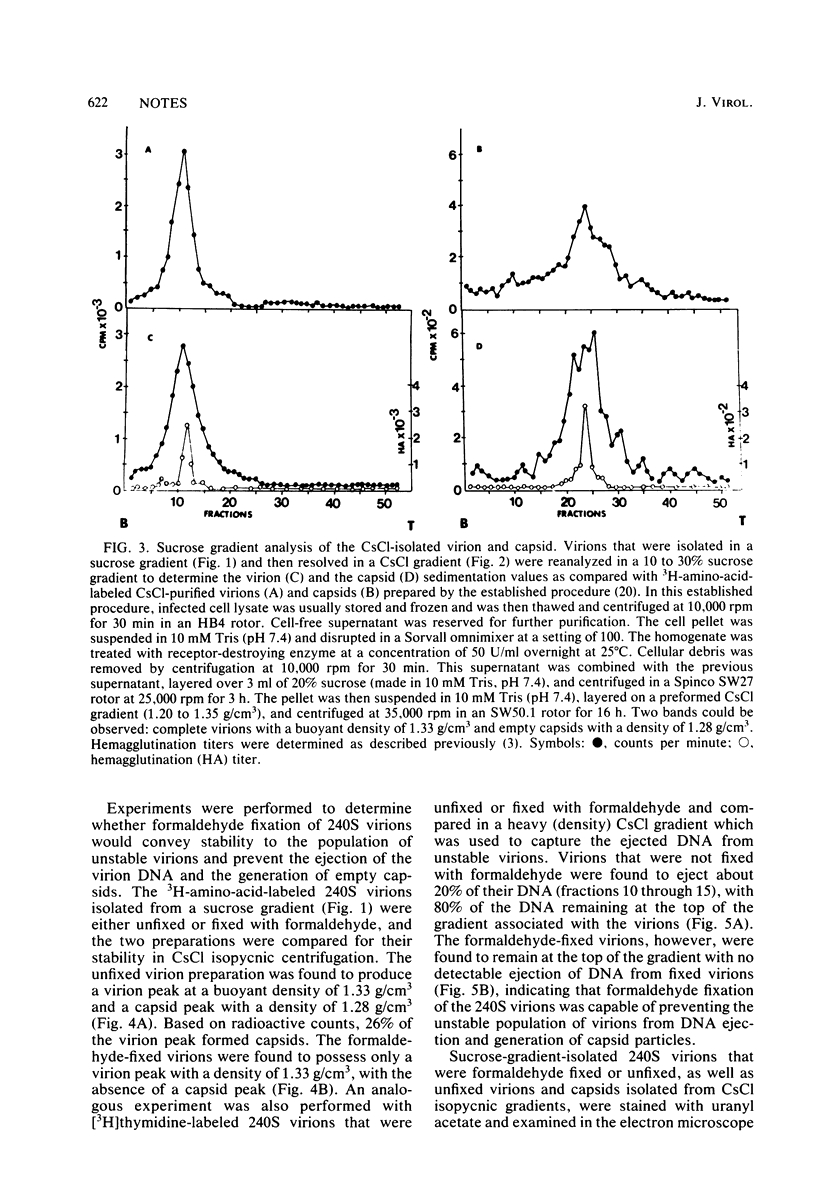
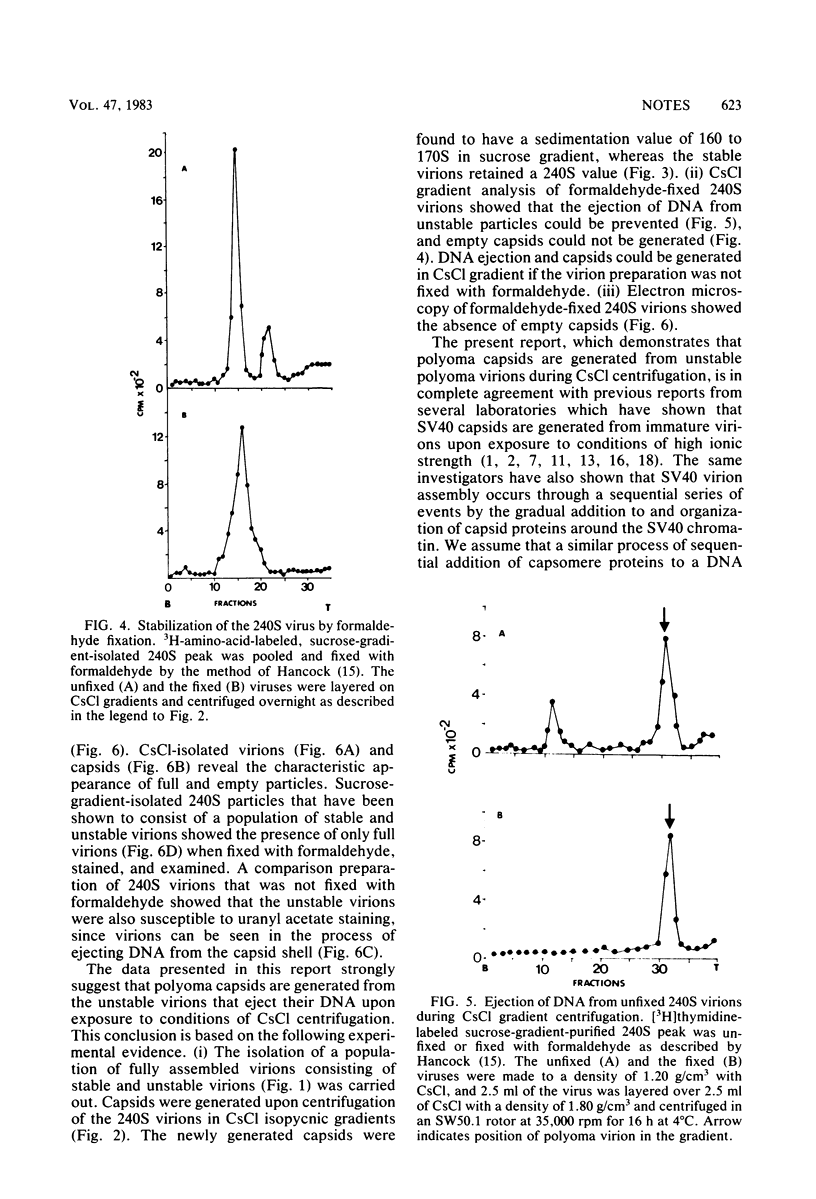
Polyomavirus was purified from infected mouse cell lysates under mild physiological conditions. When analyzed in a sucrose gradient, a major virus peak (240S) was identified. This sucrose-isolated virus could be divided into two populations based on its stability to CsCl gradient centrifugation. Members of the unstable population were shown to eject their DNA cores when subjected to CsCl gradient centrifugation, forming empty capsids, whereas the stable population was unaffected by the same CsCl treatment. Formaldehyde fixation of the 240S virus particles stabilized the virions and prevented ejection of DNA and generation of empty capsids. When formaldehyde-fixed 240S virus was examined with the electron microscope, only full virions were observed. These results indicate that polyoma capsids are not preformed in vivo, but instead are generated when infected cell lysates are subjected to harsh CsCl purification procedures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Nedospasov S. A. SV40-specific nucleoprotein complexes: heterogeneity and composition. Virology. 1981 Mar;109(2):244–256. doi: 10.1016/0042-6822(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Baumgartner I., Kuhn C., Fanning E. Identification and characterization of fast-sedimenting SV40 nucleoprotein complexes. Virology. 1979 Jul 15;96(1):54–63. doi: 10.1016/0042-6822(79)90172-7. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Separation of neutralizing and hemagglutination-inhibiting antibody activities and specificity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1980 Apr;34(1):119–129. doi: 10.1128/jvi.34.1.119-129.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Kendall J. D., Consigli R. A. In vitro reassembly of infectious polyoma virions. J Virol. 1979 Nov;32(2):640–647. doi: 10.1128/jvi.32.2.640-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M., WATSONDH The physical characteristics of polyoma virus. I. Two types of particle. Virology. 1962 Oct;18:170–176. doi: 10.1016/0042-6822(62)90002-8. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. II. Biochemical and electron microscopic analysis of simian virus 40 virion assembly. J Virol. 1979 Jul;31(1):199–208. doi: 10.1128/jvi.31.1.199-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. The effect of 5-fluoro-deoxyuridine on the replication of viral DNA and synthesis of polyoma capsid protein. J Gen Virol. 1968 May;2(3):437–441. doi: 10.1099/0022-1317-2-3-437. [DOI] [PubMed] [Google Scholar]
- Consigli R. A., Zabielski J., Weil R. Plaque assay for polyoma virus on primary mouse kidney cell cultures. Appl Microbiol. 1973 Oct;26(4):627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Baumgartner I. Role of fast-sedimenting SV40 nucleoprotein complexes in virus assembly. Virology. 1980 Apr 15;102(1):1–12. doi: 10.1016/0042-6822(80)90064-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. Intracellular SV40 nucleoprotein complexes: synthesis to encapsidation. Virology. 1980 Dec;107(2):389–401. doi: 10.1016/0042-6822(80)90306-2. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Aloni Y. Isolation and characterization of various forms of simian virus 40 DNA-protein complexes. Virology. 1980 Apr 15;102(1):107–118. doi: 10.1016/0042-6822(80)90074-4. [DOI] [PubMed] [Google Scholar]
- La Bella F., Romani M., Vesco C., Vidali G. High mobility group proteins 1 and 2 are present in simian virus 40 provirions, but not in virions. Nucleic Acids Res. 1981 Jan 10;9(1):121–131. doi: 10.1093/nar/9.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Vesco C. Late modifications of simian virus 40 chromatin during the lytic cycle occur in an immature form of virion. J Virol. 1980 Mar;33(3):1138–1150. doi: 10.1128/jvi.33.3.1138-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Vidali G., Vesco C. Histone acetylation in CV-1 cells infected with simian virus 40. Virology. 1979 Jul 30;96(2):564–575. doi: 10.1016/0042-6822(79)90112-0. [DOI] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milavetz B., Hopkins T. Simian virus 40 encapsidation: characterization of early intermediates. J Virol. 1982 Sep;43(3):830–839. doi: 10.1128/jvi.43.3.830-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancock R. Separation by equilibrium centrifugation in CsC1 gradients of density--labelled and normal deoxyribonucleoprotein from chromatin. J Mol Biol. 1970 Mar 14;48(2):357–360. doi: 10.1016/0022-2836(70)90167-1. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Bakayev V. V., Georgiev G. P. Chromosome of the mature virion of simian virus 40 contains H1 histone. Nucleic Acids Res. 1978 Aug;5(8):2847–2860. doi: 10.1093/nar/5.8.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M., Garber E., Levine A. J. Parameters affecting the stability of SV40 virions during the extraction of nucleoprotein complexes. Virology. 1979 May;95(1):256–259. doi: 10.1016/0042-6822(79)90427-6. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesco C., Fantuzzi L. F. Different histone families in intracellular SV40 nucleoprotein complexes with respect to the acetylation turnover. Virology. 1982 Apr 30;118(2):389–400. doi: 10.1016/0042-6822(82)90358-0. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Improved infectivity of reassembled polyoma virus. J Virol. 1982 Jul;43(1):337–341. doi: 10.1128/jvi.43.1.337-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]