Platensimycin (1) (Scheme 1) is a novel antibiotic lead compound recently discovered by Merck scientists from a strain of Streptomyces platensis.1a,b The potential medicinal applications1c,d and challenging structure motif, especially the cage-like tetracyclic core with several stereogenic centers, made this compound very attractive as a target for chemical synthesis. To this end, total synthesis of its racemic form was first reported by Nicolaou and co-workers,2a and later they also reported corresponding asymmetric versions.2b More recently, two other routes to the tetracyclic core structure (±)-92c,d and synthesis of a related structure2e have been reported. Whereas these reported routes all utilized intramolecular etherification reactions2a between the alcohol motifs and the alkene parts as key steps, an alternative intramolecular Robinson annulation approach seems to be more straightforward. Herein, we describe our efforts in the enantioselective synthesis of the key cage-like tetracyclic core structure of platensimycin.
Scheme 1.
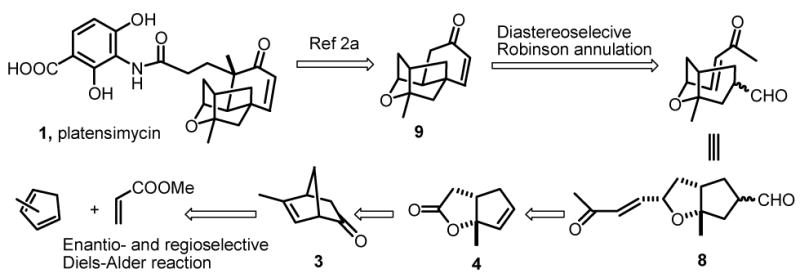
Retrosynthetic analysis.
The retrosynthetic analysis presented in Scheme 1 envisions a Robinson annulation event3 of bicyclic compound 8 to give the tetracyclic core structure 9.2a Specifically, we expect that by using proline-type catalysts, high diastereoselectivity will be obtained.4 Compound 8 could be constructed from bicyclic lactone 4 by adding two appendages in an appropriate manner. Lactone 4 has turned out to be a known compound, which was encountered in the total synthesis of a series of natural products in the hirsutene family.5 In contrast to the methods in the literature, we believe the potential precursor of lactone 4 could be ketone 3, through a Baeyer-Villiger oxidation/rearrangement sequence.6 And by utilizing our recently developed Brønsted acid-assisted chiral Lewis acid (BLA)7 catalyzed highly enantio- and regioselective Diels-Alder reaction,8 and subsequent N-nitroso aldol addition/decarboxylation sequence,9 enantiomerically pure ketone 3 could be easily prepared from inexpensive, commercially available starting materials.
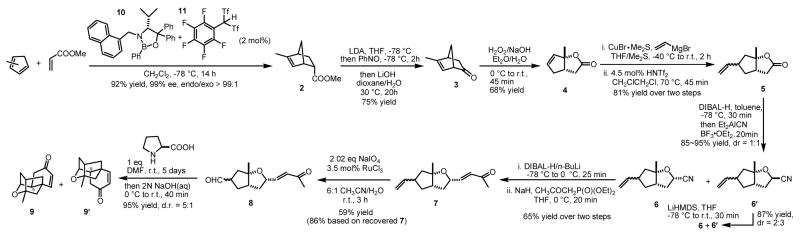
The implementation of the above mentioned approach is outlined in Scheme 2. BLA catalyst (2 mol%) prepared in situ from oxazaborolidine10 10 and carbon-based Brønsted acid11 11 promoted the Diels-Alder reaction between methyl acrylate and methyl cyclopentadiene to give adduct 2 in 92% yield with essentially complete regio-, diastereo- and enantiocontrol. The Diels-Alder adduct 2 was transformed to the desired ketone 3 in a one-pot procedure: nitroso aldol reaction of lithium enolate of 2 gave the N-nitroso adduct exclusively, which upon treatment with lithium hydroxide in dioxane/H2O underwent oxidative decarboxylation to give 3 in 75% yield after hydrolysis during work-up.12 Baeyer-Villiger oxidation of ketone 3 under basic hydrogen peroxide conditions13 gave lactone 4 in 68% yield, presumably through hydrolysis of the initially formed Baeyer-Villiger product followed by dehydrative lactonization.6
Scheme 2.
Synthetic route toward tetracyclic compound 9.
Addition of vinyl cuprate reagent14 to lactone 4 led to the corresponding carboxylic acid, which underwent an acid catalyzed lactonization with a catalytic amount of trifluoromethanesulfonimide15 to give vinyl lactone 5 as an inconsequential diastereomeric mixture (ca. 10:1 d.r.) in 81% yield over two steps.16 DIBAL-H reduction of 5 was followed by Lewis acid mediated cyanation in one pot, giving desired cyanide 6 and undesired cyanide 6′ as a separable diastereomeric mixture in 1:1 ratio with 85~95% yield. This one-pot sequence eliminated the need to go through corresponding acetate intermediates as is often seen in the literature.17 The undesired cyanide 6′ can be converted back to a 2:3 mixture of 6 and 6′ by deprotonation with LiHMDS followed by aqueous work-up. Cyanide 6 was reduced by DIBAL-H/n-BuLi to the corresponding aldehyde,18 which was immediately subjected to Wadsworth-Emmons conditions19 to give enone 7 in 65% yield over two steps. The protocol of ruthenium catalyzed oxidative cleavage of terminal olefins20 chemoselectively gave aldehyde 8 in 59% yield (86% based on recovered 7).
Gratifyingly, the key Robinson annulation event was accomplished in one pot by using L-proline as the chiral control element to mediate the initial intramolecular Michael addition, followed by sodium hydroxide treatment to finish the aldol dehydration. The tetracyclic core structure 9 and its C-9 epimer (platensimycin numbering1b) 9′ was obtained with 5:1 d.r. favoring the desired isomer. The observed preference for the enone’s si face being attacked (Figure 1) can be understood by the stereoelectronic reasons previously proposed.21 With L-proline as the matched chiral control element, such intrinsic preference is reinforced to give higher diastereoselectivity than the mismatched D-proline (3:1 d.r., favoring 9).22,23 The 1H and 13C NMR spectra of 9 are identical to those previously reported2a,c,d. Thus, our formal synthesis of platensimycin is finished.
Figure 1.
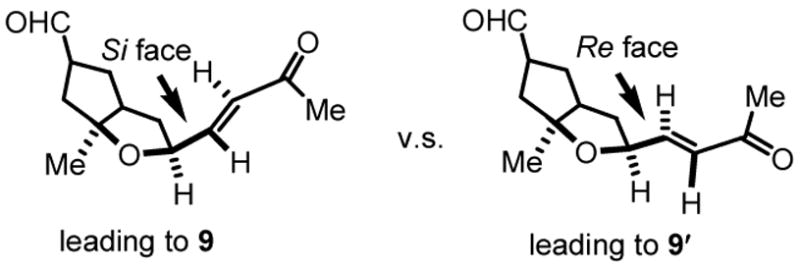
Facial selectivity for the intramolecular Michael addition.
In conclusion, an enantioselective route to the tetracyclic core structure of platensimycin is accomplished in ten steps from simple commercially available starting materials. A number of the steps in this synthesis are noteworthy or novel: (1) the regio- and enantioselective Diels-Alder reaction between methyl acrylate and methyl cyclopentadiene with only 2 mol% catalyst loading; (2) the one-pot conversion from ester 2 to ketone 3 using nitrosobenzene under mild conditions; (3) the one-pot reductive cyanation of lactone 4; (4) the stereoselective intramolecular Michael addition24 between the α-branched aldehyde moiety and the β-substituted enone part of bicyclic compound 8.
Supplementary Material
Experiment procedures and spectral data for compounds, complete author list of reference 1a. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This paper is dedicated to the memory of the late Professor Yoshihiko Ito. Partial financial support from the National Institute of Health (NIH, GM5-20121) is acknowledged. Special thanks to Ian Steele for X-ray analysis.
References
- 1.(a) Wang J, et al. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]; (b) Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. J Am Chem Soc. 2006;128:11916–11920. 15547. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]; (c) Pearson H. Nature. 2006;441:260–261. doi: 10.1038/441260a. [DOI] [PubMed] [Google Scholar]; (d) Häblich D, von Nussbaum F. ChemMedChem. 2006;1:951–954. doi: 10.1002/cmdc.200600145. [DOI] [PubMed] [Google Scholar]
- 2.(a) Nicolaou KC, Li A, Edmonds DJ. Angew Chem, Int Ed. 2006;45:7086–7090. doi: 10.1002/anie.200603892. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Li A, Edmonds DJ, Tria GS. Angew Chem, Int Ed. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]; (c) Zou Y, Chen C-H, Taylor CD, Foxman BM, Snider BB. Org Lett. 2007;9:1825–1828. doi: 10.1021/ol070563g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nicolaou KC, Tang Y, Wang J. Chem Commun. 2007:1922–1923. doi: 10.1039/b704589a. [DOI] [PubMed] [Google Scholar]; (e) Kaliappan KP, Ravikumar V. Org Lett. 2007;9:2417–2419. doi: 10.1021/ol070848t. [DOI] [PubMed] [Google Scholar]
- 3.For a classical example of Robinson annulation in total synthesis, see: Stork G, Shiner CS, Winkler JD. J Am Chem Soc. 1982;104:310–312.
- 4.(a) Fonseca MTH, List B. Angew Chem, Int Ed. 2004;43:3958–3960. doi: 10.1002/anie.200460578. [DOI] [PubMed] [Google Scholar]; (b) Hayashi Y, Gotoh H, Tamura T, Yamaguchi H, Masui R, Shoji M. J Am Chem Soc. 2005;127:16028–16029. doi: 10.1021/ja055740s. [DOI] [PubMed] [Google Scholar]; (c) List B. Chem Commun. 2006:819–824. doi: 10.1039/b514296m. [DOI] [PubMed] [Google Scholar]
- 5.(a) Curran DP, Rakiewicz DM. J Am Chem Soc. 1985;107:1448–1449. [Google Scholar]; (b) Curran DP, Rakiewicz DM. Tetrahedron. 1985;41:3843–3958. [Google Scholar]; (c) Fevig TL, Elliott RL, Curran DP. J Am Chem Soc. 1988;110:5064–5067. [Google Scholar]; (d) Weinges K, Reichert H. Synlett. 1991:785–786. [Google Scholar]; (e) Weinges K, Reichert H, Huber-Patz U, Irngartinger H. Liebigs Ann Chem. 1993:403–411. [Google Scholar]; (f) Weinges K, Braun R, Huber-Patz U, Irngartinger H. Liebigs Ann Chem. 1993:1133–1140. [Google Scholar]; (g) Weinges K, Braun R. Liebigs Ann Chem. 1994:99–101. [Google Scholar]
- 6.(a) Meinwald J, Seidel MC, Cadoff BC. J Am Chem Soc. 1958;80:6303–6305. [Google Scholar]; (b) Curran DP, Chen M. Tetrahedron Lett. 1985;26:4991–4994. [Google Scholar]; (c) Corey EJ, Weinshenker NM, Schaaf TM, Huber W. J Am Chem Soc. 1969;91:5675–5677. doi: 10.1021/ja01048a062. [DOI] [PubMed] [Google Scholar]; (d) Corey EJ, Schaff TK, Huber W, Koelliker V, Weinshenker NM. J Am Chem Soc. 1970;92:397–398. doi: 10.1021/ja00705a609. [DOI] [PubMed] [Google Scholar]; (e) Corey EJ, Cheng X. The Logic of Chemical Synthesis. John Wiley & Sons; New York: 1989. Chapter 11: Prostanoids [Google Scholar]
- 7.For a review of combined acid catalysis, see: Yamamoto H, Futatsugi K. Angew Chem, Int Ed. 2005;44:1924–1942. doi: 10.1002/anie.200460394.
- 8.Payette JN, Yamamoto H. J Am Chem Soc. 2007;129 doi: 10.1021/ja0735958. in press. For an excellent review of catalytic enantioselective Diels-Alder reactions, see: Corey EJ. Angew Chem, Int Ed. 2002;41:1650–1667. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b.
- 9.Payette JN, Yamamoto H. Manuscript in preparation.. For review of nitroso-aldol reactions, see: Yamamoto H, Momiyama N. Chem Commun. 2005:3514–3525. doi: 10.1039/b503212c.For synthetic applications of nitroso-aldol reactions to realize mild oxidation, see: Baran PS, Hafensteiner BD, Ambhaikar NB, Guerrero CA, Gallagher JD. J Am Chem Soc. 2006;128:8678–8693. doi: 10.1021/ja061660s.
- 10.Futatsugi K, Yamamoto H. Angew Chem, Int Ed. 2005;44:1484–1487. doi: 10.1002/anie.200461319. and references therein. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa A, Ishikawa T, Ishihara K, Yamamoto H. Bull Chem Soc Jpn. 2005;78:1401–1410. and references therein. [Google Scholar]
- 12.For a related oxidative decarboxylation, see: Corey EJ, Imai N, Pikul S. Tetrahedron Lett. 1991;32:7517–7520.
- 13.Weinshenker NM, Stephenson R. J Org Chem. 1972;37:3741. doi: 10.1021/jo00796a036. [DOI] [PubMed] [Google Scholar]
- 14.SN2′ type addition of organocopper reagents to compound 4 and related structures are well studied, see: Curran DP, Chen MH, Leszczweski D, Elliott LR, Rakiewicz DM. J Org Chem. 1986;51:1612–1614.
- 15.Catalytic amount of silver triflate and triflic acid can also mediate this transformation, albeit in slightly lower yields. See: Yang CG, Reich NW, Shi Z, He C. Org Lett. 2005;7:4553–4556. doi: 10.1021/ol051065f.Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org Lett. 2006;8:4179–4182. doi: 10.1021/ol061174+.
- 16.Under the cyclization conditions of compound 8, this stereocenter would be enolized to sp2 hybridized, so both epimers could give the desired products.
- 17.For review, see: Levy DE, Tang C. The Chemistry of C-Glycosides. Pergamon Press; Exeter: 1995. Chapter 2: Electrophilic Substitutions. For a recent example, see: Rychnovsky SD, Takaoka LR. Angew Chem, Int Ed. 2003;42:818–820. doi: 10.1002/anie.200390218.
- 18.Ritter T, Zarotti P, Carreira EM. Org Lett. 2004;6:4371–4374. doi: 10.1021/ol0480832. [DOI] [PubMed] [Google Scholar]
- 19.Peng X, Bondar D, Paquette LA. Tetrahedron. 2004;60:9589–9598. [Google Scholar]
- 20.Yang D, Zhang C. J Org Chem. 2001;66:4814–4818. doi: 10.1021/jo010122p. [DOI] [PubMed] [Google Scholar]
- 21.Corey EJ, Boaz NW. Tetrahedron Lett. 1985;26:6015–6018.Yamamoto Y, Nishii S, Ibuka T. J Chem Soc, Chem Commun. 1987:464–466. Hyperconjugation with the neighboring C-O or C-C σ bond would lead to the enone’s LUMO(π*) energy difference between the two shown conformers
- 22.For an insightful discussion on proline catalysis, see: Seebach D, Beck AK, Badine DM, Limbach M, Eschenmoser A, Treasurywala AM, Hobi R, Prikoszovich W, Linder B. Helv Chim Acta. 2007;90:425–470.
- 23.Extensive study of this Robinson annulation event is underway and will be reported in due course. For preliminary results, see Supporting Information.
- 24.Intermolecular reaction between such counterparts is still a remaining challenge in the area of organocatalysis, see: Melchiorre P, Jørgensen KA. J Org Chem. 2003;68:4151–4157. doi: 10.1021/jo026837p.Peelen TJ, Chi Y, Gellman SH. J Am Chem Soc. 2005;127:11598–11599. doi: 10.1021/ja0532584.Chi Y, Gellman SH. Org Lett. 2005;7:4253–4256. doi: 10.1021/ol0517729.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experiment procedures and spectral data for compounds, complete author list of reference 1a. This material is available free of charge via the Internet at http://pubs.acs.org.