Abstract
The BBB prevents the unrestricted exchange of substances between the central nervous system (CNS) and the blood. The blood-brain barrier (BBB) also conveys information between the CNS and the gastrointestinal (GI) tract through several mechanisms. Here, we review three of those mechanisms. First, the BBB selectively transports some peptides and regulatory proteins in the blood-to-brain or the brain-to-blood direction. The ability of GI hormones to affect functions of the BBB, as illustrated by the ability of insulin to alter the BBB transport of amino acids and drugs, represents a second mechanism. A third mechanism is the ability of GI hormones to affect the secretion by the BBB of substances that themselves affect feeding and appetite, such as nitric oxide and cytokines. By these and other mechanisms, the BBB-regulates communications between the CNS and GI tract.
Introduction
It has long been appreciated that the gastrointestinal (GI) tract and the central nervous system (CNS) interact. For example, Charles Darwin wrote in his classic The Expression of the Emotions in Man and Animals (1872): “The manner in which the secretions of the alimentary canal and of certain other organs….are affected by strong emotions, is another excellent instance of the direct action of the sensorium on these organs, independently of the will or of any serviceable associated habit.”
In contrast to the long-standing appreciation of the phenomenon, evidence for the mechanisms underlying gut-brain interactions have only begun to be elucidated in the last few decades. Claude Bernard noted that the brain and the heart interacted through the pneumo-gastric nerve “…so that”, as Darwin wrote, “under any excitement there will be much mutual action and reaction between these…” But for many of us, the modern era elucidating how the GI tract and CNS interacted can be traced to the work of Gibbs and Smith [1]. These authors clearly showed that the satiety effect of peripherally administered cholecystokinin was mediated through the vagus nerve. This work not only illustrated a physical connection facilitating gut-brain communication, but emphasized that this communication traveled in the gut-to-brain direction as well as in the brain-to-gut direction. This route has been extended to other substances, such as the cytokines, which can induce sickness behavior through vagal pathways and to other nerves, such as the palatine nerve, which can also relay cytokine-induced signals to the brain[2-4].
Mechanisms other than vagal afferents were slow to be elucidated. The other most obvious route, that of substances being secreted into the circulation by the brain or the brain reacting to substances secreted into the circulation by the peripheral tissues, was thought by most workers to be precluded because of the blood-brain barrier (BBB). The BBB consists of several parallel barriers, the most studied being the vascular barrier and the choroid plexus [5]. The vascular barrier is modified by the presence of intercellular tight junctions, a decrease in pinocytotic activity, and a near absence of intracellular fenestrea (Figure 1). In total, these modifications prevent the production of an ultrafiltrate, the mechanism by which serum proteins ordinarily leak into interstitial tissue beds [6]. Because the BBB is so effective at keeping large serum proteins such as albumin out of the brain, it was assumed by many that the BBB would also exclude peptides and regulatory proteins.
Figure 1.
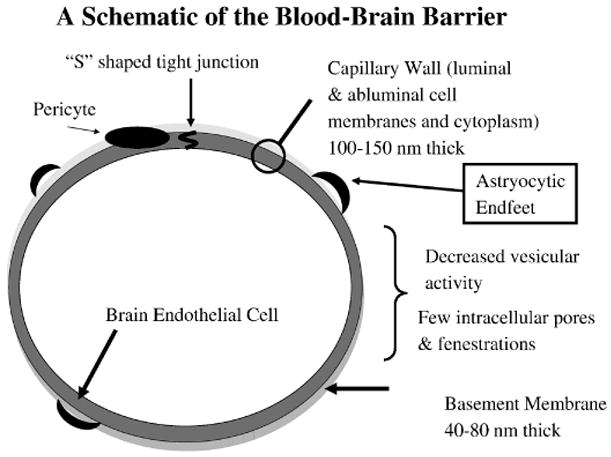
A schematic of the vascular blood-brain barrier. The physical barrier and transporters reside at the brain endothelial cell. However induction, maintenance, and modulation of barrier and transporter function is influenced by other cells types with and outside the central nervous system. Chief among these regulatory cells are the astrocytes and pericytes. The astrocytes project endfeet that form a mesh- or net-like structure surrounding the brain capillaries. Pericytes lie within the basement membrane next to the brain endothelial cells. Microglia within the brain and circulating immune cells, hormones and cytokines also likely influence BBB function.
Although direct transport across the BBB was prematurely ruled out, other mechanisms were added to the list of ways in which the CNS and GI tract can communicate. Substances can reach parts of the brain where the blood vessels do not form barriers, but are leaky as is typical of peripheral capillary beds. These circumventricular organs act as emetic centers and regulators of thirst[7;8]. Additionally, amylin exerts its anorectic effects [9] through one of the circumventricular organs, the area postrema [10]. Other GI peptide hormones, such as CCK ghrelin, and adiponectin, may also act in part through circumventricular organs. However, most work in this area agrees that a layer of cells forms a barrier between the circumventricular organs and the adjacent brain parenchyma, thus preventing substances entering the circumventricular organs from leaking into other brain regions. Circulating substances can also affect brain function indirectly by altering the blood level of a second substance which could cross the BBB. For example, insulin by lowering blood glucose levels and ACTH by elevating serum glucocorticoids can each affect a variety of CNS functions.
More recently, direct roles of the BBB in mediating communication between the CNS and GI tract have found renewed interest. Our laboratory has been very involved for over two decades in investigating the ability of peptides and regulatory proteins to cross the BBB in both the brain-to-blood and the blood-to-brain direction. The ability of brain endothelial cells themselves to directly respond to substances in the blood and in brain interstitial fluid is also an important mechanism in understanding potential gut-brain interactions. Finally, the brain endothelial cell is also now know to be capable of secreting substances, such as cytokines and nitric oxide, which have effects on appetite. It is these three direct roles of the BBB (Figure 2) in mediating brain-gut connections that the rest of this review will emphasize.
Figure 2.
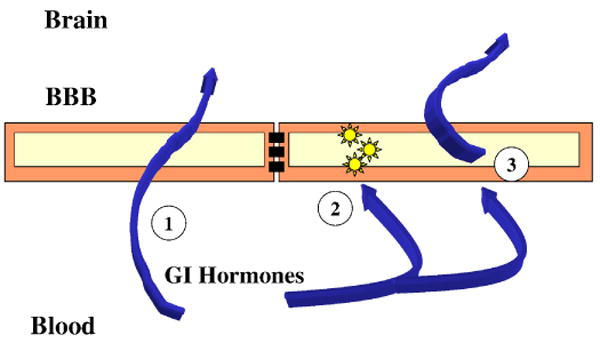
The three mechanism discussed here by which the blood-brain barrier (BBB) participates in gut-brain communication. Number 1 is by direct transfer of peptides and regulatory proteins across the BBB; number 2 is the ability of gastrointestinal (GI) hormones to alter the functioning of the brain endothelial cells which comprise the BBB; number 3 is the ability of GI hormones to alter the secretion from the BBB of substances which have effects on feeding.
Direct Permeability of the BBB to GI-related Peptides and Proteins
In 1979 Mutt et al showed that secretin when injected directly into the brain could induce pancreatic secretions at a fraction of its peripherally effective dose [11]. Thus, secretin joined the ranks of a growing number of substances originally identified in the periphery but with effects within the brain. Currently, this rank includes a long list of substances, including insulin, leptin, ghrelin, amylin, and pancreatic polypeptide. To exert their actions at receptors deep within the brain, these substances must cross the BBB. Many of these substances in this class, not surprising, have been shown to have saturable transporters located at the BBB, whereas some of the others can cross by non-saturable mechanisms. What these substances do within the brain once they have occupied their CNS receptors is not always clear. Some, such as leptin, exert what is currently thought to be their main actions within the CNS. Others, such as insulin, can have effects opposite to those that they exert in the periphery [12;13]. For example, peripheral administration of insulin will raise blood levels of insulin, reduce levels of glucose in the blood, and stimulate feeding, whereas administration of insulin into the brain has been reported by several laboratories to reduce blood levels of insulin, increase levels of glucose in the blood, and inhibit feeding. Therefore, substances like insulin which oppose through the CNS their peripheral actions may act as their own counterregulatory hormones [14].
Some substances produced in both the brain and the periphery are able to cross the BBB. Why a peptide or protein that is produced in the brain is also transported into the brain is not clear. It may be that the transported material accesses a different set of receptors than material endogenous to the CNS, reinforces only selected concentrations of endogenous material, or can form different concentration gradients in combination with endogenous material across a network of receptors.
Transport patterns across the BBB can provide hints at what are some of the actions of these substances within the CNS. For example, whereas most of the work on the CNS actions of leptin, ghrelin, and to a lesser extent insulin have concentrated almost exclusively on the hypothalamus, these substances have BBB transporters and CNS receptors located throughout the CNS [15-17]. Evidence shows that in each case these extra-hypothalamic brain receptors are operational and affect CNS functions [18-20]. Furthermore, each of these substances have BBB transporters and neuronal receptors located at the hypothalamus and can exert profound effects on cognitive functions. Therefore, it is likely that gastrointestinal hormones have multiple roles within the CNS.
Another example of BBB transport patterns providing insight into function is the finding that the majority of insulin entering the brain from the periphery does so at concentrations lower than those needed to induce hypoglycemia [21; 22]. Therefore, it is unlikely that CNS insulin acts primarily to inform the brain about potential hypoglycemia. Instead, it is likely that most of the information the brain gathers from the transport of insulin relates to physiological events. However, insulin transport is likely also to be involved in certain pathological reactions. Animals treated with lipopolysaccharide, a derivative of the cell walls of gram negative bacteria and a potent inducer of the innate immune system, increases insulin transport 2-3 fold [23]. To the extent that CNS insulin acts in a counterregulatory fashion to blood insulin, an increase in CNS insulin would induce resistance to the peripheral actions of insulin. Release of lipopolysaccharide by gram negative bacteria could increase transport of insulin across the BBB and so induce the insulin resistance seen in sepsis [24;25].
Leptin transport patterns provide several clues to the function, role, and evolution of leptin. Inhibition of leptin transport in obese animals is consistent with the development of leptin resistance at the level of the BBB [26]. The studies also show that the blood-to-brain signal of leptin to the brain is most robust at leptin levels below those considered to represent ideal body weight [27]. This, in turn, suggests that leptin evolved to be most effective at what in Western societies are low to low-normal levels. Interestingly, wild baboons living in normal, non-famine conditions also have low levels of leptin, suggesting that during most of evolution, lower levels of body fat and much lower levels of leptin were the norms [28]. We have found that part of the inhibition in leptin transport associated with obesity is caused by circulatory factors in addition to leptin itself and part of the inhibition is not explained by the immediate effects of circulatory substances [29]. Triglycerides inhibit leptin transport across the BBB and, since triglycerides tend to be high in obesity, are likely one cause of leptin resistance [30]. However, triglycerides are also high in starvation. Blockade of the anorectic leptin during starvation would convey survival value. Since animals and humans have more often been faced with starvation than obesity, it is likely that triglycerides came to represent to the CNS starvation, not obesity. In this sense, the leptin resistance of obesity as caused by hypertriglyceridemia may be a metabolic case of mistaken identity because of an unfortunate coincidence that hypertriglyceridemia occurs in both obesity and starvation.
Effects of BBB Functions
The brain endothelial cells which comprise the BBB have true receptors; that is, they have binding sites coupled to intracellular machinery that modulates their functions. Thus, brain endothelial cells not only transport insulin, they also are affected by it.
Insulin is a particularly well studied example of how a GI hormone can influence the BBB. Insulin alters the transport rate across the BBB of a number of substances, including the amino acid tryptophan [31] (and so brain levels of serotonin), the anti-HIV drug AZT [32], and leptin [33]. Insulin also inhibits alkaline phosphatase activity by the brain endothelial cell [34].
Effects on BBB Secretions
Brain endothelial cells secrete a number of substances which can affect CNS functions, including feeding behaviors, such as nitric oxide and cytokines [35-37]. Some cytokines, such as interleukin-6, are constitutively secreted, whereas others are secreted in response to stimuli. Lipopolysaccharide and the human immunodeficiency virus are examples of stimuli that increase the endothelial cell secretion of cytokines [38;39]. Additionally, cytokine secretions by the BBB are polarized; that is a cytokine can be secreted either from the brain side or the blood side of the capillary wall [40]. A more complex situation is when an endothelial cell receives stimuli from one side and secretes cytokine into the other; for example, lipopolysaccharide applied to the abluminal surface of a brain endothelial cell can induce the release of interleukin-6 from the luminal surface. As no brain cell is more than 40-50 microns distant from a brain endothelial cell, secretion of cytokines or other substances affecting appetite could be a major mechanism by which peripheral signals affect brain function.
Release of cytokines from brain endothelial cells can also be modulated by GI peptides. Adiponectin is an adipokine that is regulated by interleukin-6 and has several effects on vasculature [41-44]. It does not cross the BBB well, yet some of its effects are mediated through the CNS [45]. One way that it may do this is by its ability to inhibit the release of interleukin-6 from brain endothelial cells [46]. However, inhibition of interleukin-6 would be expected to have the opposite effects on body weight, as interleukin-6 is usually associated with decreased appetite and loss of body weight, which is the opposite effect ascribed to adiponectin. It may be that inhibition of interleukin-6 is a mechanism by which adiponectin courterregulates its main actions, much as insulin in the CNS may counteract its actions in the periphery.
Conclusion
The BBB and the cells which form it are vitally active in communications between the CNS and GI tract. By first forming a barrier, the BBB is able to restrict and control the transfer of informational molecules between the blood and the interstitial fluid of the brain. Besides having transport systems for some peptides and regulatory proteins, the BBB is also able to secrete substances that themselves have effects on feeding behavior. Additionally, GI hormones can modulate the functions of brain endothelial cells, including modulating their ability to secret behaviorally active substances.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Smith GP, Gibbs J, Jerome C, Pi-Sunyer FX, Kissileff HR, Thornton J. The satiety effect of cholecystokinin: a progress report. Peptides. 1981;2 2:57–59. doi: 10.1016/0196-9781(81)90011-5. [DOI] [PubMed] [Google Scholar]
- 2.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2003;17:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 3.Romeo HE, Tio DL, Rahman SU, Chiappelli F, Taylor AN. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication: relevance to neuroimmune surveillance of the oral cavity. J Neuroimmunol. 2001;115:91–100. doi: 10.1016/s0165-5728(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 4.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 5.Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barriers. Boca Raton: CRC Press; 1996. [Google Scholar]
- 6.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross PM, Weindl A. Peering through the windows of the brain. J Cereb Blood Flow Metab. 1987;7:663–672. doi: 10.1038/jcbfm.1987.120. [DOI] [PubMed] [Google Scholar]
- 8.Johnson AK. The neuroendocrinology of thirst and salt appetite - visceral sensory signals and mechanisms of central integration. Frontiers in Neuroendocrinology. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 9.Morley JE, Morley PMK, Flood JF. Anorectic effects of amylin in rats over the life span. Pharmacol Biochem Behav. 1993;44:577–580. doi: 10.1016/0091-3057(93)90169-t. [DOI] [PubMed] [Google Scholar]
- 10.Riediger T, Schmid HA, Lutz T, Simon E. Amylin potently activates AP neurons possibly via formation of the excitatory second messanger cGMP. Am J Physiol. 2001;281:R1833–1843. doi: 10.1152/ajpregu.2001.281.6.R1833. [DOI] [PubMed] [Google Scholar]
- 11.Mutt V, Carlquist M, Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979;25:1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- 12.Florant GL, Singer L, Scheurink AJW, Park CR, Richardson RD, Woods SC. Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiol Behav. 1991;49:335–338. doi: 10.1016/0031-9384(91)90053-q. [DOI] [PubMed] [Google Scholar]
- 13.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Current Pharmaceutical Design. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA, Kastin AJ. Physiological consequences of the passage of peptides across the blood-brain barrier. Rev Neurosci. 1993;4:365–372. doi: 10.1515/revneuro.1993.4.4.365. [DOI] [PubMed] [Google Scholar]
- 15.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 16.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 18.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 19.Diano S, Farr SA, Benoit SE, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger JB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 20.Gupta G, Azam M, Baquer NZ. Modulation of rat brain insulin receptor kinase activity in diabetes. Neurochem Int. 1992;20:487–492. doi: 10.1016/0197-0186(92)90027-o. [DOI] [PubMed] [Google Scholar]
- 21.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific enzyme immunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 22.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: Saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 23.Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- 24.Dahn MS, Lange MP, Mitchell RA, Lobdell K, Wilson RF. Insulin production following injury and sepsis. Journal of Trauma-Injury Infection & Critical Care. 1987;27:1031–1038. doi: 10.1097/00005373-198709000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Vanhorebeck I, Langouche L, Van den Berghe G. Glycemic and nonglycemic effects of insulin: how do they contribute to a better outcome of critical illness? Curr Opin Crit Care. 2005;11:304–344. doi: 10.1097/01.ccx.0000170506.61281.94. [DOI] [PubMed] [Google Scholar]
- 26.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 27.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood to brain transport of leptin in normal weight mice. Am J Physiol. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 28.Banks WA, Phillips-Conroy JE, Jolly CJ, Morley JE. Serum leptin levels in wild and captive populations of baboons (Papio): Implications for the ancestral role of leptin. J Clin Endocrinol Metab. 2001;86:4315–4320. doi: 10.1210/jcem.86.9.7874. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Current Pharmaceutical Design. 2003;9:801–809. doi: 10.2174/1381612033455350. [DOI] [PubMed] [Google Scholar]
- 30.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 31.Cangiano C, Cardelli-Cangiano P, Cascino A, Patrizi MA, Barberini F, Rossi F, Capocaccia L, Strom R. On the stimulation by insulin of tryptophan transport across the blood-brain barrier. Biochemistry International. 1983;7:617–627. [PubMed] [Google Scholar]
- 32.Ayre SG, Skaletski B, Mosnaim AD. Blood-brain barrier passage of azidothymidine in rats: effect of insulin. Res Comm Chem Path Pharmacol. 1989;63:45–52. [PubMed] [Google Scholar]
- 33.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–242. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- 34.Catalan RE, Martinez AM, Aragones MD, Miguel BG, Robles A. Insulin action on brain microvessels; effect on alkaline phosphatase. Biochem Biophys Res Commun. 1988;150:583–590. doi: 10.1016/0006-291x(88)90433-0. [DOI] [PubMed] [Google Scholar]
- 35.Mandi Y, Ocsovszki I, Szabo D, Nagy Z, Nelson J, Molnar J. Nitric oxide production and MDR expression by human brain endothelial cells. Anticancer Research. 1998;18:3049–3052. [PubMed] [Google Scholar]
- 36.Kis B, Kaiya H, Nishi R, Deli MA, Abraham CS, Yanagita T, Isse T, Gotoh S, Kobayashi H, Wada A, Niwa M, Kangawa K, Greenwood J, Yamashita H, Ueta Y. Cerebral endothelial cells are a major source of adrenomedullin. J Neuroendocrinology. 2002;14:283–293. doi: 10.1046/j.1365-2826.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- 37.Vadeboncoeur N, Segura M, Al-Numani D, Vanier G, Gottschalk M. Proinflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunology and Medical Microbiology. 2003;35:49–58. doi: 10.1111/j.1574-695X.2003.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 38.Didier N, Banks WA, Creminon C, Dereuddre-Bosquet N, Mabondzo A. Endothelin-1 production at the in-vitro blood-brain barrier during HIV infection. Neuroreport. 2002;13:1179–1183. doi: 10.1097/00001756-200207020-00022. [DOI] [PubMed] [Google Scholar]
- 39.Reyes TM, Fabry Z, Coe CL. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999;851:215–220. doi: 10.1016/s0006-8993(99)02189-7. [DOI] [PubMed] [Google Scholar]
- 40.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain, Behavior, and Immunity. 2006 doi: 10.1016/j.bbi.2005.10.005. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- 41.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. Journal of Clinical Endocrinology and Metabolism. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 43.Hattori Y, Suzuki M, Hattori S, Kasai K. Gobular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 44.Ouchi N, Nihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, and adipocytes-derived plasma protein, inhibits endothelial NF-kappaβ signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 45.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nature Medicine. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 46.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA. Adiponectin does not cross the blood-brain barrier, but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]


