Abstract
Developing sensory systems are sculpted by an activity-dependent strengthening and weakening of connections. Long-term potentiation (LTP) and depression (LTD) in vitro have been proposed to model this experience-dependent circuit refinement. We directly compared LTP and LTD induction in vitro with plasticityin vivo in the developing visual cortex of a mouse mutant of protein kinase A (PKA), a key enzyme implicated in the plasticity of a diverse array of systems.
In mice lacking the RIβ regulatory subunit of PKA, we observed three abnormalities of synaptic plasticity in layer II/III of visual cortexin vitro. These included an absence of (1) extracellularly recorded LTP, (2) depotentiation or LTD, and (3) paired-pulse facilitation. Potentiation was induced, however, by pairing low-frequency stimulation with direct depolarization of individual mutant pyramidal cells. Together these findings suggest that the LTP defect in slices lacking PKA RIβ lies in the transmission of sufficient net excitation through the cortical circuit.
Nonetheless, functional development and plasticity of visual cortical responses in vivo after monocular deprivation did not differ from normal. Moreover, the loss of all responsiveness to stimulation of the originally deprived eye in most cortical cells could be restored by reverse suture of eyelids during the critical period in both wild-type and mutant mice. Such an activity-dependent increase in response would seem to require a mechanism like potentiation in vivo. Thus, the RIβ isoform of PKA is not essential for ocular dominance plasticity, which can proceed despite defects in several common in vitro models of neural plasticity.
Keywords: visual cortex, plasticity, development, PKA, LTP, LTD, PPF
Manipulations of visual experience during a critical period in early life perturb the functional organization of connections in mammalian visual cortex through a competitive interaction between inputs serving the two eyes and the responses of their target cortical cells (Wiesel and Hubel, 1963;Movshon and Kiorpes, 1990; Shatz, 1990; Hata and Stryker, 1994). The cellular and molecular basis for this plasticity, however, remains largely unknown. Studies of learning and memory in mature animals provide several promising candidate factors that may contribute to developmental plasticity in vivo (Kandel and O’Dell, 1992). Most notably, the cAMP second messenger system has been implicated in such diverse systems as transient synaptic facilitation (Ghirardi et al., 1992; Byrne et al., 1993) and persistent structural changes inAplysia (Glanzman et al., 1990; Schacher et al., 1993; F. Wu et al., 1995), synaptogenesis in the pond snail Helisoma (Funte and Haydon, 1993), olfactory associative learning in fruit flies (Davis, 1993; DeZazzo and Tully, 1995), synaptic LTP/LTD (Huang and Kandel, 1994; Huang et al., 1994; Weisskopf et al., 1994; Brandon et al., 1995; Qi et al., 1996), and hippocampal learning behavior in vertebrates (Bourtchouladze et al., 1994; Z-L Wu et al., 1995; Abel et al., 1997; Bernabeu et al., 1997). cAMP-dependent protein kinase (PKA) can rapidly modulate synaptic efficacy by phosphorylating ion channels and receptors (Blackstone et al., 1994; Johnson et al., 1994; Colwell and Levine, 1995) and initiate protein synthesis-dependent growth processes by translocating to the nucleus (Spaulding, 1993).
To investigate a possible role for PKA in ocular dominance plasticity, we turned to a new class of tools provided by recent techniques for manipulating the mouse genome (Grant and Silva, 1994; Mayford et al., 1995). Rodent models of the plasticity of binocular responses replicate the essential aspects found in other animals: within a clear critical period during which a brief, 4-d deprivation has a saturating effect, visual experience modulates cortical responses through a correlation-based competition between inputs from the two eyes (Draeger, 1978; Fagiolini et al., 1994; Gordon and Stryker, 1996). Here, we analyzed visual cortical plasticity in the binocular zone of primary visual cortex (V1) of mice carrying a targeted gene disruption of the RIβ regulatory subunit of PKA (Brandon et al., 1995). Inactivation of the neuronal RIβ subunit gene yields mice whose total PKA catalytic activity is unimpaired, apparently because of a compensatory upregulation of the RIα subunit (Amieux et al., 1997). Nevertheless, these mice show highly selective impairment in the ability to depress synaptic transmission in the dentate gyrus and CA1 region of hippocampus (Brandon et al., 1995), and they lack a presynaptic form of LTP in the CA3 region (Huang et al., 1995), suggesting an important role for the RIβ isoform in these functionsin vitro. We therefore directly compared experience-dependent plasticity in the intact visual cortex with simple assays of LTP and LTD in neocortical slices commonly thought to reflect the mechanisms of plasticity in vivo (Tsumoto, 1992;Kirkwood et al., 1995, 1996; Singer, 1995; Katz and Shatz, 1996).
MATERIALS AND METHODS
In vitro recordings and analysis. Mice carrying a targeted disruption of the PKA RIβ gene were generated as described previously (Brandon et al., 1995). Coronal slices (400 μm) through the binocular zone of the primary visual cortex (V1) were prepared blind to genotype from animals at the peak of the critical period for monocular deprivation effects [postnatal day (P) 24–33] and maintained at 27–29°C in oxygenated (95%O2/5%CO2) artificial CSF containing (in mm): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 1.0 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, 11 glucose. Extracellular field potentials were recorded with a 1m NaCl (1–3 MΩ) electrode inserted into layer II/III, and stable baseline responses were evoked by stimulation at 0.1 Hz in layer IV or in the white matter with a glass bipolar stimulating electrode (Hensch and Stryker, 1996). To induce LTP, five episodes of theta-burst stimulation (TBS) were applied at 10 sec intervals (Kirkwood and Bear, 1994a). Each TBS consisted of four pulses at 100 Hz repeated 10 times at 5 Hz. We attempted to induce LTD and depotentiation using low-frequency stimulation (900 pulses at 1 Hz) (Dudek and Bear, 1993; Kirkwood and Bear, 1994b). At the end of each extracellular field potential experiment, the non-NMDA and NMDA glutamate receptor antagonists CNQX (Tocris) and D-APV (Sigma, St. Louis, MO) were both applied in the bath to confirm the synaptic nature of the extracellular response. Measurements of the maximum negative field potential amplitude were normalized to the baseline period before theta-burst or low-frequency stimulation and were plotted against the running time of the experiment.
Individual layer II/III cortical or hippocampal CA1 pyramidal cells were recorded with patch electrodes (5–8 MΩ) in the whole-cell voltage-clamp mode (−70mV holding potential, Axoclamp-2B), either using the “blind” technique or under direct visualization with infrared Nomarski DIC optics (Stern et al., 1992). The pipette solution contained (in mm): 122.5 cesium or potassium gluconate, 17.5 cesium or potassium chloride, 10 HEPES buffer, 0.2 EGTA, 8 NaCl, 2.0 Mg-ATP, 0.3 Na3-GTP, and 0.15% biocytin, pH 7.2 (290–300 mOsm). LTP was induced within 10 min of obtaining whole-cell access by pairing membrane potential depolarization to 0 mV with 100 synaptic stimuli at 1 Hz (Gustafsson et al., 1987; Kirkwood and Bear, 1994a; Yoshimura and Tsumoto, 1994) and then monitored at a baseline holding potential of −70 mV and stimulation at 0.1 Hz. Measurements of EPSC slope were normalized to the baseline period before pairing, and whole-cell input and series resistances were monitored for stability throughout the experiment. EPSC peak amplitudes were used to determine paired-pulse facilitation, expressed as a ratio of the second response size to the first.
In vivo recordings and analysis. Electrophysiological procedures have been described in detail elsewhere (Gordon and Stryker, 1996). In brief, mice were anesthetized with 50 mg/kg Nembutal (Abbott Labs, Irving, TX) and chlorprothixene (0.2 mg, Sigma) and placed in a stereotaxic holder. The animals breathed a mixture of oxygen and room air through a trachea tube, and additional anesthetic doses (0.15–0.25 mg) were administered to maintain a heart rate of 6–9 Hz. A 5 × 5 mm portion of the skull was removed, exposing the visual cortex, and the intact dura was covered with agarose (2.8% in saline). The corneas were protected with silicone oil, and optic disk locations were projected onto a tangent screen to determine the vertical meridian. Optic disk locations varied only slightly across animals (mean ± SD; elevation = 33.6 ± 5.7; azimuth = 65.0 ± 6.0).
Resin-coated tungsten microelectrodes (2–4 MΩ) were used to record single units from primary (V1) visual cortex, as verified by electrode track reconstructions and histological criteria. Data were obtained from the binocular zone, the region of V1 representing the central 25° of the upper portion of each visual hemifield. Receptive fields of isolated single units were plotted on a screen placed 30 cm from the animal, using a hand-held projection lamp. Cells were assigned ocular dominance scores according to the 7-point classification scheme ofHubel and Wiesel (1962): a score of 1 indicates response to contralateral eye stimulation exclusively, and a score of 7 indicates purely ipsilateral eye response. Intermediate scores (2–6) reflect the degree of binocular responsiveness. A weighted average of the bias toward one eye or the other, the contralateral bias index (CBI), was calculated for each hemisphere according to the formula: CBI = [(n1 − n7) + (2/3)(n2 − n6) + (1/3)(n3 − n5) +N]/2N, where N = total number of cells, and nx = number of cells with ocular dominance scores equal to x.
For monocular deprivation experiments, one eyelid margin was trimmed while the mice were under halothane anesthesia, and the lids were sutured shut at P25–27 for 4 d near the peak of the critical period. All recordings were made from the binocular zone of V1 contralateral to the deprived eye, blind to the genotype of the animal. Some recordings were also made blind to deprivation status. For reverse suture experiments, initial and control deprivations were performed without trimming the eyelids (for 5 d beginning P20–22), to facilitate reopening (for 4–8 d). Recordings were made from both hemispheres blind to the order in which eyes were deprived. At the end of each experiment, an overdose of Nembutal was given, and the animal was perfused.
Histological analysis. For Nissl staining, mice were transcardially perfused with 0.5 m PBS followed by 10% formalin in PBS. After post-fixation in formalin, the brain was removed, cryoprotected in 30% sucrose–10% formalin, and cut into 40 μm sections on a freezing microtome. Sections were mounted on slides, defatted, and stained with cresyl echt violet (Schmid).
For single-cell reconstructions after whole-cell patch-clamp recordings, slices were fixed in 4% paraformaldehyde for at least 24 hr before cryoprotection and resectioning at 50 μm on the freezing microtome. Sections were processed according to standard avidin–biotin complex (ABC) techniques (Vector Laboratories, Burlingame, CA), and biocytin label was visualized by the nickel-intensified diaminobenzidine (Ni-DAB) reaction (Horikawa and Armstrong, 1988).
RESULTS
Visual cortical morphology and responses in the absence of PKA RIβ
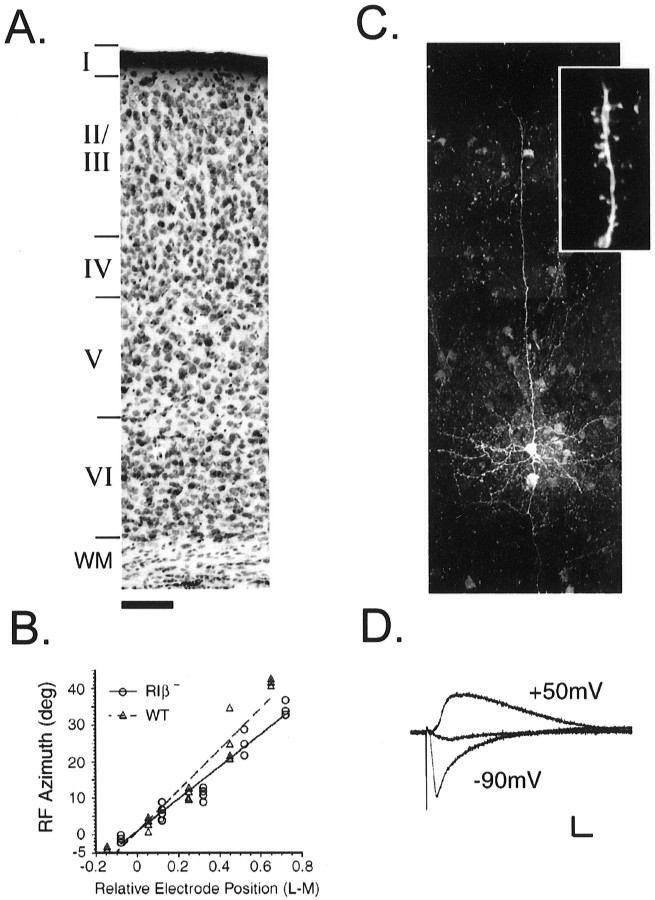
A characteristic six-layered binocular region of primary visual cortex (V1) was observed in PKA RIβ-deficient (RIβ−) mice with pyramidal cells in the supragranular layers bearing many postsynaptic spines (Fig.1A,C). Stimulation of the underlying layer IV evoked NMDA receptor-gated (NMDA-R) and non-NMDA-R-mediated synaptic currents in these cells, which could be blocked by 50 μm D-APV and 10 μm CNQX, respectively (Fig. 1D) (Stern et al., 1992). The influx of calcium through NMDA-R channels on dendritic spines is essential for the induction of conventional LTP and LTD in both the hippocampus and neocortex (Tsumoto, 1992; Singer, 1995). Thus, the visual cortex of RIβ− mice apparently expressed synaptic structures required for such plasticity in vitro.
Fig. 1.
Characteristic morphology and synaptic responses in the visual cortex of PKA RIβ− mutant mice.A, Six distinct laminae are identifiable in Nissl-stained coronal sections through the binocular zone of primary visual cortex taken from animals at the peak of the critical period. Scale bar, 100 μm. B, Visual responses are retinotopically organized in RIβ− cortex. A series of evenly spaced microelectrode penetrations were made across a portion of the lateromedial extent of V1 in each animal. Receptive field (RF)-center azimuths are plotted versus electrode position relative to the vertical meridian (n = 3–7 cortical cell RFs per penetration). The correlation coefficients for three RIβ− and four WT regressions were 0.92 ± 0.04 and 0.91 ± 0.03, respectively. C, Neurons filled with biocytin in the supragranular layers exhibit pyramidal morphology with a long apical dendrite extending to the pial surface and profuse basal processes. Numerous postsynaptic spines are readily visible (inset). Scale bar (shown in A): 45 μm; inset, 6 μm. D, Synaptic responses to underlying layer IV stimulation consist of fast non-NMDA-R and slower NMDA-R-mediated components in supragranular pyramidal cells. Whole-cell voltage-clamp recordings were first made at −90mV, and then fast non-NMDA and GABAA receptors were blocked using CNQX and bicuculline methiodide (10 μm each) to reveal slow NMDA-R currents when membrane potential was set to +50 mV. Finally, NMDA-Rs were blocked by 50 μm D-APV at +50 mV (middle trace). Calibration: 50 pA, 10 msec.
Extracellular recordings of single-unit responses in vivowere obtained blind to genotype from V1 of two RIβ− and two wild-type (WT) adult mice. A comparison of receptive field size, retinotopy, orientation selectivity, ocular dominance, and response strength revealed neuronal response properties in WT and RIβ− V1 to be indistinguishable. Receptive field size distributions in RIβ− and WT animals overlapped considerably, and the mean receptive field size from the RIβ− did not differ significantly from WT (5.9 ± 0.1 and 6.4 ± 0.3°, respectively; p = 0.25). A normal linear retinotopic arrangement was revealed by regression analysis of receptive field azimuth on electrode position for both RIβ− and WT V1 (Fig. 1B). Moreover, the scatter about this relationship was equally low for both genotypes, as demonstrated by the high correlation coefficients. The distribution of ocular dominance scores of cells in the binocular zones of nondeprived RIβ− and WT mice was also similar. The contralateral bias index (CBI), a measure of the degree to which the contralateral eye dominates the cortex, did not differ significantly for three RIβ− and four WT hemispheres (mean CBI = 0.68 ± 0.05 and 0.66 ± 0.04, respectively;p = 0.7; t test). Thus, the primary visual cortex of mice developed apparently normal receptive field size, retinotopy, and ocular dominance in the complete absence of the RIβ subunit of protein kinase A.
Impaired LTP of extracellular field potentials in PKA RIβ− mice
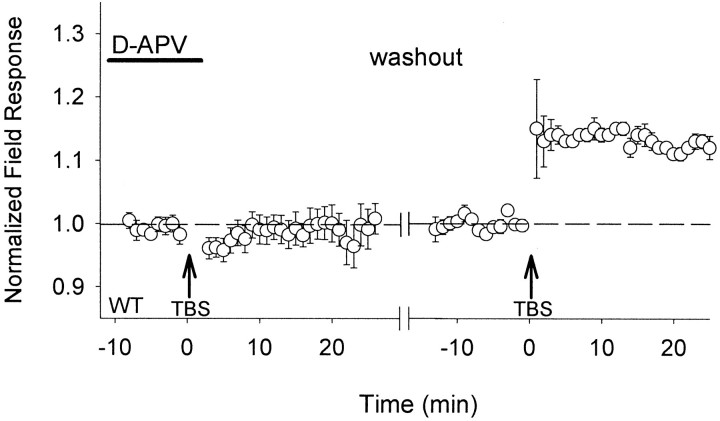
The ability to generate LTP in supragranular layers of cortex by TBS from the white matter has been proposed to reveal the mechanisms responsible for ocular dominance plasticity, because both phenomena appear to be correlated with age and visual experience (Kirkwood et al., 1995, 1996). We confirmed that activation of NMDA-R was necessary for TBS to generate LTP in slices of visual cortex from WT mice (Fig.2) (Larson and Lynch, 1988).
Fig. 2.
TBS-induced LTP of extracellular field responses via NMDA-R activation in mouse visual cortex. Theta-burst stimulation (TBS) to layer IV in the binocular zone of wild-type mouse visual cortex (left arrow) fails to potentiate supragranular field responses in the presence of D-APV (50–100 μm). The ability to generate LTP by TBS along this pathway (right arrow) is restored after washing out (40–60 min) the NMDA-R antagonist from the slice (n = 6 slices from 5 mice). Responses are normalized to the baseline period just before each TBS, and grouped data are shown as the average of all slices (± SEM), with one trial per slice.
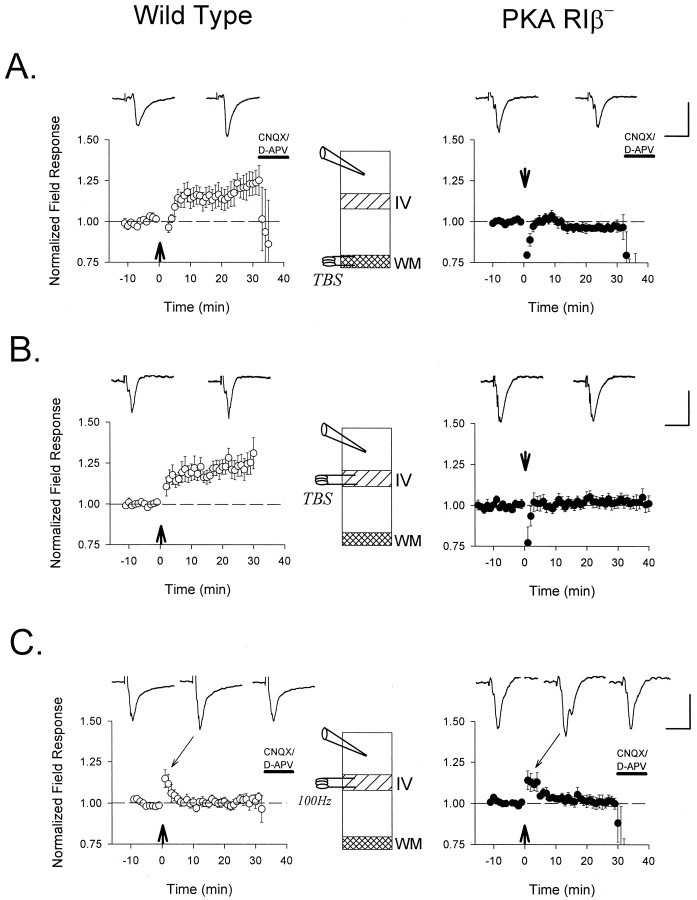
Theta-burst stimuli consistently failed to induce LTP in the mutant mice recorded blind to genotype. TBS applied to the white matter of the binocular zone (Fig. 3A) potentiated layer II/III field EPSPs in WT (normalized mean ± SEM = 1.23 ± 0.06 at 25 min after TBS; n = 6 slices from four mice) but not RIβ− animals (0.96 ± 0.03 at 25 min after TBS; n = 5 slices from three mice; p < 0.01; t test).
Fig. 3.
Defective LTP of extracellular field responses in the visual cortex of PKA RIβ− mice. TBS (arrow) applied (A) to the white matter (n = 6 and 5 slices from 4 and 3 mice, WT and RIβ−, respectively) or (B) directly to layer IV (n = 8 and 11 from 7 and 6 mice, WT and RIβ−, respectively) potentiates supragranular field response amplitudes in WT (○) but not RIβ− (•) mice recorded blind to genotype.C, More powerful tetani (four 1 sec bursts of 100 Hz;arrow) fail to induce LTP in both WT and mutant slices (n = 5 slices from 3 mice each). Representative traces 5 min before and 25 min after conditioning stimuli are shownabove each graph. Sample traces during post-tetanic potentiation are also indicated in C. Except for the experiments in B, which were continued to examine depotentiation (compare Fig. 6A), glutamate receptor antagonists CNQX (10 μm) and D-APV (50 μm) were routinely bath-applied to determine the synaptic nature of the field response. Calibration: 0.3 mV, 20 msec for each.
Maturation of inhibition in the cortical circuit has been proposed to underlie the developmental regulation of LTP induction from the white matter (Kirkwood and Bear, 1994a). To circumvent this hypothetical “plasticity gate,” we moved the stimulating electrode to layer IV (Fig. 3B). Once again TBS induced a robust potentiation in WT (1.23 ± 0.06 at 25 min after TBS; n = 8 slices in seven mice) but not in RIβ− mice (1.03 ± 0.03 at 25 min after TBS; n = 11 slices in six mice;p < 0.01; t test). This was surprising, since LTP induced by high-frequency stimulation is normal in hippocampal area CA1 of these mutants (Brandon et al., 1995). We therefore applied such a tetanus to the visual cortical slices (four bouts of 100 Hz stimuli for 1 sec, each at 10 sec intervals) (Fig.3C). However, this tetanus produced only a brief post-tetanic potentiation that decayed back to baseline in both WT and RIβ− mice (1.02 ± 0.03 and 1.01 ± 0.04 at 25 min after 100 Hz, respectively; n = 5 slices from three mice each; p > 0.1; t test). The failure of a strong tetanus to generate LTP even in wild-type animals was not surprising, because inhibition in the cortical circuit has long been known to curtail plasticity induced by high-frequency stimuli (Artola and Singer, 1987; Bear and Kirkwood, 1993). Thus, under our conditions TBS was an effective stimulus protocol for neocortical potentiationin vitro, yet it failed to produce LTP in animals lacking PKA RIβ.
LTP induced by pairing in PKA RIβ− mice
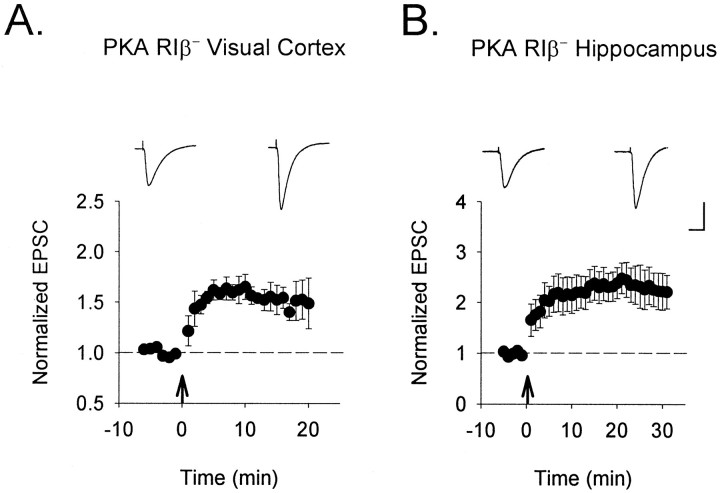
Because NMDA-R-dependent LTP in the CA1 region of RIβ− hippocampus was reported to be intact (Brandon et al., 1995) and we found functional NMDA-Rs on RIβ−cortical cells, we examined further the cause of the potentiation defect in neocortex in vitro. LTP induced by TBS in wild-type mouse visual cortex was dependent on NMDA-R activation as in other species, as indicated by its reversible blockade by the selective NMDA-R antagonist D-APV (Fig. 2) (Kirkwood and Bear, 1994a). We therefore attempted to induce LTP in RIβ− mutants by postsynaptic depolarization in whole-cell voltage-clamp mode paired with low-frequency stimulation of synaptic inputs. This procedure is known to directly relieve the magnesium blockade of postsynaptic NMDA-Rs (Gustafsson et al., 1987; Kirkwood and Bear, 1994a; Yoshimura and Tsumoto, 1994). Robust LTP using this pairing protocol could be observed in RIβ− mice both in the visual cortex and in the hippocampal CA1 region studied as a control within the same slice, consistent with the original report of intact tetanus-induced LTP in CA1 (Fig. 4) (n = 9 cells from seven mice each). Thus, postsynaptic mechanisms required for LTP induction were preserved in individual pyramidal cells lacking the RIβ subunit of PKA.
Fig. 4.
Preservation of postsynaptic LTP mechanisms in PKA RIβ− mice. A, Direct postsynaptic depolarization of supragranular pyramidal cells (from −70 to 0 mV) induces LTP in mutant visual cortex when paired with synaptic stimulation (100 pulses at 1 Hz to layer IV). B, Robust LTP is similarly induced by pairing at Schaeffer collateral synapses studied as a control within the same slice. Nine cells from each region were recorded in slices from a total of seven mice. Sample traces 5 min before and 20 min after pairing are shown above each graph. Calibration: 100 pA, 20 msec.
Defect in paired-pulse facilitation in RIβ− mice
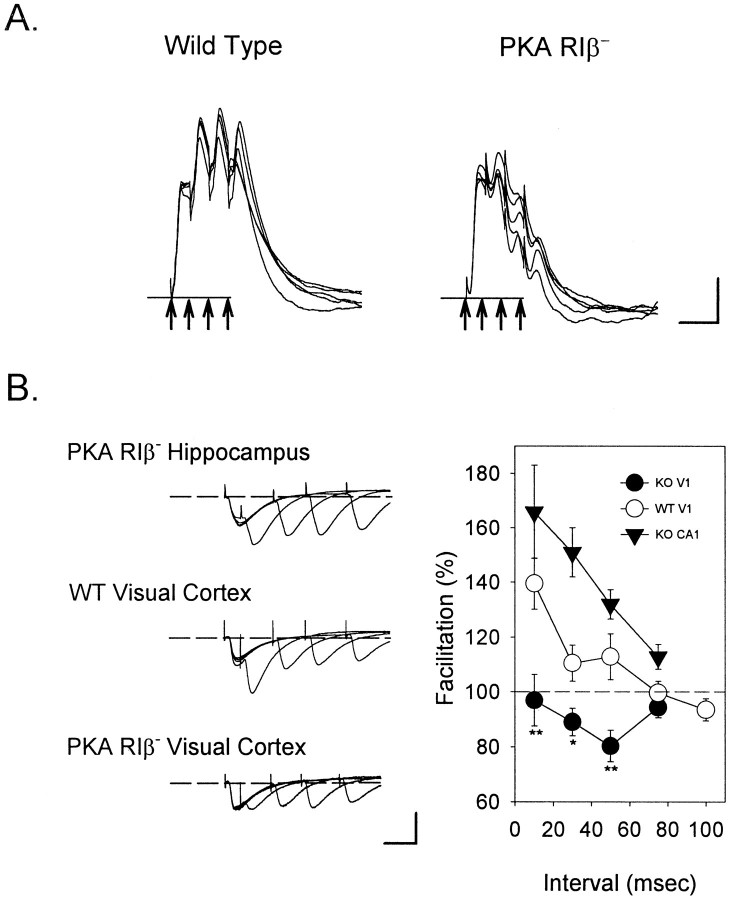
In whole-cell current-clamped supragranular pyramidal cells, responses during a theta burst exhibited a sustained facilitation in WT, but decremented strongly in RIβ− slices (Fig.5A) (n = 10 of 10 cells each). Short-term changes in neocortical synaptic strength that occur during TBS are strongly correlated with the magnitude of LTP subsequently expressed (Castro-Alamancos and Connors, 1996). To confirm this qualitative impression, we examined paired-pulse facilitation (PPF) with whole-cell voltage-clamp recordings (Andreasen and Hablitz, 1994). Ascending WT projections from layer IV to II/III exhibited a prominent facilitation only at the shortest interstimulus intervals, in agreement with recent descriptions of intracortical connections (Thomson and Deuchars, 1994; Stratford et al., 1996). PPF was pronounced at all intervals tested in mutant hippocampal area CA1, as expected from previous extracellular recordings (Brandon et al., 1995). In RIβ− visual cortex within the same slice, however, little or no PPF was observed, even at the shortest interstimulus intervals of 10 ms, which define a theta burst (Fig.5B) (n = 8 cells from three mice each;p < 0.01 WT vs RIβ− cortex;t test). This lack of facilitation may have rendered theta-burst stimuli incapable of depolarizing cells sufficiently to activate postsynaptic mechanisms that would yield LTP.
Fig. 5.
Disrupted net depolarization and paired-pulse facilitation in the visual cortex of PKA RIβ− mice.A, Whereas TBS produces a prolonged depolarization in wild-type pyramidal cells, a decrementing response is observed in the knockout cells (n = 10 of 10 cells). Whole-cell current-clamp responses to the first bursts in five episodes of TBS to layer IV are shown superimposed. Arrows indicate the four stimulus pulses delivered at 10 msec intervals. Calibration: 5 mV, 20 msec. B, Paired-pulse facilitation (PPF) is perturbed in RIβ− visual cortex but not in the hippocampus. Pairs of stimuli to layer IV elicit a prominent PPF only at 10 msec interpulse intervals in WT supragranular pyramidal cells voltage-clamped to −70 mV. In contrast, mutant V1 exhibits no PPF along this pathway, whereas it is pronounced at all intervals tested in RIβ− CA1 (n = 8 cells each; **p < 0.01, * p < 0.05;t test WT vs RIβ− cortex). Calibration: 40 pA, 20 msec.
Impaired synaptic depression in RIβ− micein vitro
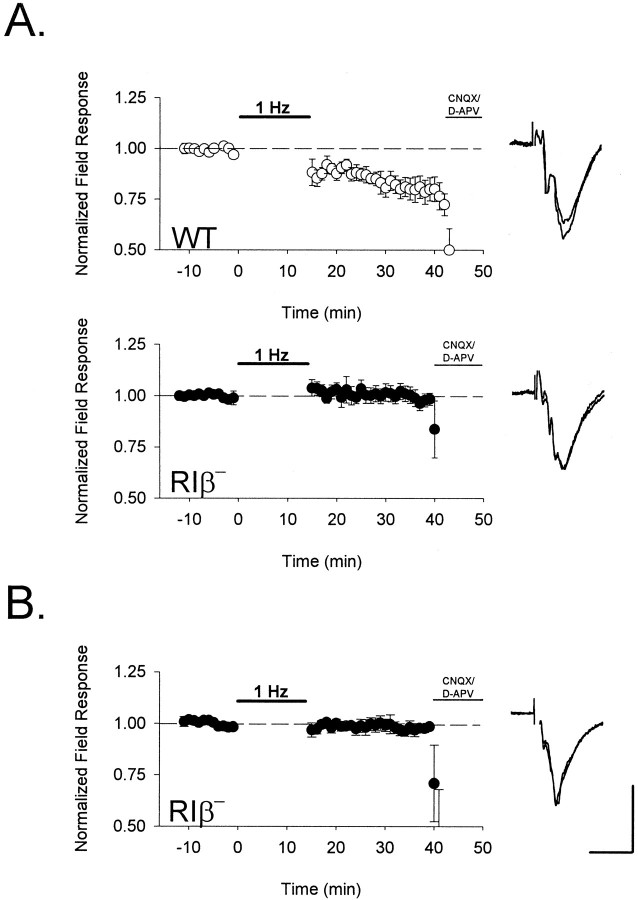
The original report of the PKA RIβ knockout mouse described an inability to generate LTD in the dentate gyrus and CA1 region of the hippocampus (Brandon et al., 1995). We found that this impairment was also present in RIβ− visual cortex in vitro. Depression (Fig. 6A) of field EPSPs in the supragranular layers by low-frequency stimulation of layer IV after an earlier TBS (Fig. 3B) was significantly disrupted in the mutant (normalized mean ± SEM = 0.80 ± 0.05 in WT vs 1.00 ± 0.04 in RIβ− at 20 min after 1 Hz stimulation; n = 8 and 11 slices from seven and six mice, respectively; p < 0.01; ttest). Similarly, low-frequency stimulation did not depress naïve, unconditioned synapses in the absence of RIβ (0.98 ± 0.03 normalized field response 20 min after 1 Hz stimulation; n = 5 slices from three mice) (Fig.6B).
Fig. 6.
Absence of synaptic depression after low-frequency stimulation in PKA RIβ− mice. Extracellular field potential amplitude was monitored in layer II/III after low-frequency stimulation (900 pulses at 1 Hz) to layer IV of visual cortex (•, RIβ−; ○, WT). A, Renormalized responses after an earlier TBS (compare Fig. 3B) were depotentiated in wild-type (n = 8 slices from 7 mice) but unchanged in RIβ− mice (n= 11 slices from 6 animals). B, Low-frequency stimulation was similarly ineffective at naïve RIβ− synapses (n = 5 slices from 3 mice). Bath application of CNQX (10 μm) and D-APV (50 μm) terminated each experiment to confirm the synaptic nature of the field response. Representative traces 5 min before and 20 min after LFS are shown superimposed to the right of each graph. Calibration: 0.3 mV, 10 msec.
Monocular deprivation and reverse suture in RIβ−mice in vivo
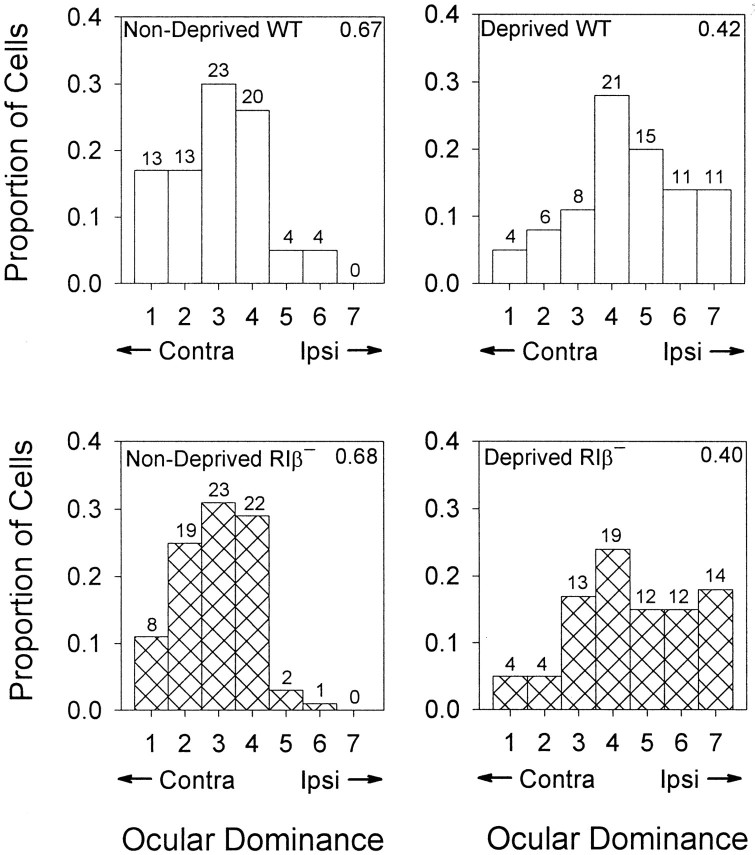
The absence of LTP and particularly synaptic depression in slices suggested that mutant mice might not exhibit the usual experience-dependent plasticity in visual cortex in vivo. We therefore examined the loss of responses in V1 to stimulation of one eye after a period of occluded vision through that eye. Three mutant and three WT mice underwent monocular deprivation by lid suture for 4 d beginning between P25 and P27. Ocular dominance distributions of neurons recorded blind to genotype from V1 contralateral to the deprived eye revealed significant and similar shifts toward the open, ipsilateral eye in both WT and RIβ− mice (Fig.7) (deprived vs nondeprived ttest; p < 0.04 for each genotype). A second procedure in which recordings were made blind to deprivation status was used for five additional RIβ− mice, two deprived and three nondeprived. Only monocularly deprived animals demonstrated a shift in ocular dominance (CBI = 0.41 ± 0.03 in two hemispheres ipsilateral to the deprived eye, and CBI = 0.68 ± 0.06 in five nondeprived hemispheres; t test; p < 0.002). Thus, functional disconnection of input from a deprived eye occurred despite the absence in vitro of several forms of plasticity, including LTP, paired-pulse facilitation, and most notably LTD.
Fig. 7.
Loss of deprived-eye responses after monocular deprivation in PKA RIβ− mice. Ocular dominance distributions were recorded blind to genotype from the binocular zone of two nondeprived adults each (left panels) of wild-type (hollow bars; n = 77 cells) and RIβ− mice (hatched bars;n = 75 cells). Both distributions shifted significantly and similarly (right panels) in response to monocular deprivation of the contralateral eye for 4 d beginning at P25–27 (n = 76 and 78 cells from 3 mice each, WT and RIβ−, respectively). Numbers of cells are indicated above each bar, and contralateral bias indices are shown in the top right-hand corner of each graph.
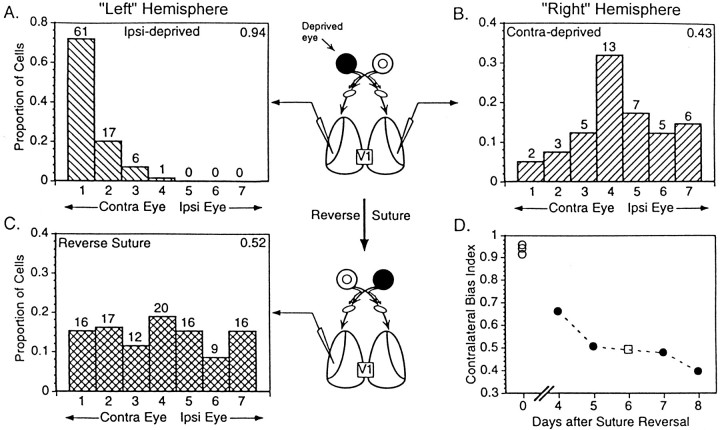
We used a reverse-suture paradigm to demonstrate whether inputs that had previously been made ineffective could again become dominantin vivo (Wiesel and Hubel, 1965). Because of the overall dominance of the contralateral eye in the normal mouse, the binocular segments of the two hemispheres differ markedly in their responses to the two eyes after monocular deprivation. In the hemisphere ipsilateral to the deprived eye, 75% of the cells are no longer driven at all by stimulation of that eye (Fig.8A), and two-thirds of the few cells that do respond to the deprived eye do so only weakly (ocular dominance group 2). This hemisphere provides suitable conditions for testing whether responses to the initially deprived eye might reemerge after a period of reverse suture. In the hemisphere contralateral to the deprived eye, however, substantial responses to the deprived eye remain after the initial deprivation (Figs. 7,8B). We therefore investigated the effects of reverse suture only in the hemisphere ipsilateral to the originally deprived eye. If as in other species strong responses to the deprived eye in this hemisphere could be restored to the majority of cells by a period of reverse suture, this change would represent an absolute increase in deprived eye responses and could not be merely a result of a loss of input from the originally open eye. Such an activity-dependent increase in response to the deprived eye might be thought to require a mechanism more similar to LTP as studied in vitro than to LTD.
Fig. 8.
Potentiation of initially deprived eye responses by reverse suture in PKA RIβ− mice. A, Ocular dominance distribution of cells recorded ipsilateral to an eye deprived of vision for 5 d beginning at P20–22 (“Left” Hemisphere). A nearly complete dominance of the RIβ− cortex by the contralateral eye occurs because of the innate bias toward contralateral eye dominance in nondeprived animals (n = 85 cells in 3 hemispheres). Numbers of cells are indicated above each bar, and contralateral bias indices are shown in the top right-hand corner of each graph. B, The shift in ocular dominance is typically less dramatic in the opposite “Right” Hemisphere (n = 41 cells in 3 mice) (see also Fig. 7, or Gordon and Stryker, 1996). C, Ocular dominance distribution of cells in RIβ− visual cortex recorded ipsilateral to the initially deprived eye (“Left” Hemisphere) reveals a strengthening of previously lost inputs after suture reversal for an additional 4–8 d (P26–34;n = 106 cells from 4 mice). D, Individually calculated CBIs of ipsilaterally deprived (○, same animals as in A) and reverse-sutured animals (•, same animals as in C) demonstrate the gradual recovery of response, similar to WT (□) with increasing duration of suture reversal.
After an initial 5 d deprivation of the ipsilateral eye early in the critical period, few cortical cells in the hemisphere ipsilateral to the deprived eye responded at all to stimuli presented to that eye (Fig. 8A) (n = 85 cells in three RIβ− hemispheres). Responses in this hemisphere to the initially deprived eye reemerged once the eye was opened and the initially open eye was sutured shut for 4–8 d (Fig. 8C). The ocular dominance distribution after reverse suture shifted significantly back toward the initially deprived eye (n= 106 cells from four RIβ− animals and 26 cells from one WT mouse; p < 0.0005; χ2 test). Furthermore, the degree of recovery was greater with successively increasing periods after suture reversal (Fig. 8D). These data demonstrate a dramatic increase in efficacy of inputs from an initially deprived eye and establish the existence of some mechanism for increasing synaptic strength in RIβ− mice in vivo, despite the absence of paired-pulse facilitation and TBS-induced LTP.
DISCUSSION
We have examined visual cortical plasticity in a mouse mutant of protein kinase A, a molecule implicated in many different forms of plasticity. Our results demonstrate an identical reduction in response after monocular deprivation for wild-type mice and those lacking the RIβ subunit. They further show that activity-dependent plasticityin vivo in the visual cortex of either genotype can selectively increase the responses to one eye, using a reverse-suture procedure. In contrast, profound deficits were found in paired-pulse facilitation, long-term synaptic depression, and TBS-induced long-term potentiation in slices of RIβ− visual cortex. Direct pairing of postsynaptic depolarization with presynaptic stimulation, however, elicited LTP in mutant pyramidal cells. These findings have several important implications: first, they argue against an essential role for the RIβ subunit in visual cortical plasticity in vivo. Second, they demonstrate a possible role for PKA RIβ in paired-pulse facilitation in visual cortical circuitry and suggest that expression of this form of short-term plasticity is not required for developmental plasticity in vivo. Finally, they illustrate that studies of potentiation and depression of extracellular field potentials in neocortex are not necessarily informative with regard to either postsynaptic LTP mechanisms in vitro or ocular dominance plasticity in vivo.
The role of PKA in visual cortical plasticityin vivo
The peak of the critical period for plasticity has been correlated with cAMP production in the visual cortex (Reid et al., 1996). Our results do not exclude a role for PKA in ocular dominance plasticity. The PKA holoenzyme is a tetramer composed of a regulatory subunit dimer, which contains the cAMP binding sites, and a single catalytic subunit bound to each regulatory subunit (Spaulding, 1993). At least four regulatory (RIα, RIβ, RIIα, RIIβ) and two catalytic (Cα, Cβ) subunits have been characterized in mice (Cadd and McKnight, 1989). Although α subunits are ubiquitously expressed, the β isoforms show a more restricted pattern of high expression in the nervous system. Interestingly, disrupting the DrosophilaRIβ homolog alone causes specific defects in olfactory learning (Goodwin et al., 1997). Because selective inhibitors of the various PKA isoforms are not available, mice carrying deletions of subunits other than RIβ or in combination should provide valuable insight. Spatially restricted reductions in PKA activity could also be assessed by expressing an inhibitory form of the regulatory subunit of PKA in mice (Abel et al., 1997).
PKA RIβ and intracortical signaling
Defective paired-pulse facilitation in PKA mutant visual cortexin vitro is consistent with a presynaptic function for RIβ. In one view (Zucker, 1989; Fisher et al., 1997) (but see Wang and Kelly, 1996; Bao et al., 1997), PPF is mediated by residual calcium produced by action potential invasion of the presynaptic terminal bouton that enhances transmitter release to a closely following spike. Changes in intracellular cAMP concentration are tightly coupled to calcium influx (Cooper et al., 1995), and RIβ is the regulatory subunit isoform that confers the greatest cAMP sensitivity to PKA (Cadd et al., 1990). Our finding that facilitation on the millisecond time scale was disrupted at cortical synapses lacking RIβ (Fig. 5) is consistent with this interpretation. Stronger tetani were capable of producing only post-tetanic potentiation, a short-term presynaptic enhancement lasting a few minutes (Fig. 3C) (Zucker, 1989), possibly via the upregulated RIα subunit, which is activated at three- to sevenfold higher concentrations of cAMP (Cadd et al., 1990;Amieux et al., 1997). Ultrastructural localization of the various PKA subunit isoforms to the presynaptic terminal or elsewhere will aid in our understanding of their functions in cortical circuitry.
Several additional lines of evidence support a presynaptic role for PKA and RIβ in synaptic facilitation. Normal presynaptic PKA activity directly modulates the secretory machinery during facilitation (Trudeau et al., 1996). Thus, it is noteworthy that LTP is defective at the mossy fiber synapse in hippocampal area CA3 but not CA1 of the RIβ− mutant studied here (Huang et al., 1995). Mossy fiber LTP has recently been shown to be a presynaptic phenomenon mediated by the cAMP pathway (Huang et al., 1994; Weisskopf et al., 1994). Presynaptic reductions in neurotransmitter release from primary afferent terminals in the spinal cord and periphery also best explain the decreased inflammation and pain behavior in PKA RIβ−mice (Malmberg et al., 1997). In contrast, postsynaptic PKA activity was preserved in visual cortical pyramidal cells lacking RIβ, because norepinephrine abolished spike frequency adaptation as in WT cells (Madison and Nicoll, 1982; T. Hensch, unpublished observations).
Interestingly, a gradual loss of facilitation in favor of paired-pulse suppression has been correlated with the end of sensitivity to monocular deprivation in rats (Ramoa and Sur, 1996). Altered cortical inhibition may contribute to such a decline in PPF from layer IV to II/III (Metherate and Ashe, 1994; Ramoa and Sur, 1996). Indeed, enhanced intracortical inhibition in RIβ− neocortex could have prevented the induction of both LTP (Artola and Singer, 1987; Bear and Kirkwood, 1993) and LTD (Dudek and Friedlander, 1996), since PKA can potentiate (Kano and Konnerth, 1992) as well as depress GABAA receptor currents (Porter et al., 1990). Although this possibility remains to be examined further, the abolition of paired-pulse facilitation clearly does not curtail ocular dominance plasticity.
In vitro models of ocular dominance plasticity
Although the onset of plasticity in vivo is clearly not related to the capacity for LTP generation in visual cortex, the end of the critical period has been correlated with a decreased ability to potentiate supragranular responses from the white matter (Kato et al., 1991; Kirkwood et al., 1995, 1996). The maturation of an inhibitory “plasticity gate” in middle cortical layers has been proposed to account for the loss of plasticity both in vivo and in vitro (Kirkwood and Bear, 1994a; Kirkwood et al., 1995). Although an analogous “gate” is shut in RIβ− mice in vitro, monocular deprivation and reverse suture produce robust plasticity in the intact animal. A similar dissociation between LTPin vitro and activity-dependent plasticity in vivo is seen in juvenile mice lacking α-calcium/calmodulin-dependent kinase II (αCaMKII): barrel field reorganization is intact (Glazewski et al., 1996) and ocular dominance plasticity is impaired in only half the animals (Gordon et al., 1996), whereas neocortical LTP (after TBS) is reported to be consistently reduced (Kirkwood et al., 1997).
A further dissociation between LTP in vitro and neural plasticity in vivo is evident in recent comparisons between hippocampal spatial learning behavior and NMDA-R-dependent LTP (Bannerman et al., 1995; Barnes, 1995; Saucier and Cain, 1995;Nosten-Bertrand et al., 1996; Holscher et al., 1997b) or presynaptic mossy fiber LTP in PKA RIβ mutants (Huang et al., 1995). Several manipulations that prevent depression of field potentials by low-frequency stimulation have also failed to predict the plasticity found in the intact animal (NMDA-R: Kasamatsu et al., 1998; metabotropic glutamate receptors: Hensch and Stryker, 1996; Yokoi et al., 1996; PKA Cβ and RIβ: Huang et al., 1995; Qi et al., 1996;Brandon et al., 1995; the present study). We conclude that assaying synaptic efficacy changes in extracellular field responses is not an accurate indicator of experience-dependent plasticity in vivo.
NMDA-R-dependent plasticity mechanisms could still play a vital role in cortical development, because the intracellular machinery to generate LTP in individual pyramidal cells was preserved in RIβ−mutants. At thalamocortical synapses of barrel cortex, pairing low-frequency presynaptic stimulation with postsynaptic depolarization induces LTP only during the critical period for plasticity in vivo (Crair and Malenka, 1995; Isaac et al., 1997). Pairing paradigms have also provided direct evidence that LTP is important for the activity-dependent formation of glutamatergic synapses in the hippocampus (Durand et al., 1996), refuting earlier extracellular studies claiming that LTP occurs only at later stages of hippocampal development (Harris and Teyler, 1984; Bekenstein and Lothman, 1991;Dudek and Bear, 1993; Jackson et al., 1993; Battistin and Cherubini, 1994). The differential efficacy with which TBS and pairing protocols induce LTP in RIβ− mutants underscores previous observations in rat anterior cingulate cortex that potentiation of extracellular field potentials does not necessarily reflect the status of postsynaptic LTP mechanisms in the cortex (Sah and Nicoll, 1991).
We may conclude at a minimum that the simple patterns of stimulation used to potentiate and depress extracellular field potentials in slices may not correspond to patterns of activity that are important for plasticity to occur in the intact brain. For example, TBS is believed to mimic intrinsic patterns of activity in the hippocampus (Rose and Dunwiddie, 1986; Holscher et al., 1997a), but it is not clear whether this is also true for visual cortex. Distinct presynaptic release probabilities underlie differences in short-term plasticity between the hippocampus and neocortex (Finlayson and Cynader, 1995;Castro-Alamancos and Connors, 1997). We have further observed that ascending connections in the binocular zone of visual cortex are more sensitive to RIβ gene deletion than Schaeffer collateral synapses in hippocampal area CA1. A simple extrapolation of findings from the hippocampus in vitro to the neocortex in vivotherefore may be misleading. The unique properties of thalamocortical circuits must be better understood if we are to explore mechanisms of their experience-dependent development.
Footnotes
J.A.G. was an ARCS Foundation scholar and was supported by a National Institutes of Health Medical Scientist Training Program fellowship. T.K.H. was a Howard Hughes Medical Institute Predoctoral Fellow. This work was supported by National Institutes of Health grants to G.S.M. (GM32875) and M.P.S. (EY02874), and a Human Frontiers Science Program grant to M.P.S. We thank Drs. R. A. Nicoll and M. Fagiolini for critical comments on this manuscript.
Correspondence should be addressed to Dr. Takao K. Hensch, Laboratory for Neuronal Circuit Development, Brain Science Institute (RIKEN), 2-1 Hirosawa, Wako-shi, Saitama 351-01, Japan.
Dr. Hensch’s present address: Laboratory for Neuronal Circuit Development, Brain Science Institute (RIKEN), 2-1 Hirosawa, Wako-shi, Saitama 351-01, Japan.
Dr. Gordon’s present address: Columbia Presbyterian Medical Center, Chief Resident’s Office, Milstein Hospital Building, Rm 5-006, 177 Fort Washington Avenue, New York, NY 10032-3784.
Dr. Brandon’s present address: Laboratory of Genetics, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, CA 92037.
REFERENCES
- 1.Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 2.Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS. Compensatory regulation of RIα protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen M, Hablitz JJ. Paired-pulse facilitation in dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- 4.Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 6.Bao J-X, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
- 7.Barnes CA. Involvement of LTP in memory: are we “searching under the streetlight”? Neuron. 1995;15:751–754. doi: 10.1016/0896-6273(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 8.Battistin T, Cherubini E. Developmental shift from long-term depression to long-term potentiation at mossy fibre synapses in the rat hippocampus. Eur J Neurosci. 1994;6:1750–1755. doi: 10.1111/j.1460-9568.1994.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 9.Bear MF, Kirkwood A. Neocortical long-term potentiation. Curr Opin Neurobiol. 1993;3:197–202. doi: 10.1016/0959-4388(93)90210-p. [DOI] [PubMed] [Google Scholar]
- 10.Bekenstein JW, Lothman EW. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Dev Brain Res. 1991;63:245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- 11.Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J Neurosci. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schultz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 14.Brandon EP, Zhuo M, Huang Y-Y, Qi M, Gerhold KA, Burton KA, Kandel ER, McKnight GS, Idzerda RL. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RIb subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne JH, Zwartjes R, Homayouni R, Critz SD, Eskin A. Roles of second messenger pathways in neuronal plasticity and in learning and memory. In: Shenolikar ES, Nairn AC, editors. Advances in second messenger and phosphoprotein research. Raven; New York: 1993. pp. 47–107. [PubMed] [Google Scholar]
- 16.Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 17.Cadd G, Uhler MD, McKnight GS. Holoenzymes of cAMP-dependent protein kinase containing the neural form of type I regulatory subunit have an increased sensitivity to cyclic nucleotides. J Biol Chem. 1990;265:19502–19506. [PubMed] [Google Scholar]
- 18.Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci USA. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Alamancos MA, Connors BW. Distinct forms of short-term plasticity at excitatory synapses of hippocampus and neocortex. Proc Natl Acad Sci USA. 1997;94:4161–4166. doi: 10.1073/pnas.94.8.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cyclic AMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper DMF, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 22.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 23.Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- 24.DeZazzo J, Tully T. Dissection of memory formation: from behavioral to pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–217. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- 25.Draeger UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978;41:28–42. doi: 10.1152/jn.1978.41.1.28. [DOI] [PubMed] [Google Scholar]
- 26.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature adult hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 28.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 29.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark-rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 30.Finlayson PG, Cynader MS. Synaptic depression in visual cortex tissue slices: an in vitro model for cortical neuron adaptation. Exp Brain Res. 1995;106:145–155. doi: 10.1007/BF00241364. [DOI] [PubMed] [Google Scholar]
- 31.Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends Neurosci. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- 32.Funte LR, Haydon PG. Synaptic target contact enhances presynaptic calcium influx by activating cAMP-dependent protein kinase during synaptogenesis. Neuron. 1993;10:1069–1078. doi: 10.1016/0896-6273(93)90055-v. [DOI] [PubMed] [Google Scholar]
- 33.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and non-depressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 34.Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- 35.Glazewski S, Chen C-M, Silva A, Fox K. Requirement for a-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–423. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin SF, Del Vecchio M, Velinzon K, Hogel C, Russell SRH, Tully T, Kaiser K. Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:8817–8827. doi: 10.1523/JNEUROSCI.17-22-08817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon JA, Cioffi D, Silva AJ, Stryker MP. Deficient plasticity in the primary visual cortex of α-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron. 1996;17:491–499. doi: 10.1016/s0896-6273(00)80181-6. [DOI] [PubMed] [Google Scholar]
- 39.Grant SGN, Silva AJ. Targeting learning. Trends Neurosci. 1994;17:71–75. doi: 10.1016/0166-2236(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 40.Gustafsson B, Wigstrom H, Abraham WC, Huang Y-Y. Long-term potentiation in the hippocampus using depolarizing potentials as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris KM, Teyler TJ. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol (Lond) 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hata Y, Stryker MP. Control of thalamocortical afferent rearrangement by postsynaptic activity in developing visual cortex. Science. 1994;265:1732–1735. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 43.Hensch TK, Stryker MP. Ocular dominance plasticity under metabotropic glutamate receptor blockade. Science. 1996;272:554–557. doi: 10.1126/science.272.5261.554. [DOI] [PubMed] [Google Scholar]
- 44.Holscher C, Anwyl R, Rowan M. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997a;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holscher C, McGlinchey L, Anwyl R, Rowan M. HFS-induced long-term potentiation and depotentiation in area CA1 of the hippocampus are not good models for learning. Psychopharmacology. 1997b;130:174–182. doi: 10.1007/s002130050226. [DOI] [PubMed] [Google Scholar]
- 46.Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y-Y, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 48.Huang Y-Y, Li X-C, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y-Y, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 50.Hubel D, Wiesel TN. Receptive fields, binocular interaction, and functional architecture in the cat’s visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaac JTR, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 52.Jackson PS, Suppes T, Harris KM. Stereotypical changes in the pattern and duration of long-term potentiation expressed at postnatal days 11 and 15 in the rat hippocampus. J Neurophysiol. 1993;70:1412–1419. doi: 10.1152/jn.1993.70.4.1412. [DOI] [PubMed] [Google Scholar]
- 53.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandel ER, O’Dell TJ. Are adult learning mechanisms also used for development? Science. 1992;258:243–245. doi: 10.1126/science.1411522. [DOI] [PubMed] [Google Scholar]
- 55.Kano M, Konnerth A. Potentiation of GABA-mediated currents by cAMP-dependent protein kinase. NeuroReport. 1992;3:563–566. doi: 10.1097/00001756-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Kasamatsu T, Imamura K, Mataga N, Hartveit E, Heggelund U, Heggelund P. Roles of N-methyl-d-aspartate receptors in ocular dominance plasticity in developing visual cortex: re-evaluation. Neuroscience. 1998;82:687–700. doi: 10.1016/s0306-4522(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 57.Kato N, Artola A, Singer W. Developmental changes in the susceptibility to long-term potentiation of neurones in rat visual cortex slices. Dev Brain Res. 1991;60:43–50. doi: 10.1016/0165-3806(91)90153-a. [DOI] [PubMed] [Google Scholar]
- 58.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 59.Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994a;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994b;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkwood A, Lee HK, Bear MF. Co-regulation of long term potentiation and experience- dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 62.Kirkwood A, Rioult MG, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 63.Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of αCaMKII mutant mice. Proc Natl Acad Sci USA. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson J, Lynch G. Role of N-methyl-d-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- 65.Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharges in the hippocampus. Nature. 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- 66.Malmberg A, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the RIβ subunit of PKA. J Neurosci. 1997;17:7462–7470. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayford M, Abel T, Kandel ER. Transgenic approaches to cognition. Curr Opin Neurobiol. 1995;5:141–148. doi: 10.1016/0959-4388(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 68.Metherate R, Ashe JH. Facilitation of an NMDA receptor-mediated EPSP by paired-pulse stimulation in rat neocortex via depression of GABAergic IPSPs. J Physiol (Lond) 1994;481:331–348. doi: 10.1113/jphysiol.1994.sp020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Movshon JA, Kiorpes L. The role of experience in visual development. In: Coleman RJ, editor. Development of sensory systems in mammals. Wiley; New York: 1990. pp. 155–202. [Google Scholar]
- 70.Nosten-Bertrand N, Errington ML, Murphy KPSJ, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris RGM, Silver J, Stewart CL, Bliss TVP, Morris RJ. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature. 1996;379:826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- 71.Porter NM, Twyman RE, Uhler MD, Macdonald RL. Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron. 1990;5:789–796. doi: 10.1016/0896-6273(90)90338-g. [DOI] [PubMed] [Google Scholar]
- 72.Qi M, Zhuo M, Skalhegg BS, Brandon EP, Kandel ER, McKnight GS, Idzerda RL. Impaired hippocampal plasticity in mice lacking the Cβ catalytic subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramoa AS, Sur M. Short-term synaptic plasticity in the visual cortex during development. Cereb Cortex. 1996;6:640–646. doi: 10.1093/cercor/6.4.640. [DOI] [PubMed] [Google Scholar]
- 74.Reid SNM, Daw NW, Gregory DS, Flavin H. cAMP levels increased by activation of metabotropic glutamate receptors correlate with visual cortical plasticity. J Neurosci. 1996;16:7619–7626. doi: 10.1523/JNEUROSCI.16-23-07619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- 76.Sah P, Nicoll RA. Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol (Lond) 1991;433:615–630. doi: 10.1113/jphysiol.1991.sp018446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saucier D, Cain DP. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature. 1995;378:186–188. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- 78.Schacher S, Kandel ER, Montarolo P. cAMP and arachidonic acid simulate long-term structural and functional changes produced by neurotransmitters in Aplysia sensory neurons. Neuron. 1993;10:1079–1088. doi: 10.1016/0896-6273(93)90056-w. [DOI] [PubMed] [Google Scholar]
- 79.Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 80.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 81.Spaulding SW. The ways in which hormones change cyclic adenosine 3′, 5′-monophosphate-dependent protein kinase subunits, and how such changes affect cell behavior. Endocr Rev. 1993;14:632–650. doi: 10.1210/edrv-14-5-632. [DOI] [PubMed] [Google Scholar]
- 82.Stern P, Edwards FA, Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol (Lond) 1992;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stratford KJ, Tarczy-Hornoch K, Martin KAC, Bannister NJ, Jack JJB. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- 84.Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 85.Trudeau L-E, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 86.Tsumoto T. Long-term potentiation and long-term depression in the neocortex. Prog Neurobiol. 1992;209:209–228. doi: 10.1016/0301-0082(92)90011-3. [DOI] [PubMed] [Google Scholar]
- 87.Wang JH, Kelly PT. Regulation of synaptic facilitation by postsynaptic Ca2+/CaM pathways in hippocampal CA1 neurons. J Neurophysiol. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]
- 88.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 89.Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 90.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 91.Wu F, Friedman L, Schacher S. Transient versus persistent functional and structural changes associated with facilitation of Aplysia sensorimotor synapses are second messenger dependent. J Neurosci. 1995;15:7517–7527. doi: 10.1523/JNEUROSCI.15-11-07517.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu Z-L, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci USA. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 94.Yoshimura Y, Tsumoto T. Dependence of LTP induction on postsynaptic depolarization: a perforated patch-clamp study in visual cortical slices of young rats. J Neurophysiol. 1994;71:1638–1645. doi: 10.1152/jn.1994.71.5.1638. [DOI] [PubMed] [Google Scholar]
- 95.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]