Abstract
Exogenous administration of the neurotrophins brain-derived neurotrophic factor (BDNF) or neurotrophin-4/5 (NT-4/5), or blockade of their endogenous actions, have been reported to affect the anatomic organization and physiological responses of neurons in developing mammalian primary visual cortex. Experimental alteration of levels of these neurotrophic factors can also influence the morphology of the geniculocortical afferents that project from the lateral geniculate nucleus (LGN) to primary visual cortex. BDNF and NT-4/5 are ligands of the TrkB tyrosine kinase receptor. Although multiple populations of cortical neurons express TrkB, it is not known whether geniculocortical afferents express this receptor on their axon branches in visual cortex. We have anatomically labeled geniculocortical afferents of postnatal day 40 kittens with the anterograde neuronal tracer Phaseolus vulgaris leucoagglutinin (PHA-L) and performed double-label immunofluorescence with a panel of anti-TrkB antibodies. Confocal microscopy and object-based colocalization analysis were used to measure levels of TrkB-like immunoreactivity (IR) on geniculocortical afferents in layer IV of primary visual cortex. By using a conservative analysis involving a comparison of measured colocalization with the amount of colocalization expected based on random overlap of TrkB puncta and PHA-L-labeled afferents, 3 of 5 anti-TrkB antibodies tested showed significant colocalization with the geniculocortical axons. Results for the other two antibodies were indeterminate. The indices obtained for colocalization of TrkB and geniculocortical afferents were also compared with the equivalent index obtained for GAD65, a protein that has a similar overall expression pattern to that of TrkB but is not expressed on geniculocortical axons. This analysis indicated that TrkB was present on geniculocortical axons for all five TrkB antibodies tested. TrkB-like IR was also observed on neuronal somata in the LGN. These results indicate that TrkB receptors on geniculocortical afferents are potential mediators of the actions of BDNF and NT-4/5 in developing visual cortex.
Keywords: BDNF, brain-derived neurotrophic factor, critical period, lateral geniculate body, neurotrophin, striate cortex
Experimental manipulations of the amounts of the neurotrophic factors brain-derived neurotrophic factor (BDNF) or neurotrophin-4/5 (NT-4/5) can influence many aspects of the structure and function of developing primary visual cortex (McAllister et al., 1999). The tyrosine kinase receptor TrkB is a high affinity receptor for these neurotrophins (Barbacid, 1994). Administration of exogenous BDNF or NT-4/5 to primary visual cortex with osmotic minipumps caused desegregation of ocular dominance columns in kitten (Cabelli et al., 1995), but this effect was not observed in adult cats for BDNF (Hata et al., 2000). Although monocular deprivation (MD) during a critical period of development normally causes a shift in binocular responses of primary visual cortical neurons in favor of the nondeprived eye, combining local infusion of BDNF or NT-4/5 with MD has complex effects on ocular dominance plasticity, orientation selectivity, and visual responsiveness (Galuske et al., 2000; Gillespie et al., 2000; Lodovichi et al., 2000). NT-4/5 delivered locally to visual cortex of developing ferrets, but not BDNF, prevented MD from inducing atrophy of lateral geniculate nucleus (LGN) neurons representing the deprived eye (Riddle et al., 1995). Administration of high concentrations of exogenous neurotrophins could cause effects that are pharmacological but not physiological. However, experiments in which endogenous neurotrophin activity is diminished by fusion proteins containing the extracellular domain of TrkB coupled to immunoglobulin heavy chain (TrkB-IgG) directly address the role of neurotrophins in normal brain development, and infusion of TrkB-IgG into kitten primary visual cortex inhibited the later stages of ocular dominance column formation (Cabelli et al., 1997).
Collectively, these results are consistent with a model of visual cortical development and plasticity in which axons compete with each other for access to limiting amounts of a trophic factor that is produced and released by postsynaptic cortical neurons in an activity-dependent manner (Maffei et al., 1992; Shatz, 1997). In this model, afferents with sufficient levels or appropriate patterns of activity would receive or respond to trophic support. It is known that ocular dominance column development and plasticity result from an activity-dependent competition in visual cortex between neural pathways representing the two eyes (Shatz, 1990). The morphology of geniculocortical afferents undergoes activity-dependent change during ocular dominance column formation (Antonini and Stryker, 1993a) and MD (Antonini and Stryker, 1993b). In addition, geniculocortical afferents represent the last stage in the visual pathways that is strictly monocular, and they converge onto common postsynaptic binocular target cells in layer IV. Therefore, geniculocortical afferents are a likely candidate to mediate competitive interactions in visual cortical development and plasticity. Although the powerful effects of TrkB ligands on the arrangement of these afferents suggest that they might express TrkB receptors, ligands of receptors that the afferents lack can also produce profound effects. For example, cortical infusion of the GABAA receptor agonist muscimol indirectly alters afferent morphology through its actions on cortical neurons, even though the afferents do not express this receptor (Hata et al., 1999). If geniculocortical afferents compete for a limiting amount of BDNF and/or NT-4/5 released by cortical neurons, they should express TrkB receptors on their axon branches in layer IV of primary visual cortex. We have tested this prediction by anatomically labeling geniculocortical afferents and have found significant amounts of TrkB-like immunoreactivity (IR) on these axons in layer IV of kitten primary visual cortex.
MATERIALS AND METHODS
Experimental animals
Five kittens were used for this study. All were from the breeding colony at the University of California, San Francisco, and had normal pigmentation. One animal was monocularly deprived for 7 days (P33-P40), but equal amounts of data were collected from sections containing deprived or nondeprived labeled geniculocortical afferents. Data from this animal constituted 12 of the 156 image pairs used for colocalization analysis in this study. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Committee on Animal Research, University of California, San Francisco.
Labeling of geniculocortical afferents and immunofluorescence
Iontophoretic injections of the neuronal tracer Phaseolus vulgaris leucoagglutinin (PHA-L; Gerfen and Sawchenko, 1984) were made into lamina A of the LGN of P28 kittens. Detailed descriptions of this procedure have been published (Antonini and Stryker, 1993a; Silver and Stryker, 1999). The tracer was taken up by geniculate cell bodies and transported anterogradely over a 12-day period to geniculocortical axons in layer IV of primary visual cortex. On P40, animals were deeply anesthetized with an intraperitoneal injection of pentobarbital (100 mg/kg). They were then perfused transcardially, and tissue blocks containing the LGNs and visual cortex were sectioned coronally as previously described (Silver and Stryker, 1999).
Most of the primary visual cortical sections were incubated in a blocking solution containing 0.1 M sodium phosphate with 0.9% sodium chloride (phosphate-buffered saline, PBS, pH 7.4), 2% bovine serum albumin (Sigma, St. Louis, MO), 20% normal donkey serum (Sigma), 5% sucrose, 0.5% Triton X-100, and 0.05% thimerosal (Sigma). The blocking solution for a minority of sections contained 20 mM potassium PBS (KPBS, pH 7.4), 2.5% BSA, 0.5% Triton X-100, 3% normal horse serum (Vector, Burlingame, CA), and 0.05% thimerosal. After a 1-hour incubation at room temperature, sections were transferred to blocking solution containing goat IgG anti-PHA-L antibody (Vector; dilution of 1:500) and one of the following primary antibodies (Fig. 1): rabbit IgG anti-TrkB23 (Yan et al., 1994; 6.2 μg/ml), rabbit IgG anti-TrkB146 (Cabelli et al., 1996; 5.5 μg/ml), rabbit IgG anti-TrkB348 (McCarty and Feinstein, 1998; 6.5 μg/ml), rabbit IgG anti-TrkB606 (Costantini et al., 1999; 6.7 μg/ml), rabbit IgG RTB (Huang et al., 1999a; provided by Dr. Louis Reichardt; dilution of 1:100), or mouse IgG monoclonal anti-GAD65 (Chang and Gottlieb, 1988; dilution of 1:5). TrkB23, TrkB146, TrkB348, and TrkB606 antibodies were kindly provided by Drs. Monte Radeke and Stuart Feinstein. The anti-GAD65 antibodies in a GAD-6 hybridoma supernatant were obtained from the Developmental Studies Hybridoma Bank, Departments of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and Biological Sciences, University of Iowa, Iowa City, IA, under contract N01-HD-6-2915 from the NICHD.
Fig. 1.
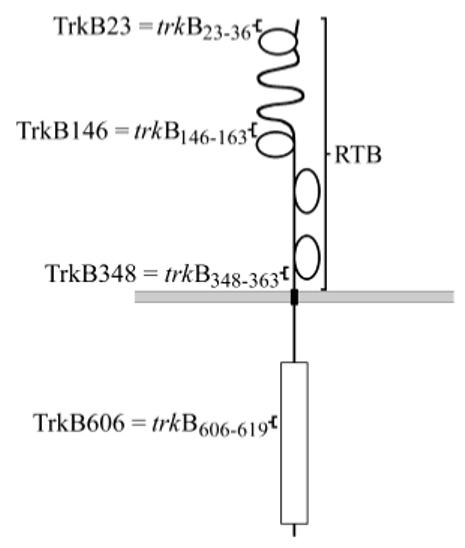
Anti-TrkB antibodies shown on schematic TrkB receptor. The RTB antibody was raised against the biochemically purified extracellular domain of rat TrkB after heterologous expression in COS-7 cells and was used as an antiserum. The other anti-TrkB antibodies were generated by immunization with synthetic peptides corresponding to specific domains of the rat TrkB amino acid sequence and were affinity purified by using the same peptide. The TrkB606 antibody should recognize only the tyrosine kinase-containing full-length isoform, whereas the others should recognize both full-length and truncated isoforms.
Sections of LGN were treated as above except the primary antibody solution consisted of mouse IgG monoclonal anti-microtubule-associated protein 2 (MAP2, Huber and Matus, 1984; Sigma; dilution of 1:500) and one of the anti-TrkB antibodies. All sections were incubated in primary antibody solutions for 48 hours at 4°C, washed three times for 10 minutes each in PBS or KPBS, and transferred to blocking solution containing two of the following secondary antibodies: Cy3-conjugated rabbit anti-goat IgG (for anti-PHA-L primary antibody, Jackson, West Grove, PA; dilution of 1:100), Cy3-conjugated donkey anti-mouse IgG (for anti-MAP2 antibody, Jackson; dilution of 1:100), Cy5-conjugated donkey anti-rabbit IgG (for anti-TrkB primary antibodies, Jackson; dilution of 1:100), or biotinylated horse anti-mouse IgG (for anti-GAD65 primary antibody, Vector; dilution of 1:200). For sections in which one of the secondary antibodies would recognize the other (for example, donkey anti-rabbit IgG and rabbit anti-goat IgG), the sections were sequentially labeled with two consecutive overnight incubations of one secondary antibody each separated by three washes for 10 minutes each in PBS or KPBS.
After an overnight incubation at 4°C, sections labeled with only Cy3- or Cy5-conjugated antibodies were washed three times for 10 minutes each in PBS and then mounted on gelatinized microscope slides from tap water. Sections labeled with biotinylated secondary antibody were washed three times for 10 minutes each in KPBS and then incubated overnight at 4°C in KPBS containing Cy5-conjugated streptavidin (Jackson; dilution of 1:100) and 0.05% thimerosal. After a final series of three washes for 10 minutes each in KPBS, these sections were mounted as described above. The mounting medium consisted of 5% n-propyl gallate (Sigma) and 10% (v/v) 0.1M PBS in glycerol. Coverslips were sealed with clear nail polish. For each antibody, parallel control experiments were performed in which the primary antibody was omitted. In all cases, there was no detectable signal due to the binding of secondary antibodies or streptavidin directly to the tissue (data not shown).
Colocalization analysis
To measure colocalization of TrkB-like IR or GAD65 label with PHA-L-labeled afferents, images were collected and processed as described in Silver and Stryker (2000). All image processing was performed on a Macintosh computer running the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/). Stacks of optical sections separated by 1 μm were obtained with an MRC600 or MRC1024 confocal microscope (Bio-Rad, Hercules, CA) by using aperture sizes of 1 or 2.16 mm. Pixel- or object-based colocalization analyses were carried out by using a customized version of NIH Image that is freely available for public use at http://phy.ucsf.edu/∼idl/colocalization.htm .
The colocalization procedures have been previously described (Silver and Stryker, 2000) and will only be summarized here. One optical section was chosen for colocalization measurements and was designated the reference section. Because the objects of interest were geniculocortical axons located in the neuropil, all pixels located within cell bodies or blood vessels were masked and removed from further analysis. Portions of PHA-L-labeled axons located within the reference section were manually traced by comparing the reference section to the optical sections immediately above and below. TrkB and GAD65 images were thresholded so that the brightest 2% of the neuropil pixels were retained and all others were set to 0. For pixel-based analysis, the thresholded TrkB or GAD65 image was simply overlaid with the traced PHA-L-labeled axon segments, and the intensities of the colocalized pixels were summed.
For object-based analysis, the pixel intensity values of the optical sections immediately above and below the reference section were reassigned so that the histogram of pixel intensity values matched that of the reference section. The rank order of pixel intensity values was not altered. Individual TrkB- or GAD65-positive puncta were segmented from each other in the xy plane of the reference section, and the location of puncta along the z-axis was determined by comparing the mean pixel intensity of the segmented puncta in the reference section with corresponding pixels in the optical sections immediately above and below.
RESULTS
TrkB-like immunoreactivity is present in LGN cell bodies of P40 kitten
Sections of kitten LGN were double labeled with anti-MAP2 antibodies and one of a panel of anti-TrkB antibodies (Fig. 1). The specificity of the anti-TrkB antibodies used in this study has been extensively documented by the laboratories that provided us with the antibodies, in several species and by using a variety of techniques (TrkB23: Cabelli et al., 1996; Fryer et al., 1996; Yan et al., 1997; Costantini et al., 1999; TrkB146: Cabelli et al., 1996; TrkB348: McCarty and Feinstein, 1998; TrkB606: Costantini et al., 1999; RTB: Huang et al., 1999a). Although the MAP2 protein is primarily found in dendrites, the anti-MAP2 antibody faintly labels neuronal somata as well (Fig. 2; see also Huber and Matus, 1984). The four anti-TrkB antibodies tested all showed punctate label in cell bodies of neurons in lamina A of the LGN (Fig. 2). TrkB-like IR has been previously observed in LGN neuronal somata in developing and adult ferret (Cabelli et al., 1996) and adult rat (Yan et al., 1997). TrkB-like IR was also observed in the nucleus. The nuclear label is probably artifact, because Western blotting of subcellular fractions of adult rat cerebral cortex demonstrated that the nuclear fraction contains proteins recognized both by an antiserum raised against the extracellular domain of TrkB and an antiserum raised against the intracellular domain, suggesting that these nuclear proteins are immunologically similar to TrkB (Aoki et al., 2000). However, they are distinct from TrkB, as they have a different molecular weight from the known TrkB isoforms, and, unlike TrkB, they were not found in subcellular fractions of synaptic membrane or postsynaptic density (Aoki et al., 2000).
Fig. 2.
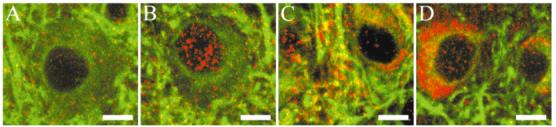
TrkB-like immunoreactivity (IR) is present on cell bodies in the lateral geniculate nucleus. False-color double-label immunofluorescent images are shown for TrkB23 (A), TrkB146 (B), TrkB348 (C), and TrkB606 (D). Red is TrkB-like IR, and green indicates MAP2-like IR, which strongly labels dendrites but also allows the visualization of the cytoplasmic region of cell bodies as diffuse label surrounding unlabeled cell nuclei. For all anti-TrkB antibodies, the label is primarily punctate in neuronal cell bodies and neuropil. Nuclear labeling is probably artifactual (see text). Scale bars = 10 μm in A-D.
Visual inspection of images is insufficient to determine whether TrkB is colocalized with geniculocortical afferents
A panel of anti-TrkB antibodies was used to establish whether TrkB-like IR is present on geniculocortical afferents in layer IV of kitten primary visual cortex. Geniculocortical axons were anterogradely labeled with iontophoretic injections of PHA-L into lamina A of the LGN. After anterograde transport of the tracer, PHA-L-containing afferents were labeled with an anti-PHA-L antibody. Double-label immunofluorescence and confocal microscopy were used to obtain thin optical sections from layer IV of visual cortex. The amount of colocalization of PHA-L-labeled afferents with the GAD65 protein was used as a negative control. GAD65 is an isoform of glutamic acid decarboxylase and is found primarily in presynaptic terminals of inhibitory intracortical neurons (Esclapez et al., 1994). Therefore, it is not colocalized with the excitatory geniculocortical afferents (Silver and Stryker, 1999).
Qualitative visual examination of double-labeled confocal images of layer IV of kitten visual cortex revealed that there was substantial punctate TrkB-like IR in the neuropil (Fig. 3A-E) and in cell somata (data not shown). This localization is in agreement with previous studies of developing and adult ferret visual cortex (Cabelli et al., 1996) and adult rat cortex (Yan et al., 1997). Additionally, in rat hippocampal neuropil, TrkB-positive puncta corresponding to vesicular compartments have been reported (Drake et al., 1999).
Fig. 3.
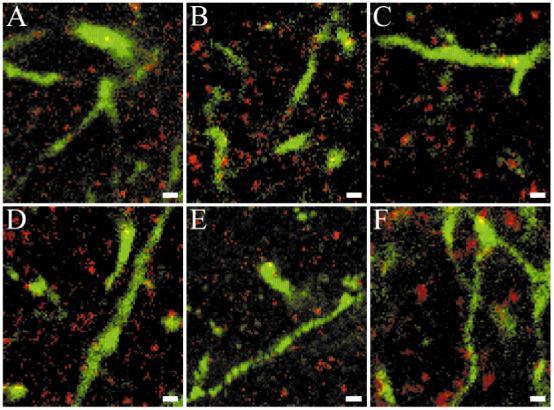
Visual image inspection is inadequate to determine whether TrkB-like immunoreactivity is colocalized with geniculocortical afferents. False-color double-label immunofluorescent confocal images from layer IV of kitten visual cortex are shown in red for TrkB23 (A), TrkB146 (B), TrkB348 (C), TrkB606 (D), RTB (E), and GAD65 (F) and in green for Phaseolus vulgaris leucoagglutinin (PHA-L)/-labeled geniculocortical afferent arbors (A-F). Although apparent colocalization of TrkB-positive puncta with PHA-L-labeled afferents can be observed for all antibodies, it is also present for GAD65. Scale bars = 1 μm in A-F.
Occasionally, a TrkB-positive punctate structure appeared to be colocalized with a PHA-L-labeled axon branch. However, visual inspection of the images was inadequate for establishing to what extent these apparent colocalizations were actual. Even though one of the advantages of confocal microscopy is the exclusion of signal from structures located above and below the focal plane, there is always some contribution from nearby structures and this can lead to artifactual colocalizations. In this case, the danger of artifactual colocalizations is high, because the TrkB label consists of small puncta, the PHA-L-labeled axons make up a small proportion of the neuropil, and there are high levels of punctate TrkB label throughout the neuropil surrounding the PHA-L-labeled geniculocortical afferents. Therefore, it is difficult to determine how much of the apparent colocalization observed by using visual inspection is artifact and how much (if any) is genuine. In fact, GAD65-positive puncta occasionally appeared to be colocalized with PHA-L-labeled afferents (Fig. 3F), even though there is no genuine colocalization of these two labels (Silver and Stryker, 1999). To reduce the contribution of artifact to our estimates of colocalization, we used quantitative procedures previously found adequate for colocalizing synaptic vesicle proteins and PHA-L-labeled geniculocortical afferents (Silver and Stryker, 2000).
Object-based colocalization analysis demonstrates that TrkB-like IR is present on geniculocortical afferents in layer IV of kitten primary visual cortex
Both pixel-based and object-based methods were used to measure the amounts of colocalization of TrkB or GAD65 label with geniculocortical afferents (see Materials and Methods section and Silver and Stryker, 2000). For pixel-based analysis, the thresholded TrkB or GAD65 image was overlaid on the traced PHA-L-labeled axons, and the intensities of the colocalized pixels were summed. For object-based analysis, the TrkB- or GAD65-positive puncta were considered to be individual objects and were assigned boundaries in three-dimensional space, and only those puncta that were located entirely within a traced axon segment in all three dimensions were counted as colocalized.
To determine the amount of colocalization expected based on random overlap of the two labels, colocalization analysis was performed on a PHA-L field and a TrkB or GAD65 field from an entirely different location. This is referred to as the shuffled condition, and the difference between the colocalization indices obtained from pairs of shuffled images and those from pairs of images from the same location (the experimental condition) provides an estimate of actual colocalization. The experimental conditions for all TrkB antibodies showed greater colocalization than the shuffled controls. For object-based analysis, this difference was significant (P < 0.05) for three of the five anti-TrkB antibodies, at the threshold of significance for another (TrkB606; P = 0.050), and indeterminate for the fifth (TrkB23; P = 0.10) (Fig. 4A). GAD65, which is known to be absent from geniculocortical axons, was significantly anticolocalized (P < 0.05). Pixel-based analysis was less conclusive, with colocalization indices smaller in every case than those from the object-based analysis (Fig. 4B). The statistical confidence was also smaller for five of six antibodies (Fig. 4B), demonstrating the value of an objectbased colocalization analysis that uses three-dimensional information.
Fig. 4.
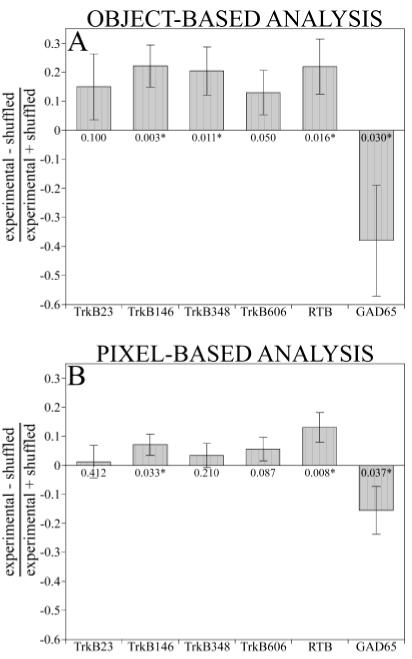
Quantitative colocalization analysis reveals the presence of TrkB-like immunoreactivity on geniculocortical afferents. The amount of colocalization of TrkB or GAD65 with the Phaseolus vulgaris leucoagglutinin-labeled afferents was computed by using either object- or pixel-based analyses and expressed as a contrast index (Silver and Stryker, 2000). Positive values of this index indicate colocalization, negative values indicate anticolocalization, and values not significantly different from 0 are indeterminate. A: Object-based analysis. B: Pixel-based analysis. Sample sizes are 18 pairs of images for TrkB606 and 12 pairs of images each for the other antibodies. Error bars represent standard errors of the difference, and P values based on one-tailed t tests are shown for each condition. Asterisks indicate statistical significance at the P < 0.05 level.
Although the results from the object-based analysis indicate significant amounts of colocalization of TrkB and geniculocortical afferents for three of five antibodies, the absence of significant differences between experimental and shuffled conditions for the other two antibodies does not suggest a lack of colocalization. The three possible outcomes of this analysis are significant colocalization, significant anticolocalization, and indeterminacy. The GAD65 antibody was significantly anticolocalized with the afferents (experimental colocalization indices were significantly lower than shuffled indices), but the results are inconclusive concerning whether the antibodies TrkB23 and TrkB606 are expressed on geniculocortical afferents.
However, this analysis is a very conservative one. The computed colocalization values are relative comparisons between the experimental and shuffled conditions and are, therefore, a function of the amount of colocalization of TrkB with labeled geniculocortical afferents as well as the overall amount of TrkB label in the cortical neuropil. For this reason, no definitive statements concerning the absolute amount of colocalization can be made. This relative measure of colocalization is necessary to remove many sources of variability that often plague quantitative immunofluorescence studies, including quality of perfusion of the tissue, amount of antibody penetration, differences in gain and black level microscope settings, and amount of tissue photobleaching (Silver and Stryker, 2000). Because there are high levels of TrkB protein expression in the cortical neuropil, the amount of colocalization expected due to random overlap (the shuffled colocalization index) is also high. Because the experimental colocalization index needs to significantly exceed the shuffled index to produce a positive colocalization result, the considerable TrkB label in the cortical neuropil biases the results toward indeterminacy while substantially decreasing the likelihood of false-positive colocalization results.
The GAD65 colocalization values provide a quantitative estimate of the amount of apparent colocalization measured when there is no genuine colocalization with the geniculocortical afferents. Because the overall pattern of GAD65 puncta resembles that obtained with the anti-TrkB antibodies (compare Fig. 3F with Fig. 3A-E), the amounts of colocalization (expressed as the difference between the experimental and shuffled colocalization indices divided by their sum, see Fig. 4) can be directly compared. For this comparison, if an anti-TrkB antibody has a colocalization value that is significantly higher than that obtained for GAD65, it is considered to be colocalized with the geniculocortical afferents. Although this analysis is not as conservative as the previous one, it is more direct, because it involves comparison with a case in which there is no colocalization (GAD65) instead of a comparison with the amount of colocalization expected due to random overlap (the shuffled condition).
The results of this analysis show that the colocalization indices obtained for the anti-TrkB antibodies by using object-based analysis were higher than the corresponding measure for GAD65 for all five antibodies tested (P < 0.05 for TrkB23, P < 0.01 for TrkB606, and P < 0.005 for the other three antibodies). Because the anti-TrkB antibodies were generated against several different portions of the protein, the fact that they produced similar colocalization results strengthens the general conclusion that TrkB is located on geniculocortical afferent arbors.
DISCUSSION
We have shown that TrkB-like IR is present in kitten LGN neuronal cell bodies and geniculocortical afferents in layer IV of primary visual cortex. Therefore, these axons have the potential to respond directly to BDNF and NT-4/5. This finding is consistent with a model of visual cortical development and plasticity in which axons compete with each other for access to a neurotrophic factor released by cortical target cells based on their activity (Maffei et al., 1992). Several other findings are generally compatible with this model, although others pose problems (Hata et al., 1999). Monocular deprivation decreases levels of BDNF mRNA (juvenile and adult rat: Bozzi et al., 1995; kitten and adult cat: Lein and Shatz, 2000) and BDNF-like IR (juvenile rat: Rossi et al., 1999) in portions of visual cortex representing the deprived eye. In addition, BDNF is released from cultured hippocampal neurons after depolarization (Goodman et al., 1996) and is present in dendrites of rat visual cortical neurons (Rossi et al., 1999). Finally, administration of BDNF to juvenile rat visual cortical slices enhances basal excitatory synaptic transmission for some layer IV neurons (Carmignoto et al., 1997). Although this potentiating effect of BDNF appears to be presynaptic, it is not known whether BDNF was acting primarily on thalamocortical or corticocortical synapses in this preparation (Carmignoto et al., 1997).
If competition occurs among geniculocortical afferents for BDNF and/or NT-4/5 released by visual cortical neurons, one might expect that levels of these neurotrophins would be different in layer IV than the other cortical layers, because layer IV is the main site of termination of these axons. Indeed, levels of BDNF mRNA (juvenile rat: Bozzi et al., 1995; adult rat: Castrén et al., 1992; kitten and adult cat: Lein et al., 2000) and protein (adult rat: Conner et al., 1997) are lower, and NT-4/5 mRNA levels are higher (adult mouse: Bozzi and Borrelli, 1999), in layer IV than in other cortical laminae.
The high levels of expression of TrkB on cortical neurons obviously suggest possible alternative actions of TrkB ligands in visual cortex. In ferret visual cortical slices, the growth of cortical dendrites can be substantially altered by exposure to BDNF or NT-4/5 (McAllister et al., 1995) or TrkB-IgG (McAllister et al., 1997), and overexpression of BDNF causes destabilization of dendritic spines in the transfected neurons (Horch et al., 1999). In addition, direct application of BDNF or NT-4/5 to rat layer V cortical neurons causes depolarization and firing of action potentials with a latency of less than 10 msec (Kafitz et al., 1999), and transgenic mice overexpressing BDNF in cortical neurons show an accelerated development of inhibition in visual cortex and a precocious critical period for visual cortical plasticity (Hanover et al., 1999; Huang et al., 1999b).
It is also possible that at least some of the effects of BDNF and NT-4/5 in visual cortex are mediated by nonvisual inputs. BDNF infusion into frontoparietal cortex of adult rats increased the sprouting of serotonergic axons within the infused area (Mamounas et al., 1995). In addition, BDNF can be retrogradely transported from rat occipital cortex to the serotonergic dorsal and medial raphe nuclei as well as the cholinergic medial septum and diagonal band of Broca (Sobreviela et al., 1996). Clearly, more experiments are required to precisely define the populations of axons and neurons that mediate the effects of TrkB ligands on cortical development and plasticity.
Four of the five anti-TrkB antibodies used in this study were raised against extracellular portions of TrkB and should, therefore, recognize both truncated and full-length forms of the receptor. However, the TrkB606 antibody should be specific for the full-length form, because it was generated by using a peptide corresponding to a portion of the intracellular region that is not present in truncated receptors (Fig. 1). Although the conservative analysis based on comparison of experimental and shuffled conditions produced a nearly significant result for colocalization of TrkB606 and geniculocortical afferents, the comparison with the GAD65 colocalization value indicated significant levels of TrkB606 IR on these axons. This finding suggests that at least some of the TrkB expressed on geniculocortical afferents in primary visual cortex of P40 kittens is the full-length form and is consistent with the possibility that TrkB ligands binding to the afferents could induce signal transduction involving TrkB tyrosine kinase activity.
ACKNOWLEDGMENTS
Michael A. Silver was a Howard Hughes Medical Institute Predoctoral Fellow. The authors thank Monte Radeke and Stuart Feinstein for providing the TrkB23, TrkB146, TrkB348, and TrkB606 antibodies and Louis Reichardt for providing the RTB antibody. In addition, M.A.S. would like to thank Antonella Antonini and Karen MacLeod for excellent surgical assistance.
Grant sponsor: National Institutes of Health; Grant number: EY02874.
LITERATURE CITED
- Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993a;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993b;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G-W, Lin S-Y, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Borrelli E. Absence of the dopamine D2 receptor leads to a decreased expression of GDNF and NT-4 mRNAs in restricted brain areas. Eur J Neurosci. 1999;11:1275–1284. doi: 10.1046/j.1460-9568.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Pizzorusso T, Cremisi F, Rossi FM, Barsacchi G, Maffei L. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Allendoerfer KL, Radeke MJ, Welcher AA, Feinstein SC, Shatz CJ. Changing patterns of expression and subcellular localization of TrkB in the developing visual system. J Neurosci. 1996;16:7965–7980. doi: 10.1523/JNEUROSCI.16-24-07965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of TrkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory transmission in rat visual cortex. J Physiol (Lond) 1997;498:153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-C, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini LC, Feinstein SC, Radeke MJ, Snyder-Keller A. Compartmental expression of TrkB receptor protein in the developing striatum. Neuroscience. 1999;89:505–513. doi: 10.1016/s0306-4522(98)00287-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length TrkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJK, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Galuske RAW, Kim D-S, Castrén E, Singer W. Differential effects of neurotrophins on ocular dominance plasticity in developing and adult cat visual cortex. Eur J Neurosci. 2000;12:3315–3330. doi: 10.1046/j.1460-9568.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Crair MC, Stryker MP. Neurotrophin-4/5 alters responses and blocks the effect of monocular deprivation in cat visual cortex during the critical period. J Neurosci. 2000;20:9174–9186. doi: 10.1523/JNEUROSCI.20-24-09174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Tsumoto T, Stryker MP. Selective pruning of more active afferents when cat visual cortex is pharmacologically inhibited. Neuron. 1999;22:375–381. doi: 10.1016/s0896-6273(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Hata Y, Ohshima M, Ichisaka S, Wakita M, Fukuda M, Tsumoto T. Brain-derived neurotrophic factor expands ocular dominance columns in visual cortex in monocularly deprived and nondeprived kittens but does not in adult cats. J Neurosci. 2000;20:RC57. doi: 10.1523/JNEUROSCI.20-03-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999a;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999b;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huber G, Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984;4:151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J Neurosci. 2000;20:1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J Comp Neurol. 2000;420:1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Feinstein SC. Activation loop tyrosines contribute varying roles to TrkB autophosphorylation and signal transduction. Oncogene. 1998;16:1691–1700. doi: 10.1038/sj.onc.1201688. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Bozzi Y, Pizzorusso T, Maffei L. Monocular deprivation decreases brain-derived neurotrophic factor immunoreactivity in the rat visual cortex. Neuroscience. 1999;90:363–368. doi: 10.1016/s0306-4522(98)00463-1. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Cowan WM. Neurotrophins and visual system plasticity. In: Jessell TM, Zipursky SL, editors. Molecular and cellular approaches to neural development. Oxford University Press; Oxford: 1997. pp. 509–524. [Google Scholar]
- Silver MA, Stryker MP. Synaptic density in geniculocortical afferents remains constant after monocular deprivation in the cat. J Neurosci. 1999;19:10829–10842. doi: 10.1523/JNEUROSCI.19-24-10829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Stryker MP. A method for measuring colocalization of presynaptic markers with anatomically labeled axons using double label immunofluorescence and confocal microscopy. J Neurosci Methods. 2000;94:205–215. doi: 10.1016/s0165-0270(99)00145-4. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Pagcatipunan M, Kroin JS, Mufson EJ. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: relationship to BDNF mRNA expressing neurons. J Comp Neurol. 1996;375:417–444. doi: 10.1002/(SICI)1096-9861(19961118)375:3<417::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with Trk receptor expression. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]


