Abstract
The responses of cells in the visual cortex to stimulation of the two eyes changes dramatically following a period of monocular visual deprivation (MD) during a critical period in early life. This phenomenon, referred to as ocular dominance (OD) plasticity, is a widespread model for understanding cortical plasticity. In this study, we designed stimulus patterns and quantification methods to analyze OD in the mouse visual cortex using optical imaging of intrinsic signals. Using periodically drifting bars restricted to the binocular portion of the visual field, we obtained cortical maps for both contralateral (C) and ipsilateral (I) eyes and computed OD maps as (C - I)/(C + I).We defined the OD index (ODI) for individual animals as the mean of the OD map. The ODI obtained from an imaging session of less than 30 min gives reliable measures of OD for both normal and monocularly deprived mice under Nembutal anesthesia. Surprisingly, urethane anesthesia, which yields excellent topographic maps, did not produce consistent OD findings. Normal Nembutal-anesthetized mice have positive ODI (0.22 ± 0.01), confirming a contralateral bias in the binocular zone. For mice monocularly deprived during the critical period, the ODI of the cortex contralateral to the deprived eye shifted negatively towards the nondeprived, ipsilateral eye (ODI after 2-day MD: 0.12 ± 0.02, 4-day: 0.03 ± 0.03, and 6- to 7-day MD: -0.01 ± 0.04). The ODI shift induced by 4-day MD appeared to be near maximal, consistent with previous findings using single-unit recordings. We have thus established optical imaging of intrinsic signals as a fast and reliable screening method to study OD plasticity in the mouse.
Keywords: Mouse, Optical imaging, Intrinsic signals, Critical period, Monocular deprivation
Introduction
Ocular dominance(OD) plasticity induced by monocular eyelid suture is one of the best studied models of cortical plasticity (Wiesel & Hubel, 1965). Previous studies from our laboratory have shown that OD plasticity is present in the mouse and follows similar rules as in higher mammals, thus establishing the mouse as a valid model system of OD plasticity (Gordon & Stryker, 1996). Benefiting from the power of mouse genetics and molecular biology techniques, studies in the mouse have provided information regarding the cellular and molecular mechanisms underling OD plasticity and identified many molecules that control the timing of the critical period (Hanover et al., 1999; Huang et al., 1999; Fagiolini & Hensch, 2000; Fagiolini et al., 2004) and that underlie the synaptic changes induced by monocular deprivation (MD) (Pham et al., 1999; Taha et al., 2002; Cancedda et al., 2003). The knowledge learned from studying mouse OD plasticity has provided important insights for understanding mammalian cortical plasticity in general (Hensch, 2004). With techniques for manipulating mouse genome become even more sophisticated, targeted disruption of specific genes can be achieved in a tissue-specific and time-specific manner. Studying OD plasticity in the mouse promises to be increasingly fruitful.
Given the large and growing pool of transgenic mice carrying mutated genes relevant to cortical plasticity, a fast and reliable screening method is needed to monitor OD plasticity in the mouse. Currently, the standard method used to study mouse OD plasticity is single-unit recording of cortical neuron action potentials (Gordon & Stryker, 1996; Cancedda et al., 2003; Fagiolini et al., 2004). It provides single-cell resolution of cortical responses and information on response properties. Consequently, it requires relatively long recording sessions to collect data from a sufficient number of neurons. In addition, single-unit recording is technically challenging. Visual-evoked potential (VEP) recording offers a faster and easier alternative, in which integrated population synaptic currents are recorded from the surface of the visual cortex in response to visual patterns modulated in space and time (Porciatti et al., 1999). VEP recording electrodes can also be implanted chronically and record from awake mice repetitively (Sawtell et al., 2003).One limitation of the VEP recording technique is that no spatial information can be obtained. Another method for measuring cortical OD is behavioral analysis of performance in a visual task (Prusky & Douglas, 2003). The necessity for animal training may limit its use, and there can be difficulties in ascribing behavior to a particular brain structure.
Kalatsky and Stryker (2003) have recently developed a fast method of intrinsic imaging to study mouse cortical retinotopy. Using a temporally periodic stimulus and Fourier analysis to extract the response at the stimulus frequency, retinotopic maps can be obtained with imaging less than 10 min (Kalatsky & Stryker, 2003). In this study, therefore, we seek to establish optical imaging of intrinsic signals as a fast and reliable method to measure OD plasticity in the mouse visual cortex. We have designed stimulus patterns and quantification methods to analyze OD in the mouse visual cortex. Our results demonstrate that the cortical responses recorded by imaging are biased towards the contralateral eye and that an imaging session of less than 30 min gives reliable measures of OD for both normal and monocularly deprived mice.
Materials and methods
Monocular deprivation (MD)
Thirty-two inbred C57Bl/6 mice were used in this study. During the procedure of MD, mice of P26-P28 were anesthetized with 2-3% isoflurane (Abbott, North Chicago, IL) in oxygen. In all cases, the right eyes were sutured shut with three mattress sutures, according to published protocols (Gordon & Stryker, 1996). Animals were checked daily to make sure that the eyes remained closed.
Surgical preparations for optical imaging
Mice were anesthetized with an intraperitoneal injection of Nembutal (50 mg/kg) or urethane (1.0 g/kg in 10% saline solution), supplemented by chlorprothixene (0.2 mg/mouse i.m). In addition, lidocaine (2% xylocaine jelly) was applied locally to all incisions. Atropine (5mg/kg in mouse) and dexamethasone (0.2 mg/mouse) were injected subcutaneously. The animals were placed in a stereotaxic apparatus, and their temperature was maintained at 37.5°C and electrocardiograph leads were attached to monitor the heart rate continuously throughout the experiment. A tracheotomy was performed. In the experiments using Nembutal, the mice were artificially ventilated with room air. In the experiments using urethane, mice breathed a mixture of O2 and room air though the trachea tube. A craniotomy was made over the visual cortex of left hemisphere, contralateral to the deprived eye in monocularly deprived mice. Low-melting point agarose (3% in saline) and a glass coverslip were placed over the exposed area. The animals were euthanized with overdose of Nembutal at the end of experiments. All experimental procedures were approved by the UCSF Committee on Animal Research.
Optical imaging and visual stimuli
The mouse visual cortical responses were recorded using the imaging method recently developed in our laboratory (Kalatsky & Stryker, 2003). In this method, a temporally periodic stimulus is continuously presented to the animal, and the cortical response at the stimulus frequency is extracted by Fourier analysis.
Optical images of cortical intrinsic signal were obtained using Dalsa 1M30 CCD camera (Dalsa, Waterloo, Canada) controlled by custom software. Using a 135 × 50 mm tandem lens (Nikon, Inc., Melville, NY) configuration, we imaged a cortical area of 4.6 × 4.6 mm2. The surface vascular pattern and intrinsic signal images were visualized with illumination wavelengths set by a green (546 ± 10 nm) or red (610 ± 10 nm) interference filter, respectively. After acquisition of a surface image, the camera was focused 400-600 μm below the pial surface. An additional red filter was interposed between the brain and the CCD camera, and intrinsic signal images were acquired. Frames were acquired at the rate of 30 fps and then binned by 4 frames temporally, reducing sampling rate for the stored images to 7.5 Hz. The frames were stored as 512 × 512 pixel images after binning the 1024 × 1024 camera pixels by 2 × 2 pixels spatially.
A high refresh rate monitor (Nokia Multigraph 445X, 1024 × 768 @ 120 Hz) was placed in front of the animal to display visual stimuli. The monitor was 25 cm away from the mouse, with its midline aligned to the animal. Drifting thin bars (2 deg) were generated by a Matrox G450 board (Matrox Graphics, Inc., Quebec, Canada), controlled by custom software and displayed on the stimulus monitor. The spatial frequency of the drifting bar was 1 cycle/80 deg, and temporal frequency 1 cycle/8 s or 1 cycle/6 s. In a typical experiment, the stimuli were shown across the full screen first to obtain cortical retinotopic maps for the contralateral eye (See Fig. 1B for the iso-azimuth and iso-elevation lines), and then the bars were shown only within the binocular visual field to assess the ocular dominance (See Results). Eye position and the alignment of the two eyes are known to be stable under our recording conditions (Gordon & Stryker, 1996). The validity of the measured eye positions was confirmed by the fact that the border between V1 and V2 had a value of 0 azimuth.
Fig. 1.
Visual stimulus paradigm for imaging ocular dominance of cortical responses. (A) Monitor placement and stimulus patterns used to stimulate both ipsilateral and contralateral eyes for imaging OD. The monitor (40 cm × 30 cm) was placed 25 cm in front of the mouse, with its midline (defined as 0 deg, marked by a cross) aligned to the midline of the animal. Drifting thin bars (2 deg wide, spatial frequency of 1 cycle/80 deg, and temporal frequency of 1 cycle/6-8 s) were displayed in the binocular visual field (marked by dotted lines, -5 deg to 15 deg on the monitor) to assess OD. (B) Iso-azimuth (Solid lines) and iso-elevation (dashed lines) obtained from full-screen stimulation through the contralateral eye. The spacing of the contour lines is 2.5 deg. The scale bar applies to C and D as well. (C) Cortical response magnitude to the restricted stimuli through the ipsilateral eye (left eye). (D) Response through the contralateral eye. The gray scale shown to the right is used to visualize the response magnitude as fractional change in reflection ×104.
Data analysis
Detailed procedures for quantifying ocular dominance in the mouse visual cortex are presented in the Result session.
All data are reported as means ± SEM. Significance was determined using two-tailed paired t-test for comparison between two groups. For multigroup comparison, one-way ANOVA and Bonferroni posttest (for those data where the P value of the ANOVA was <0.05) were performed to determine significance level.
Results
The purpose of this study was to establish optical imaging of intrinsic imaging as a fast and reliable tool to probe visual cortical plasticity in the mouse. Fast optical imaging of the intrinsic signal of cortical activity has recently been demonstrated with a protocol using temporally periodic stimulation and Fourier analysis to extract the response at the stimulus frequency, resulting in high resolution retinotopic maps from less than 10 min of imaging data (Kalatsky & Stryker, 2003). In the following sections, we first describe the stimulus patterns and quantification methods used to measure OD plasticity in the mouse using this imaging method, and then present the time course of monocular deprivation of cortical responses during the critical period of visual development.
Stimulus patterns and quantification of ocular dominance using optical imaging
A major concern of using imaging to measure response magnitude in small cortical areas is a possible contamination of results by the spread of optical signals. We therefore reasoned that a full-field stimulus is not appropriate to quantify OD in the mouse, because the eye contralateral to the cortex being imaged sees more of the visual field than the ipsilateral eye. Indeed, previous studies with single-unit recording found that cells with receptive fields (RFs) outside the central-most 25 deg were much less likely to be driven well by the ipsilateral eye (Gordon & Stryker, 1996). As a result, the stimulus outside the binocular visual field would evoke cortical responses only through the contralateral eye. Such responses might spread into the binocular region of the visual cortex and bias the OD score by overestimating the contralateral eye response. Furthermore, Gordon and Stryker have also shown that brief MD induced the largest shift of the contralateral bias index (CBI) for cells with RFs closest to the vertical meridian (Gordon & Stryker, 1996). Therefore, to measure OD plasticity induced by MD accurately with optical imaging, we further restricted our visual stimulus to be close to the vertical meridian representation in the contralateral visual field, consistent with the procedure used to assess OD in microelectrode studies.
To this end, we placed the stimulus monitor 25 cm in front of the mouse, with its vertical midline aligned to the animal’s head and thus to the vertical meridian of the visual field (Figs. 1A & 1B). Because we always imaged the left visual cortex, the stimulus, consisting of moving bars, was shown between -5 deg to 15 deg azimuth (the vertical meridian defined as 0 deg with negative values for left visual field) and full-screen elevations from +35 deg to -35 deg (Fig. 1A). Following the method used for imaging retinotopy in the mouse (Kalatsky & Stryker, 2003), thin bars 2 deg wide moved continuously and periodically upward or downward (Spatial frequency of 1/80 deg, and temporal frequency 1 cycle/6-8 s). The phase and amplitude of cortical responses at the stimulus frequency were extracted by Fourier analysis after eliminating slow drifts in the optical signal with a duration of more than one cycle. With maps evoked by drifting bars moving upward and downward, we computed the map of absolute retinotopy of elevation using the method of stimulus reversal (Kalatsky & Stryker, 2003). The map of response magnitude was obtained by averaging the response amplitudes of individual pixels from maps to upward and downward moving bars.
To minimize the effect of the animal’s changing depth of anesthesia on scoring OD, we employed the following measures. First, we shortened the duration of each run (for one eye to one direction stimulus) to about 5 min (300-320 s). Second, we arranged the sequence of one imaging session such that the first and fourth runs were for the same eye (two directions, upward and downward), and second and third for the other eye. This way, one session of measuring OD was less than 30 min, and the average of first and last maps was compared to that of the second and third. As an example, the maps of absolute phase and average magnitude to the ipsilateral and contralateral eye of a normal adult mouse are shown in Fig. 1. The maximum response magnitude to the ipsilateral eye stimulation (Shown in fraction change of reflectance) is about 1.45×10-4, much weaker than that to the contralateral eye (2.44×10-4, Figs. 1C & 1D), as has been described electrophysiologically.
To quantify OD from the optically recorded responses, we used the response magnitude map evoked by stimulation of the ipsilateral eye as the reference to select the binocularly responsive region of interest within the primary visual cortex. First, the ipsilateral eye magnitude map was smoothed to reduce pixel shot noise by low-pass filtering using a uniform kernel of 5×5 pixels. The smoothed map was then thresholded (At 30-40% of peak response amplitude) to eliminate the background noise and to delineate the region that produced the strongest response to the ipsilateral eye (Figs. 2A & 2B). We then computed ocular dominance score, (C - I)/(C + I), for every pixel in this region, where C and I representing the raw response magnitude of each pixel to the contralateral and ipsilateral eyes, respectively. A two-dimensional map of the OD score is shown in Fig. 2C. Finally, the ocular dominance index (ODI) was calculated as the average of (C - I)/(C + I) for all responsive pixels. Consequently, the ODI ranges from -1 to 1, where a positive ODI indicates a contralateral bias, and a negative ODI an ipsilateral bias. This index is related to the contralateral bias index (CBI) used elsewhere by CBI = (ODI + 1)/2. For example, in the 2-D map of OD shown in Fig. 2C, most pixels have values greater than zero (Fig. 2D) and the ODI is 0.29, confirming a contralateral bias in the binocular zone of the visual cortex in the normal mouse. In experiments where more than one session of four runs was performed, the ODI was calculated for each session and the values were averaged.
Fig. 2.
Quantification of cortical responses to determine OD. (A) Responsive area of the ipsilateral eye magnitude map shown in Fig. 1. The area was selected by smoothing the ipsilateral eye map with a low-pass filter and then thresholding it at 30% of peak response amplitude. The nonsmoothed magnitude of the pixels within the responsive area is shown (gray scale shown to the right). (B) Response magnitude map of the contralateral eye of the same responsive area as in A shown in the same gray scale. (C) OD map in the region of interest. The OD score was calculated as (C - I)/(C + I) for every responsive pixel and plotted in gray scale, with C and I representing the non-smoothed response magnitudes of each pixel from the contralateral and ipsilateral eye maps, respectively. (D) Histogram of the OD scores for all responsive pixels. The average of (C - I)/(C + I) for all pixels is 0.29, confirming a strong contralateral bias in the binocular zone of the visual cortex in this adult mouse. The scale bar applies to all the panels showing maps (A, B, & C).
Imaging mouse ocular dominance plasticity during the critical period
We used monocular eyelid suture to induce plasticity in the contralateral visual cortex starting from P26-P28, the peak of critical period of the mouse (Gordon & Stryker, 1996). This hemisphere was chosen in most previous microelectrode studies as most reliable for assaying OD plasticity on account of the strong contralateral bias of normal cortex. Microelectrode studies had revealed effects of deprivation in both hemispheres, but the loss of ipsilateral eye responses was much harder to measure with consistency because they were relatively weak to begin with (Gordon & Stryker, 1996). For consistency, we also chose this hemisphere for our imaging studies. Our imaging and quantification method reveals that normal mice during the critical period (P19-P32) have ODIs similar to adult mice (Figs.3 & 4A; ODI: 0.22 ± 0.01, n = 6), confirming the results of previous electrophysiological studies. Furthermore, in mice deprived for a brief period of time during the critical period, the ODI of the cortex contralateral to the deprived eye shifted negatively, that is, the cortical responses shifted to favor the nondeprived, ipsilateral eye (Figs.3 & 4A). For the two examples shown in Fig. 3, the 2-D maps of OD scores show fine structures of local ocular dominance (Figs. 3B & 3E). Such local variations in OD across the binocular zone were observed in many animals, but the patterns were not consistent among different animals or even between runs in the same animal.
Fig. 3.
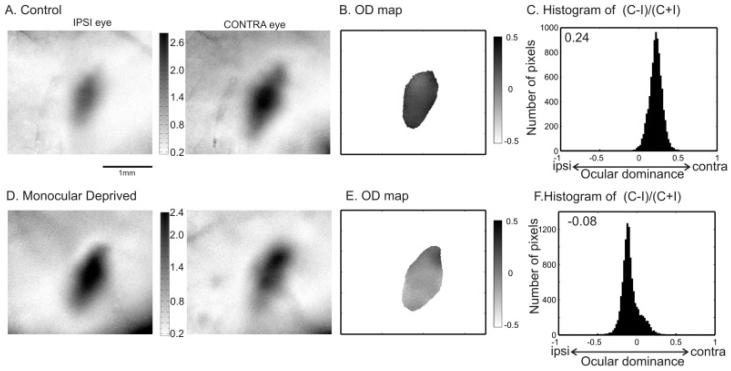
Imaging OD plasticity during the critical period. The map of response magnitudes to the ipsilateral and contralateral eyes are shown for a control mouse (P30) in A and for a monocularly deprived mouse (MD from P28 to P35) in D. The 2-D OD maps of the two mice are computed and plotted in B and E, respectively. Figs. C and F show the histograms of the OD scores obtained from nonsmoothed maps. The ODI is 0.24 for the control mouse, indicating a strong contralateral bias; -0.08 for the MD mouse, showing a slight bias towards the nondeprived, ipsilateral eye. The scale bar applies to all the panels showing maps (A, B, D, & E).
Fig. 4.
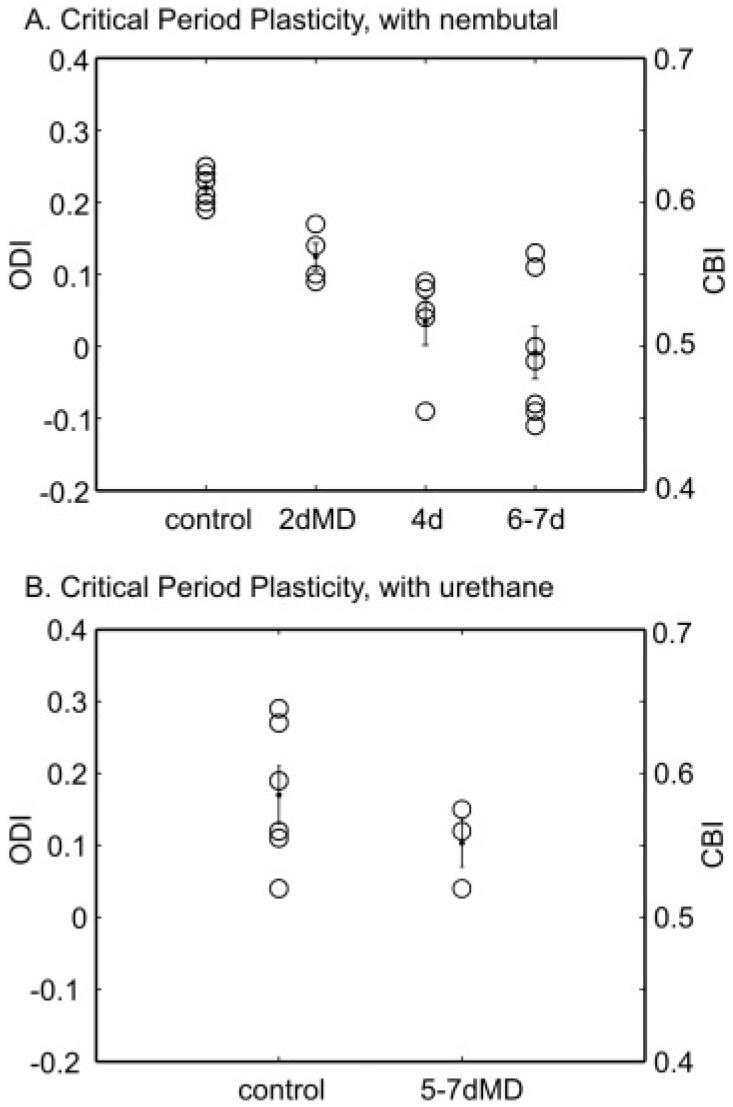
Effect of MD duration on OD shift revealed by imaging. (A) The ODI of each animal (open circle) is plotted against the duration of MD which started between P26 and P28. The mean and SEM of each group are also shown (dots).The ODI is related to the CBI used for analyzing single unit data by CBI = (ODI + 1)/2, so the corresponding CBI is also shown as the right axis for this and the following figures. Four-day MD induced a significant shift in ODI towards to the nondeprived eye (Control: 0.22 ± 0.01 vs. 4-day: 0.03 ± 0.03, P value < 0.05). The shift caused by 4-day MD is not significantly different from that by deprivation longer than 6 days (-0.01 ± 0.04, P > 0.05). In addition, 2-day MD did not induce a significant shift of the ODI (2-day: 0.12 ± 0.02, P > 0.05). (B) Same plot for results obtained from experiments using urethane as anesthesia. The ODI calculated from the imaging data is not significantly different between the control (0.17 ± 0.04, n = 6) and MD group (0.10 ± 0.03, n = 3; P value = 0.32).
In summary, a 4-day MD induced a significant shift in ODI towards the nondeprived eye compared to the control mice (Control: 0.22 ± 0.01 vs. 4-day: 0.03 ± 0.03, P value < 0.05). The shift caused by 4-day MD appeared to be nearly saturating, because it was not significantly different from that by 6- to 7-day deprivation (ODI: -0.01 ± 0.04, P > 0.05). In addition, 2-day MD did not induce a significant shift of the ODI (0.12 ± 0.02, P > 0.05 compared to control animals). All these results are consistent with previous electrophysiological data (Gordon & Stryker, 1996). Thus, optical imaging can be used as a reliable tool to monitor cortical plasticity in the mouse visual cortex.
Because mice anesthetized by urethane are more stable than those by Nembutal and therefore provide better optical maps of visual topography (Kalatsky & Stryker, 2003), we also examined OD in urethane-anesthetized mice. To our surprise, the ODIs of nondeprived mice anesthetized by urethane (0.17 ± 0.04, n = 6) were much more scattered than those of Nembutal-anesthetized mice (Fig. 4B). Furthermore, imaging of the urethane-anesthetized mice did not reveal consistent ODI shifts after 5-7 days of MD (0.10 ± 0.03, n = 3; P value = 0.32, t-test). Since it is known that single-unit recording studies (Gianfranceschi et al., 2003) and pattern VEP recordings (Huang et al., 1999; Sawtell et al., 2003) in urethane-anesthetized mice reveal OD plasticity like that using Nembutal anesthesia, the difference between the two anesthetics in the plasticity imaging assay is likely to result from differences in hemodynamic mobilization, which influences the response magnitude recorded by intrinsic imaging.
Finally, we compared the relative responses to stimulation of the two eyes using the restricted stimuli above with those evoked by full-field stimuli. In the same cortical areas of same animals, the ODIs in response to full-screen stimuli were much more strongly biased to the contralateral eye (0.24 ± 0.07, vs. restricted stimuli: 0.08 ± 0.11, n = 5), confirming our rationale for limiting the stimulation for ocular dominance measurements to the binocular visual field.
Discussion
In this study, we have designed stimulus patterns and quantification methods for analyzing OD in the mouse visual cortex. The ODI obtained from an imaging session of less than 30 min gives reliable measures of OD for both normal and monocularly deprived mice, consistent with the results of previous studies using single-unit recording.
Current methods used to study mouse OD plasticity include single-unit recording (Gordon & Stryker, 1996; Fagiolini et al., 2004), pattern VEP recording (Porciatti et al., 1999), and behavior analysis (Prusky & Douglas, 2003). Compared to these methods, our imaging method offers many advantages. First, the new imaging paradigm developed by Kalatsky and Stryker (2003) has increased spatial resolution and reduced the experimental time tremendously. These advantages persist when we apply it to measuring OD. We were able to obtain high resolution of cortical maps using spatially restricted stimuli with minutes of imaging. The ODI computed from response magnitudes of optical maps provides a good indicator of OD in Nembutal-anesthetized mice. Second, the imaging method is much less invasive compared to electrophysiology recordings. In fact, cortical responses can be imaged through intact skull (Kalatsky & Stryker, 2003), thus the damage to the cortical circuits is minimal. Consequently, it is possible to image the same mouse repetitively to follow changes of individual animals induced by MD. Such chronic recording has been achieved for VEP recording (Sawtell et al., 2003). The imaging method certainly offers much better spatial information, because VEP is only recorded at one site within the binocular zone. Lastly, simplicity of the imaging system design makes it readily accessible.
We also compared the ODI obtained by the optical imaging method to the contralateral bias index (CBI) from single-unit recordings (Fig. 4). Because the CBI is essentially the ratio of contralateral eye response (C) to the sum of contralateral and ipsilateral (I) responses, the CBI is related to the ODI by CBI = (ODI + 1)/2. The contralateral bias calculated by the imaging method is smaller than the single-unit data, in which the CBI is around 0.7 for normal mice in the critical period (Gordon & Stryker, 1996), corresponding to ODI = 0.4 (Compared to ODI = 0.22 we obtained for imaging). Thus the shift towards to the deprived ipsilateral eye is also smaller (Single unit CBI: from 0.7 to about 0.45, compare to Fig. 4A). The difference may be caused by the different nature of signals acquired by the two methods. Imaging of intrinsic signals measures changes in cortical light reflectance following local neural activation (Grinvald et al., 1986), presumably including both synaptic and spiking activities. A recent observation by Yazaki-Sugiyama and Hensch supports this hypothesis. They recorded intracellularly from layer II/III cells from the binocular zone of mouse visual cortex and compared the subthreshold responses with suprathreshold spikes of individual cells. They reported that the integrated subthreshold response was less selective for OD than the corresponding suprathreshold spike discharge (Yazaki-Sugiyama & Hensch, SFN abstract, 2004), consistent with the smaller ODI obtained here by optical imaging.
The variability of ocular dominance among normal animals in relation to the size of the OD shift is slightly greater with imaging (16%) than with microelectrode recording of samples of 30 neurons per animals (12%, Gordon & Stryker, 1996), but the imaging takes much less time. We expect that the noninvasive nature of optical imaging will in the future allow routine chronic imaging of ocular dominance, and these repeated measurements should further reduce variability.
In summary, we have established optical imaging of intrinsic signals as a fast and reliable screening method to probe OD plasticity in the mouse. It thus has the potential to greatly accelerate the studies using transgenic mice to investigate the molecular and cellular mechanisms underlying ocular dominance plasticity.
Acknowledgments
Jianhua Cang is an Aventis Pharmaceuticals Fellow of the Life Sciences Research Foundation. Valery Kalatsky was a Sloan-Swartz Fellow in Theoretical Neuroscience. This work was supported by grants from the NIH to M.P. Stryker.
References
- Cancedda L, Putignano E, Impey S, Maffei L, Ratto GM, Pizzorusso T. Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK. Journal of Neuroscience. 2003;23:7012–7020. doi: 10.1523/JNEUROSCI.23-18-07012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proceedings of the National Academy of Sciences of the U.S.A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. Journal of Neuroscience. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. Journal of Neuroscience. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual Reviews of Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Kalatsky VA, Stryker MP. New paradigm for optical imaging: Temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- Pham TA, Impey S, Storm DR, Stryker MP. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron. 1999;22:63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Research. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. European Journal of Neuroscience. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. Journal of Neurophysiology. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Hensch TK. Program No. 155.9. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Balanced sub-threshold excitation-inhibition yields biased ocular dominance of spike selectivity in mouse visual cortex. [Google Scholar]