Abstract
We have investigated the process leading to differentiation of PC12 cells. This process is known to include extension of neurites and changes in the expression of subsets of proteins involved in cytoskeletal rearrangements or in neurosecretion. To this aim, we have studied a PC12 clone (trk-PC12) stably transfected with the nerve growth factor receptor TrkA. These cells are able to undergo both spontaneous and neurotrophin-induced morphological differentiation. However, both undifferentiated and nerve growth factor-differentiated trk-PC12 cells appear to be completely defective in the expression of proteins of the secretory apparatus, including proteins of synaptic vesicles and large dense-core granules, neurotransmitter transporters, and neurotransmitter-synthesizing enzymes. These results indicate that neurite extension can occur independently of the presence of the neurosecretory machinery, including the proteins that constitute the fusion machine, suggesting the existence of differential activation pathways for the two processes during neuronal differentiation. These findings have been confirmed in independent clones obtained from PC12-27, a previously characterized PC12 variant clone globally incompetent for regulated secretion. In contrast, the integrity of the Rab cycle appears to be necessary for neurite extension, because antisense oligonucleotides against the neurospecific isoform of Rab-guanosine diphosphate-dissociation inhibitor significantly interfere with process formation.
INTRODUCTION
Extension and remodeling of neurites are essential processes in the development and correct functioning of the nervous system that play an important role in axonal pathfinding and targeting, synapse formation and stabilization, neuronal plasticity, and axonal regeneration (Prochiantz, 1995; Tanaka and Sabry, 1995). In spite of considerable experimental effort, the molecular mechanisms underlying neurite extension are far from being clarified, although the past few years have seen significant advances in identifying relevant molecules and signaling pathways underlying the establishment of neuronal polarity (Higgins et al., 1997; Valtorta and Leoni, 1999).
A number of growth-associated proteins are increased in their expression during neurite outgrowth, and some of them are believed to be essential for the process to occur (Skene, 1989). Furthermore, neurite extension requires the addition of new membrane to the growing processes to allow for the necessary membrane expansion. This is thought to occur by incorporation of exocytic vesicles into the plasma membrane (Futerman and Banker, 1996). It is not clear whether these vesicles belong to the so-called regulated pathway of secretion or to the constitutive pathway, which operates in all cell types. Developing neurons in culture exhibit high rates of exocytosis and endocytosis of synaptic vesicles (or precursors thereof) (Matteoli et al., 1992). However, the protein components of these organelles are normally not found on the plasma membrane, suggesting either that sorting and selective retrieval occur after exocytosis or that other organelles are responsible for the addition of new membrane to the growing neurite. Indeed, experiments carried out with in vitro preparations of growth cones have identified the existence of a population of large plasmalemma precursor vesicles that might be responsible for membrane expansion (Pfenninger and Friedman,1993). The possibility that at least the phospholipid components of the axonal plasma membrane are locally synthesized has also been raised (Posse de Chaves et al., 1995).
The observation that target membrane-soluble N-ethylmaleimide-sensitive factor-associated protein receptors (t-SNAREs) are required for axonal outgrowth both in cell culture and in vivo (Osen-Sand et al., 1993, 1996; Igarashi et al., 1996) suggests that, whatever the nature of the organelles involved, the mechanism of fusion with the plasma membrane is similar to that observed for the fusion of synaptic vesicles in mature nerve terminals. In addition, neurite extension has been shown to require the integrity of the Rab cycle, because suppressing the expression of Rab-guanosine diphosphate-dissociation inhibitor α (Rab-GDI α), a regulator of Rab function (Pfeffer et al., 1995), strongly inhibits process formation and axonal stabilization (D’Adamo et al., 1998).
The rat pheochromocytoma cell line PC12 is a well-characterized model for the study of neuron-like differentiation and signal transduction downstream to neurotrophin receptors. Upon exposure to nerve growth factor (NGF), PC12 cells decrease their rate of proliferation, extend neurites, and express a battery of neuronal genes, including components of the neuronal cytoskeleton, voltage-gated ion channels, and neurotransmitter-synthesizing enzymes, ultimately resulting in the production of a sympathetic neuron-like phenotype (Greene and Tischler, 1976; Greene and Kaplan, 1995).
Besides representing a useful experimental paradigm for the study of neuronal differentiation, PC12 cells are also a widely utilized model for the study of neurosecretion. At least two pathways of regulated secretion exist in these cells: that of the large dense-core granules (LDCGs), which store and release catecholamines, ATP, and proteins of the granin family; and that of the small clear vesicles (synaptic-like microvesicles), which are responsible for release of acetylcholine (Shafer and Atchison, 1991). Although the former organelles are homologous to neuronal large dense-core vesicles, the latter represent the neuroendocrine counterpart of synaptic vesicles, because they contain a similar, although not identical, set of membrane proteins (Valtorta and Benfenati, 1995).
In the past few years, considerable progress has been made in the understanding of the molecular mechanisms involved in the biogenesis of secretory organelles (Bauerfeind and Huttner, 1993) and in the elucidation of the molecular basis of their docking and fusion with the plasma membrane (Hay and Scheller, 1997; Jahn and Hanson, 1998; Benfenati et al., 1999). However, the picture is far from being complete.
Furthermore, the mechanisms that underlie the development of a neurosecretory phenotype are not as yet understood. Data obtained on a variant PC12 clone, PC12-27, which is incompetent for both branches of regulated secretion, suggest that the achievement of a secretory phenotype depends on the coordinate regulation of the expression of a large set of proteins responsible for neurosecretion, as though a “master switch” existed (Corradi et al., 1996).
We have studied a PC12 clone, trk-PC12, that overexpresses the NGF receptor TrkA. In these cells, receptor overexpression causes an overall marked increase in the activation of the TrkA signal transduction cascade and in NGF-induced differentiation, judged both as neurite extension and as activation of the early and late steps of NGF signaling downstream to TrkA (Hempstead et al., 1992). The focus of our study has been to try and understand the molecular relationships and interactions between two crucial aspects of neuronal differentiation: the structural and functional rearrangements of the cytoskeleton underlying the production of a polarized cell, and the maturation of a secretory apparatus for the regulated release of neurotransmitter.
Similar to what has been observed in the case of PC12-27, in trk-PC12 cells the machinery for regulated secretion is completely absent, in spite of a potentiated response to the differentiating action of NGF. The ability of trk-PC12 cells to extend neurites in the absence of the machinery for regulated secretion gives some insight into the origin of the membrane and the molecular requirements underlying neurite outgrowth.
MATERIALS AND METHODS
Materials
The trk-PC12 cell line, developed by Hempstead et al. (1992), was a kind gift of A. Pandiella (University of Salamanca, Salamanca, Spain), and the PC12-27 clone was a gift of J. Meldolesi (University of Milan, Milan, Italy).
Anti-p38/synaptophysin rabbit polyclonal antibody was produced as described (Valtorta et al., 1988). The following antibodies were generously donated to us: anti-synapsin II mouse monoclonal and anti-Rab3a rabbit polyclonal antibodies (P. Greengard, The Rockefeller University, New York, NY); anti-synaptotagmin rabbit polyclonal antibody (M. Matteoli, Consiglio Nazionale delle Richerche Center for Cellular and Molecular Pharmacology, Milan, Italy); anti-vesicle-associated membrane protein (VAMP)-1, anti-VAMP-2, anti-synaptosome-associated protein of 25 kDa (SNAP-25), and anti-syntaxin-1 rabbit polyclonal antibodies (C. Montecucco, University of Padova, Padova, Italy); anti-chromogranin B mouse monoclonal and anti-secretogranin II rabbit polyclonal antibodies (P. Rosa, Consiglio Nazionale delle Richerche Center for Cellular and Molecular Pharmacology, Milan, Italy); anti-tyrosine hydroxylase and anti-choline acetyltransferase rabbit polyclonal antibodies (J. Meldolesi); anti-N-kinesin and anti-tau rabbit polyclonal antibodies (F. Navone, Consiglio Nazionale delle Richerche Center for Cellular and Molecular Pharmacology, Milan, Italy); anti-paxillin mouse monoclonal antibody (I. De Curtis, San Raffaele Scientific Institute, Milan, Italy); isoform-specific anti-syntaxin mouse monoclonal and rabbit polyclonal antibodies (M.K. Bennett, University of California, Berkeley, CA); and anti-p75 low-affinity neurotrophin receptor rabbit polyclonal antibody (G. Della Valle, University of Bologna, Bologna, Italy). The following antibodies were purchased from the indicated sources: anti-growth-associated protein of 43 kDa (GAP-43) and anti-tubulin mouse monoclonal antibodies (Boehringer Mannheim, Mannheim, Germany); anti-focal adhesion kinase (FAK) (2A7) mouse monoclonal antibody (Upstate Biotechnology, Lake Placid, NY); anti-Trk (C-14) and anti-MAP kinase rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Rab-GDI α rabbit polyclonal antibody (Zymed, San Francisco, CA); anti-neurofilament 200-kDa polyclonal antibody (Sigma, St. Louis, MO); peroxidase-conjugated goat anti-mouse and goat anti-rabbit antibodies (Bio-Rad, Hercules, CA); and fluorescein isothiocyanate-conjugated goat anti-mouse, Texas Red-conjugated goat anti-rabbit, and biotin-conjugated goat anti-mouse antibodies and Texas Red-conjugated streptavidin (Jackson, West Grove, PA). Bodipy-phallacydin was from Molecular Probes (Eugene, OR).
The ECL chemiluminescence detection system was from Amersham (Buckinghamshire, United Kingdom), the BCA protein assay reagent was from Pierce (Rockford, IL), and the avidin-biotin blocking kit was from Vector Laboratories (Burlingame, CA). [2,5,6-3H]Dopamine, [α-32P]dCTP, and [35S]sulfate were from Amersham. NGF (2.5 S) from mouse submaxillary glands was from Boehringer Mannheim, and botulinum neurotoxin C (BotNC) was a gift of C. Montecucco (University of Padova, Padova, Italy).
PCR-grade purified antisense oligonucleotides to GDI1, coding for Rab-GDI α (sequences 5′-CCT TGG TAC CAG CGC CCG CTC TTC and 5′-CAT GGT CAG GCC TTG GTA CCA GCG C) and the corresponding sense oligonucleotides (sequences 5′-GAA GAG CGG GCG CTG GTA CCA AGG and 5′-GCG CTG GTA CCA AGG CCT GAC CAT G), were from Applied Biosystems (Milan, Italy).
The mammalian episomal expression vector containing the TRKA cDNA (pC9TRK) was a kind gift of G. Della Valle (Bologna, Italy). Briefly, it was constructed by inserting the full-length human TRKA cDNA, isolated by EcoRI digestion from pLM6 plasmid (Martin-Zanca et al., 1989), into pCEP9β under control of the cytomegalovirus promoter. pIRES-1hyg was from Clontech (Palo Alto, CA).
All other chemicals were of the highest grade available.
Cell Cultures and Neurite Extension Experiments
Cells were grown on plastic dishes at 37°C in a 5% CO2 humidified atmosphere in DMEM (Biowhittaker, Verviers, Belgium) supplemented with 10% fetal calf serum, 5% horse serum (Hyclone, Logan, UT), and 100 U/ml penicillin/streptomycin (Biowhittaker). For the immunofluorescence studies, cells were plated on poly-l-ornithine (10 μg/ml)-treated coverslips and cultured in the same medium. Where indicated, cells were treated with medium supplemented with NGF (50 ng/ml) and resupplemented every other day.
For neurite extension experiments, cells were viewed with a phase-contrast microscope and photographed every 12 h.
For the quantitative analysis of neurite extension, phase-contrast photographs of at least six fields of each sample were taken every 24 h and acquired with an HP ScanJet 6100C scanner (Hewlett-Packard, Palo Alto, CA). To measure the length of the processes, the public domain image analysis program NIH Image was used (developed at the U.S. National Institutes of Health, Bethesda, MD, and available at http://rsb.nih.gov/nih-image), with substantial modifications. The total length of neurites per cell was determined by measuring all the processes present in a field, normalized by the number of cell bodies. The data were then statistically analyzed using Student’s t test.
Immunoblot Analysis
Cells were solubilized by scraping with solubilization buffer (1% SDS, 2 mM EDTA, 10 mM HEPES-Na, pH 7.4) and immediately frozen in liquid nitrogen. After thawing, lysates were boiled for 3 min and sonicated. Equal amounts of proteins were subjected to SDS-PAGE (Laemmli, 1970) and transferred to nitrocellulose as previously described (Towbin et al., 1979).
Filters were then blocked for 1 h at room temperature with 5% nonfat dry milk in Tris-buffered saline (50 mM Tris-HCl, pH 7.4, 200 mM NaCl) and incubated for 2 h with the primary antibody at the appropriate concentration, washed five times for 5 min with Tris-buffered saline supplemented with a detergent (either 0.1% Triton X-100 or 0.1% Tween 20) and incubated for 1 h with the appropriate peroxidase-conjugated secondary antibody (1:10,000), washed five times for 5 min with Tris-buffered saline supplemented with the same detergent, and finally developed by chemiluminescence. After exposure to x-ray films, filters were occasionally subjected to stripping and reprobing, according to the manufacturer’s instructions.
RNA Extraction and Northern Blot Analysis
RNA extraction was performed according to the single-step method (Chomczynski and Sacchi, 1987). For Northern blotting, 10–20 μg of total RNA were separated on 1% agarose, 2.2 M formaldehyde denaturing gels and transferred onto Hybond-N nylon membranes (Amersham).
Probes were a kind gift of B. Borgonovo (San Raffaele Scientific Institute, Milan, Italy) and were prepared from reverse transcriptase-PCR fragments as previously described (Corradi et al., 1996), labeled with [α-32P]dCTP (3000 Ci/mmol) by random-priming oligolabeling (Feinberg and Vogelstein, 1984). Hybridizations were carried out at 65°C in hybridization buffer (125 mM Na2HPO4, 1 mM EDTA, 250 mM NaCl, 7% SDS, 10% polyethylene glycol, 1% BSA) supplemented with 100 μg/ml denatured salmon sperm DNA and 106 cpm/ml radiolabeled probe. Filters were then washed twice for 20 min in 2× SSC (300 mM NaCl, 30 mM sodium citrate) and 0.1% SDS, once for 20 min in 1× SSC and 0.1% SDS, and twice for 20 min in 0.1× SSC and 0.1% SDS and finally exposed for x-ray autoradiography.
Immunofluorescence Analysis
Cells were fixed for 20 min at room temperature in 4% formaldehyde (freshly prepared from paraformaldehyde) dissolved in 120 mM sodium phosphate buffer, pH 7.4, and 4% sucrose. After washing three times for 10 min in PBS (180 mM NaCl, 10 mM sodium phosphate buffer, pH 7.4), cells were incubated for 2 h with the primary antibody at the appropriate concentration in goat serum dilution buffer (450 mM NaCl, 20 mM sodium phosphate buffer, pH 7.4, 15% goat serum, 0.3% Triton X-100), washed three times in a high-salt buffer (500 mM NaCl, 20 mM sodium phosphate buffer, pH 7.4), incubated for 1 h with Bodipy-phallacydin (1:50) and either a goat anti-mouse fluorescein isothiocyanate-conjugated or a goat anti-rabbit Texas Red-conjugated antibody (1:100) in goat serum dilution buffer, and washed three times in high-salt buffer, once in PBS, and finally once in 5 mM sodium phosphate buffer, pH 7.4. The coverslips were then mounted in 70% glycerol with phenyl-ethylenediamine (1 mg/ml) as an antibleaching agent and viewed in a Zeiss Photomicroscope III equipped with epifluorescence optics (Zeiss, Oberkochen, Germany).
For anti-FAK immunofluorescence, an amplified avidin-biotin protocol was used, as previously described (Burgaya et al., 1995).
Electron Microscopy
Pellets were fixed for 1 h at room temperature in 1% formaldehyde (freshly prepared from paraformaldehyde), 2% glutaraldehyde, and 100 mM sodium phosphate buffer, pH 7.4, washed twice for 1 h in 100 mM sodium phosphate buffer, pH 7.4, and postfixed for 1 h at 4°C with 2% OsO4 in 100 mM cacodylate buffer, pH 7.4. They were subsequently washed four times for 10 min with 0.1 M sodium veronal buffer, pH 7.4, and block stained for 50 min at 4°C with 0.5% uranyl acetate in 100 mM sodium veronal buffer, pH 7.4, dehydrated, and flat embedded in 50% Epon 812 overnight and 100% Epon 812 overnight at 60°C, essentially as described (Ceccarelli et al., 1973).
Silver-gray sections were cut on an Ultracut microtome (Reichert-Jung, Vienna, Austria), double stained with 4% uranyl acetate and 0.4% lead citrate, and examined in a Hitachi H-7000 electron microscope (Hitachi, Tokyo, Japan).
Antisense Oligonucleotide Experiments
Cells (20,000 per well) were seeded into 35-mm plastic dishes the day before the addition of the oligonucleotides. At time 0, the medium was changed with normal growth medium, medium supplemented with NGF (50 ng/ml), or medium supplemented with NGF plus either antisense oligonucleotides to GDI1 or the corresponding sense oligonucleotides to a final concentration of 50 μM. At intervals of 12 h, further additions of the oligonucleotides (25 μM) were applied to the same culture media, up to 48 h. NGF was resupplemented after 24 h.
At the end of the experiment, cells from the different samples were lysed, and equal amounts of proteins were subjected to SDS-PAGE and transferred to nitrocellulose. Filters were then processed for immunoblotting with an anti-Rab-GDI α antibody.
Stable Transfections
Transfections were carried out using the cationic polymer polyethylenimine 800 (PEI 800), which was recently described as an efficient transfection agent (Boussif et al., 1995). Cells (50,000 per well) were seeded into 24-well plastic dishes the day before transfection. Medium (containing serum) was changed immediately before the addition of the transfection mix.
PEI 800 (10 equivalents/μg DNA) and the plasmid DNA (9 μg of DNA per well) were separately diluted in 150 mM NaCl in Eppendorf tubes, vortexed, quickly spun, and left for 5 min at room temperature. The solution containing PEI 800 was then delicately added to the tube containing DNA, vortexed, left for 15 min at room temperature, and then added to the cells. The 24-well dishes were centrifuged at 1000 rpm for 5 min at room temperature and incubated at 37°C for 6–8 h. Medium was then changed to normal culture medium (containing serum) for 48 h and finally substituted with medium containing the selecting agent, either G418 (700 μg/ml) or hygromycin B (350 μg/ml).
Miscellaneous Techniques
[35S]Sulfate labeling experiments for the analysis of the constitutive secretory pathway and [2,5,6-3H]dopamine uptake experiments were performed essentially as previously described (Lee and Huttner, 1983; Pozzan et al., 1984; Tooze and Huttner, 1990).
RESULTS
trk-PC12 Cells Lack the Machinery for Regulated Secretion
To analyze membrane trafficking underlying neurite extension in trk-PC12 cells, lysates from these cells were tested by Western blotting for expression of several molecules involved in neurosecretion. When compared with wild-type PC12, trk-PC12 cells showed strongly reduced or undetectable levels of all the antigens examined (Figure 1A and Table 1). These included integral membrane proteins of synaptic-like microvesicles such as p38/synaptophysin, synaptotagmin, the vesicle soluble N-ethylmaleimide-sensitive factor-associated protein receptors (v-SNAREs) VAMP-1 and VAMP-2, the peripheral membrane proteins of the same organelles, synapsin II and Rab3a, and the t-SNAREs syntaxin 1 and SNAP-25 (Südhof, 1995; Valtorta and Benfenati, 1995). Also the granins (chromogranin B and secretogranin II), the main cargo components of LDCGs (Rosa and Gerdes, 1994), were reduced to undetectable levels in trk-PC12 cells. Treatment with NGF for 24 h, which induced morphological differentiation of these cells, did not significantly affect the levels of the proteins of the neurosecretory apparatus. Consistent results were obtained by immunofluorescence analysis of the cells after fixation under analogous conditions (Table 1).
Figure 1.
(A) Western blot analysis of the expression of markers of synaptic-like microvesicles, LDCGs, and plasma membrane proteins related to the apparatus for regulated secretion. Protein (100 μg) from either wild-type PC12 (WT) or trk-PC12 (Trk+) cells lysed under control conditions or after treatment with NGF (50 ng/ml) for 24 h was loaded into each lane. Although all markers analyzed are present in wild-type PC12 cells, their levels are below the threshold of detection in trk-PC12 cells, both before and after NGF treatment. p38, p38/synaptophysin; StgI, synaptotagmin I; SynII, synapsin II; CgB, chromogranin B; SgII, secretogranin II. (B) Northern blot analysis of representative markers of the secretory apparatus. Total RNA (20 μg) extracted from wild-type PC12 or trk-PC12 cells under control conditions or after treatment with NGF for 24 h was loaded into each lane and analyzed using probes specific for p38/synaptophysin (p38) or secretogranin II (SgII) mRNAs. The mRNAs coding for either protein product appear to be barely visible or undetectable in trk-PC12 cells. Northern blot signals were compared with those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Table 1.
Western blot and immunofluorescence analysis of the expression of components of the machinery for neurosecretion in wild-type PC12 and trk-PC12 cells
| Antigen | WT/UN
|
WT/NGF
|
Trk+/UN
|
Trk+/NGF
|
||||
|---|---|---|---|---|---|---|---|---|
| WB | IF | WB | IF | WB | IF | WB | IF | |
| p38 | ++++ | ++++ | +++ | +++ | − | − | − | − |
| SynIIa | − | − | − | − | − | − | − | − |
| SynIIb | ++ | +++ | ++ | + | − | − | − | − |
| StgI | +++ | n.d. | +++ | n.d. | − | n.d. | − | n.d. |
| VAMP-1 | + | ++ | +/− | + | − | − | − | − |
| VAMP-2 | ++ | +++ | ++ | ++ | +/− | + | − | +/− |
| Syntaxin-1 | ++ | n.d. | ++ | n.d. | − | n.d. | − | n.d. |
| SNAP-25 | +++ | +++ | +++ | +++ | − | − | − | − |
| Rab3a | ++ | n.d. | + | n.d. | − | n.d. | − | n.d. |
| CgB | ++ | ++++ | + | +++ | − | − | − | − |
| SgII | ++++ | ++++ | ++++ | ++++ | − | − | − | − |
| TyrH | +++ | n.d. | +++ | n.d. | +/− | n.d. | +/− | n.d. |
| ChAT | ++ | n.d. | ++ | n.d. | +/− | n.d. | + | n.d. |
WT/UN, wild-type PC12/untreated; WT/NGF, wild-type PC12/NGF 24 h; Trk+/UN, trk-PC12/untreated; Trk+/NGF, trk-PC12/NGF 24 h; n.d., not determined; p38, p38/synaptophysin; SynIIa and SynIIb, synapsin IIa and IIb; StgI, synaptotagmin I; VAMP-1 and VAMP-2, vesicle-associated membrane protein-1 and -2; SNAP-25, synaptosome-associated protein of 25 kDa; CgB, chromogranin B; SgII, secretogranin II; TyrH, tyrosine hydroxylase; ChAT, choline acetyltransferase.
The expression of the t-SNARE family of the syntaxins (Bennett et al., 1993) was investigated more thoroughly by Western blot analysis using isoform-specific antibodies. Whereas isoforms 1–4 could be detected in wild-type PC12 cells, only syntaxins 2 and 4 were present in trk-PC12 cells, and syntaxins 1 and 3 were undetectable (Table 2).
Table 2.
Western blot analysis of the expression of the isoforms of the syntaxin family of t-SNAREs in wild-type PC12 and trk-PC12 cells
| Isoform | WT/UN | WT/NGF | Trk+/UN | Trk+/NGF |
|---|---|---|---|---|
| Syntaxin-1 | +++ | +++ | − | − |
| Syntaxin-2 | ++++ | ++++ | ++++ | ++++ |
| Syntaxin-3 | ++ | ++ | − | − |
| Syntaxin-4 | ++ | ++ | ++ | ++ |
WT/UN, wild-type PC12/untreated; WT/NGF, wild-type PC12/NGF 12 h; Trk+/UN, trk-PC12/untreated; Trk+/NGF, trk-PC12/NGF 12 h.
Northern blot analysis revealed that the mRNAs coding for the proteins tested were present in wild-type PC12 cells, whereas their levels were dramatically reduced in trk-PC12. NGF treatment of wild-type cells caused a small but reproducible reduction in the levels of all the transcripts analyzed (Figure 1B and our unpublished results).
Furthermore, although LDCGs could be observed by electron microscopy in wild-type PC12, these organelles were absent in trk-PC12 cells (Figure 2), suggesting a blockade in their biogenesis.
Figure 2.
Electron micrographs showing sections of wild-type PC12 (WT) and trk-PC12 (Trk+) cells. Although LDCGs (identified by their dense content; arrowheads) are abundant in wild-type PC12 cells, they are absent in trk-PC12 cells. On the contrary, clathrin-coated vesicles, as well as all other cellular organelles, can be observed in both cell types. Bar, 500 nm.
From a functional point of view, trk-PC12 cells were deficient in neurotransmitter internalization, indicating that the defect in regulated secretion also involved plasma membrane transporters. Indeed, although a time-dependent [3H]dopamine uptake could be easily detected in the case of wild-type PC12, it was absent in trk-PC12 cells. Consistently, K+ depolarization was unable to induce any release of radioactive dopamine by trk-PC12 cells (our unpublished results).
Analysis of Neurite Extension by trk-PC12 Cells
As previously demonstrated (Hempstead et al., 1992), trk-PC12 cells showed a potentiated neurite outgrowth response when exposed to NGF, in terms of both kinetics and final extent. Indeed, although several days of NGF treatment were required for process formation by wild-type cells, a few hours were sufficient in the case of trk-PC12 cells, and after 3 d of treatment nearly 100% of the cells bore neurites several cell body diameters in length (our unpublished results).
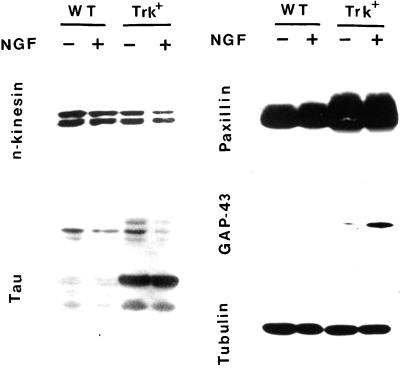
When tested for expression of a panel of cytoskeletal proteins and adhesion molecules, both under control conditions and after treatment with NGF for 24 h, trk-PC12 cells showed normal to increased levels of the antigens tested with respect to wild-type cells (Figure 3). In particular, the microtubule-associated molecule tau and the focal adhesion protein paxillin were present at higher levels. An interesting observation comes from the expression pattern of the growth cone marker GAP-43 (Benowitz and Routtenberg, 1997), which was undetectable in wild-type PC12 cells, both under control conditions and after 24 h of NGF treatment, but was already present in basal culture conditions in trk-PC12 cells and was markedly up-regulated after exposure of the cells to the neurotrophin.
Figure 3.
Western blot analysis of the expression of markers related to the apparatus for neurite extension. Total cell homogenates were processed as described in Figure 1. Both cytoskeletal markers and proteins involved in adhesion processes are present at equal or increased levels in trk-PC12 (Trk+) with respect to wild-type PC12 (WT) cells. Note the presence of the growth cone marker GAP-43 in trk-PC12 cells under control conditions and its increased expression after 24 h of NGF treatment.
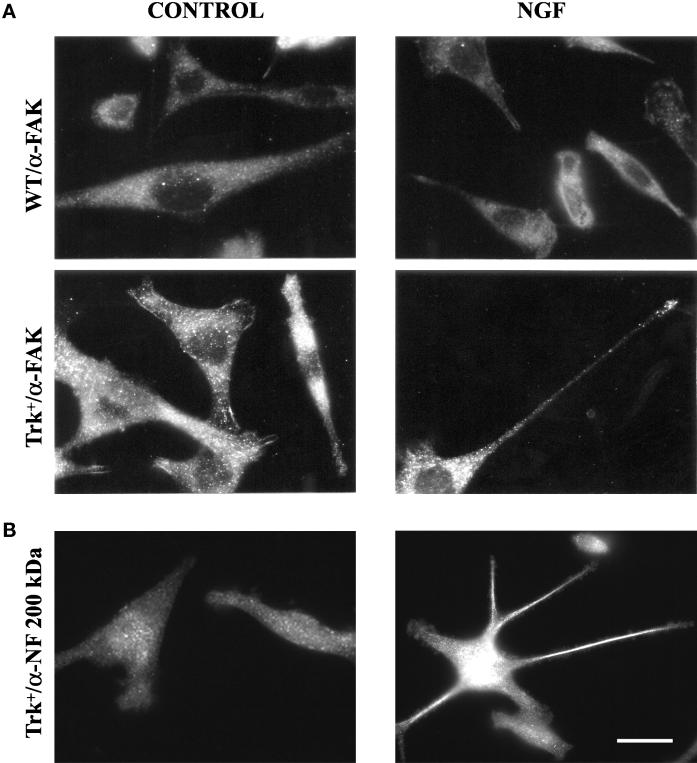
Increased levels of FAK (Hanks and Polte, 1997; Ilic et al., 1997) were present in trk-PC12 cells. The kinase was enriched at structures similar to focal contacts under control conditions and partly relocalized to growth cones in NGF-treated cells, as shown by immunofluorescence (Figure 4A). Furthermore, processes were highly enriched in neurofilaments (Figure 4B), indicating that they possess several characteristics specific to neurites.
Figure 4.
(A) Immunofluorescence analysis of FAK expression. Wild-type (WT) or trk-PC12 (Trk+) cells fixed under control conditions (left panels) or after treatment with NGF for 24 h (right panels) were analyzed for expression of focal adhesion kinase (α-FAK). Increased levels of FAK immunostaining can be observed in trk-PC12 cells, partially colocalizing with structures similar to focal adhesions under control conditions and relocalizing to the growth cone after 24 h of NGF treatment. (B) Immunofluorescence analysis of neurofilament expression and localization in trk-PC12 cells. Control and NGF-treated trk-PC12 cells were analyzed for expression of the 200-kDa neurofilament subunit (α-NF 200 kDa). Although under control conditions the protein is expressed at low levels and appears diffuse (left panel), it is strongly up-regulated after NGF treatment and enriched in the growing processes (right panel). Bar, 14 μm.
Membrane Trafficking in trk-PC12 Cells
An important piece of information on membrane dynamics in trk-PC12 cells comes from the analysis of the secretion of heparan sulfates, which are released in the medium through the constitutive flow (Tooze and Huttner, 1990). Pulse–chase experiments performed on cells labeled with [35S]sulfate indicated a time-dependent accumulation of radioactivity in the medium, which was potentiated in trk-PC12 with respect to wild-type cells (Figure 5). Secretion was blocked if the cells were shifted to 18°C (a condition known to block budding from the trans-Golgi network) and subsequently recovered when the cells were brought back to 37°C, according to a behavior diagnostic of vesicle-mediated constitutive release.
Figure 5.
Analysis of constitutive secretion by metabolic labeling of heparan sulfates. Cells were pulse labeled for 10 min with [35S]sulfate (0.7 μCi/ml) in sulfate-free medium. Equal volumes of cell lysates and media from wild-type (WT) and trk-PC12 (Trk+) cells were collected after 30, 60, and 90 min of chasing of the radioactive label at 37°C, after 30 and 60 min of chasing at 18°C, or after 60 min of chasing at 18°C followed by a 30-min shift at 37°C (+30′). A time-dependent accumulation of radioactivity in the medium can be observed at 37°C, which is blocked if the cells are incubated at 18°C. The block is relieved after returning the cells to 37°C. The constitutive flow appears potentiated in trk-PC12 (bottom panel) with respect to wild-type PC12 cells (top panel).
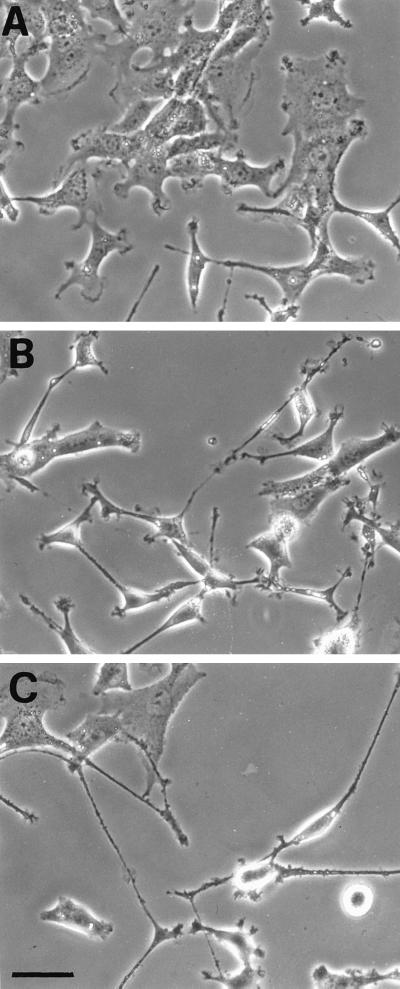
The potential dispensability of t-SNAREs for neurotrophin-dependent neurite outgrowth in trk-PC12 cells was further suggested by the observation that the process was resistant to blockade by BotNC, a metalloendopeptidase that specifically cleaves syntaxins 1–3 (Schiavo et al., 1995) and SNAP-25 (Williamson et al., 1996). Indeed, a concentration of the toxin (0.5 nM) known to be sufficient for complete cleavage of t-SNAREs in neurons appeared to be unable to prevent neurite extension in NGF-treated trk-PC12 cells (Figure 6). Because the biochemical demonstration of cleavage of the t-SNAREs by the toxin is complicated by the strong reduction in their expression levels, this experiment cannot be considered conclusive, but it offers further evidence of the dispensability of the t-SNAREs for neurite outgrowth in these cells.
Figure 6.
Morphological analysis of the effect of BotNC on NGF-dependent neurite outgrowth in trk-PC12 cells. Cells were observed and photographed by phase-contrast microscopy under control conditions (A) or after 48 h of NGF treatment either in the absence (B) or in the presence (C) of BotNC (0.5 nM). The toxin is apparently unable to prevent NGF-dependent neurite extension in trk-PC12 cells. Bar, 70 μm.
In contrast, PD98059, a selective inhibitor of MAPK kinase (Pang et al., 1995), the upstream kinase activating the MAPK pathway (Crews et al., 1992), efficiently blocked neurite extension by trk-PC12 cells without causing any apparent cytotoxicity (our unpublished results). In these cells, the molecule was effective both on the neurotrophin-induced extension of neurites and on the neurite outgrowth that can be observed under basal conditions. The inhibition of process formation was accompanied by a strong decrease in the NGF-stimulated levels of MAPK tyrosine phosphorylation (our unpublished results).
The role of the Rab family of proteins in membrane trafficking in trk-PC12 cells was investigated by interfering with the expression of Rab-GDI α. To this aim, we used antisense oligonucleotides complementary to sequences surrounding the initiation of translation of the rat GDI1 gene, previously reported to significantly reduce the levels of expression of Rab-GDI α (D’Adamo et al., 1998). Exposure of the cells to antisense oligonucleotides (50 μM), but not to the corresponding sense oligonucleotides, significantly slowed the kinetics of NGF-dependent neurite extension (Figure 7A) without affecting cell viability (our unpublished results). Western blot analysis of the corresponding samples demonstrated a selective reduction in the levels of expression of the α isoform and a complementary increase in the levels of the β isoform (Figure 7B).
Figure 7.
Analysis of the effect of antisense and sense oligonucleotides to GDI1 on NGF-dependent neurite outgrowth in trk-PC12 cells. (A) The length of neurites was measured on digitized images of trk-PC12 cells photographed by phase-contrast microscopy after 24 and 48 h of treatment with NGF in the presence of either 50 μM antisense oligonucleotides to GDI1 message (AS) or the corresponding sense oligonucleotides (S). Bars represent means ± SE. A.U., arbitrary units. (B) Western blot analysis of the corresponding samples probed with an anti-GDI α antibody. Note the selective reduction in the levels of the α isoform and the compensatory increase in the levels of the β isoform in the antisense-treated cells.
Analysis of the Role Played by TrkA Overexpression in the Neurosecretory Defect Observed in trk-PC12 Cells
To determine whether TrkA overexpression plays any causal role in the neurosecretory deficit observed in trk-PC12, other PC12 clones were prepared by transfecting wild-type PC12 cells with an expression vector carrying the cDNA of the human TRKA gene (pC9TRK). Mock transfectants were also obtained in parallel using the empty vector.
Several positive clones expressing TrkA at various levels were obtained. No concomitant changes in the levels of the downstream mediators of the TrkA signal transduction cascade (such as MAPK) were observed, although TrkA overexpression was often accompanied by a reduction in the levels of the low-affinity neurotrophin receptor p75, as previously observed (Hempstead et al., 1992; our unpublished results).
The new clones were then screened for expression of the regulated secretion apparatus. No apparent qualitative changes in the expression of several antigens tested could be detected with respect to wild-type PC12 cells. Western blot analysis of v-SNARE and t-SNARE expression showed heterogeneity in the levels of these molecules in the different clones, but this heterogeneity was apparently unrelated to the levels of TrkA expression. Similarly, heterogeneity in p38/synaptophysin and secretogranin II levels could be demonstrated by immunofluorescence, seemingly as a result of clonal differences (our unpublished results).
Analysis of Neurite Extension in Independent PC12 Clones Displaying a Neurosecretory Defect
To determine whether the proficiency of trk-PC12 cells to extend neurites in spite of the deficit in regulated secretion is a general phenomenon, an independent PC12 variant clone, PC12-27, was studied. This clone was previously described (Clementi et al., 1992; Corradi et al., 1996) and, like trk-PC12, it is globally incompetent for regulated secretion.
Unfortunately, PC12-27 clones are poorly responsive to NGF (Clementi et al., 1993), most probably because of their low levels of expression of TrkA. Thus, stable clones (PC12-27TrkA) overexpressing TrkA were generated by transfecting PC12-27 cells with pC9TRK. Because PC12-27 clones are already resistant to G418, the TrkA-overexpressing clones were selected by double transfection with a vector encoding hygromycin resistance (pIRES-1hyg).
Several positive clones were obtained, displaying various levels of the receptor. TrkA overexpression per se did not affect the neurosecretory defect of the cells, as demonstrated by Western blot analysis using representative markers of regulated secretion (Figure 8A). Indeed, all the PC12-27TrkA clones analyzed showed undetectable levels of the synaptic-like microvesicle protein p38/synaptophysin, the granule protein secretogranin II, and the t-SNARE syntaxin 1.
Figure 8.
(A) Western blot analysis of PC12-27TrkA clones. Total cell homogenates were processed as described in Figure 1. As a reference, equal amounts of lysates from wild-type PC12 (WT), trk-PC12, and PC12-27 cells were loaded. Although all markers analyzed are present in wild-type PC12 cells, their levels are below the threshold of detection in trk-PC12, PC12-27, and all PC12-27TrkA clones examined. p38, p38/synaptophysin; SgII, secretogranin II. (B) NGF-dependent neurite extension by PC12-27 and PC12-27/108 clones. Cells were photographed by phase-contrast microscopy under control (CONT) conditions or after treatment with NGF (50 ng/ml) for 72 h. Neurite outgrowth was quantitatively analyzed on digitized images. Bars represent means ± SE. A.U., arbitrary units. (C) Immunofluorescence analysis of neurofilament expression and localization in NGF-treated PC12-27/108 cells. Cells were fixed and analyzed for expression of the 200-kDa neurofilament subunit (α-NF 200 kDa) that appears to be enriched in the distal part of the neuritic shaft and in growth cones. Bar, 14 μm.
PC12-27TrkA clones were then exposed to NGF (50 ng/ml) to quantitatively evaluate their morphological response to neurotrophin treatment. As in the case of trk-PC12 cells, in a representative PC12-27TrkA clone (PC12-27/108) a few hours of treatment were sufficient to observe neurite outgrowth, and after 3 d nearly 100% of the cells bore neurites that were several cell body diameters in length and that stained positively for neurofilaments (Figure 8, B and C).
Also, untransfected PC12-27 showed a small but significant neurite extension (Figure 8B). In these cells, neurite outgrowth could be better appreciated after 6–10 d of NGF treatment (our unpublished results). However, also at these time points the effect was limited in extent as a result of the poor sensitivity of PC12-27 to the growth-inhibitory action of NGF (Clementi et al., 1993), preventing their withdrawal from the cell cycle.
DISCUSSION
The switch that turns a proliferating neuroblast into a differentiated neuron is associated with several changes in the physiology of the cell. On the one hand, we have a round, proliferating cell in which all secretory processes occur in an unpolarized manner; on the other hand, we have a quiescent, highly polarized and compartmentalized cell in which secretion is a regulated and focal event.
A useful model system for the study of neuronal differentiation is represented by PC12 cells. We have studied a PC12 clone stably transfected with the cDNA of the human TRKA gene, trk-PC12. In these cells, TrkA is constitutively active, albeit at low levels, and therefore the cells undergo spontaneous differentiation and extend neurites. Neurite extension is strongly accelerated by the presence of NGF in the medium (Hempstead et al., 1992).
trk-PC12 Cells Lack the Machinery for Regulated Secretion
Regulated secretion is a process that is present in several cell types, and in neurons and neuroendocrine cells it utilizes two distinct organelles: the synaptic vesicles and the LDCGs, each characterized by a specific molecular makeup (Valtorta and Benfenati, 1995).
Analysis of the molecular components of the machinery for regulated secretion in trk-PC12 cells by Western blotting and immunocytochemistry demonstrates the virtual absence of both synaptic vesicle and granule proteins and of the t-SNAREs syntaxin 1, syntaxin 3, and SNAP-25. In addition, the cells are incapable of performing neurotransmitter uptake.
Taken together, our observations indicate the existence, in wild-type PC12 cells, of one or more levels of coordinate regulation of the engine for neuroexocytosis, which has been perturbed in trk-PC12 cells, similar to what was previously reported for another PC12 clone, PC12-27 (Corradi et al., 1996). The results obtained with the Northern blot experiments suggest that the reduction in the levels of the protein products is due to changes in either mRNA stability or in the rate of transcription of the corresponding genes. The absence of the proteins involved in regulated secretion correlates also with the morphological absence of LDCGs, demonstrated by electron microscopy, indicating a block in the biogenesis of these organelles.
Because both pathways of regulated secretion are affected, these data suggest that this function is globally orchestrated. The existence of a coordinate form of regulation for the apparatus of neuroexocytosis is confirmed by cell fusion experiments, showing the lack of functional complementation between trk-PC12 and PC12-27 cells (Borgonovo et al., 1998).
TrkA overexpression by itself does not seem able to recapitulate the phenotype observed in trk-PC12 cells. Analysis of a battery of stable transfectants expressing the receptor at various levels shows the presence of proteins of the secretory machinery in all clones, irrespective of TrkA levels, indicating the absence of a causal role for the receptor in the neurosecretory defect observed in trk-PC12 cells.
Neurite Extension Is Potentiated in trk-PC12 Cells
Unexpectedly, the absence of regulated secretion does not seem to interfere with the ability of trk-PC12 cells to perform other functions related to the neuronal differentiation program, and particularly with the ability to extend neurites.
When analyzed for expression of some of the components involved in adhesion and neurite outgrowth, trk-PC12 cells display normal or even higher levels of proteins of the actin- and microtubule-based cytoskeleton, of adhesion molecules, and of proteins involved in growth cone function. Indeed, the kinetics of neurite extension are clearly accelerated with respect to wild-type cells: neurites become evident within hours of NGF treatment in trk-PC12 cells.
Altogether, the paradoxical picture that emerges from the analysis of trk-PC12 cells is that of a clone highly proficient in neurite extension despite being deficient in the machinery for regulated secretion. Thus, it seems likely that the neuronal differentiation program is not activated by a single switch but is composed of a series of subprograms, including maturation of the regulated secretion apparatus and neurite formation, whose executions are mutually independent.
The proficiency to extend neurites in the absence of the apparatus for regulated secretion, demonstrated in the case of trk-PC12 cells, has been further confirmed in an independent PC12 variant clone, PC12-27. Indeed, although PC12-27 cells respond to neurotrophin treatment with a slow kinetics, likely as a result of the low levels of TrkA expression, they become highly proficient in neurite outgrowth once the receptor is overexpressed. However, TrkA overexpression does not reverse the neurosecretory deficit of PC12-27 cells.
Membrane Trafficking in trk-PC12 Cells
The competence to perform neurite extension in the absence of regulated secretion raises questions as to the origin of the membrane that is added to the plasma membrane during process formation. Supposedly, in trk-PC12 cells the membrane required for neurite outgrowth does not come from vesicles derived from the regulated pathway. Indeed, LDCGs are molecularly and morphologically absent, and synaptic-like microvesicles lack their normal molecular makeup. The defect in regulated secretion does not extend to the constitutive secretory pathway, which appears to be fully functional and even potentiated in trk-PC12 with respect to wild-type cells. Therefore, it can be hypothesized that the new membrane coming from the constitutive flow can be utilized for neurite extension.
Previous work, carried out on neurons in culture, demonstrated that elimination of the t-SNAREs induced rapid growth cone collapse and impaired neurite outgrowth in chick dorsal root ganglion and retina explants (Igarashi et al., 1996) and inhibited neurite growth of cultured rat cortical neurons (Osen-Sand et al., 1996), suggesting that the introduction of the new membrane required for neurite formation would come from t-SNARE-dependent vesicle fusion processes. In this context, particularly relevant is the absence in trk-PC12 cells of the t-SNAREs syntaxin 1 and 3 and SNAP-25. This result suggests that, in these cells, regulated secretion-independent and apparently t-SNARE-independent neurite extension can take place, raising the possibility that the machinery for regulated secretion, and particularly the t-SNAREs, may not be essential in absolute terms for this function. Indeed, dendritic growth is apparently less affected by cleavage of the t-SNAREs (Osen-Sand et al., 1996), suggesting the existence of multiple pathways, also in neurons. Furthermore, it has been reported that cleavage of the v-SNAREs produced no observable effects on axon growth either in situ (Sweeney et al., 1995) or in culture (Ahnert-Hilger et al., 1996; Osen-Sand et al., 1996), in spite of the efficient blockade of neurotransmitter release, in line with what we have observed in trk-PC12 cells.
The dispensability of the t-SNAREs in neurite extension is further indicated by the experiments using BotNC, which specifically cleaves syntaxins 1–3 (Schiavo et al., 1995) and SNAP-25 (Williamson et al., 1996). A concentration of the toxin sufficient to achieve complete cleavage of the t-SNAREs in neurons is not able to prevent NGF-dependent neurite extension in trk-PC12 cells.
In addition, experiments carried out in a polarized epithelial cell line, Madin–Darby canine kidney (MDCK) cells, suggest the existence of SNARE-independent fusion processes. Although the basolateral pathway in MDCK cells, proposed to correspond to the dendritic sorting pathway of neurons (Dotti and Simons, 1990), requires SNARE integrity, apical delivery of proteins is not affected by cleavage of these molecules (Ikonen et al., 1995). The demonstration that in MDCK cells specific v-SNARE and t-SNARE isoforms, which are insensitive to clostridial toxins, colocalize in the apical region of the cell (Galli et al.,1998) has prompted the idea that these proteins might be responsible for docking and fusion of the exocytic vesicles destined to this compartment. However, an involvement of these proteins in docking and fusion still awaits support from functional experiments.
Because t-SNAREs and v-SNAREs are represented by families of proteins comprising several isoforms (Hay and Scheller, 1997), it cannot be ruled out that other, less commonly expressed or as yet unrecognized v-SNAREs and t-SNAREs are present in trk-PC12 cells and mediate the fusion of exocytic vesicles with the plasma membrane. Indeed, in the case of the syntaxins, although syntaxins 1 and 3 are not expressed by trk-PC12 cells, syntaxins 2 and 4 appear to be expressed. Furthermore, the observation that in trk-PC12 cells only two syntaxin variants are coregulated with the rest of the neurotransmitter release machinery suggests the possibility that the various isoforms may differently relate to neurosecretion and neurite extension.
Another family of proteins involved in membrane trafficking that could play a role in neurite extension and neurotransmitter release in neurons and neuroendocrine cells is that of the Rabs (Novick and Zerial, 1997; Woodman, 1998), and particularly Rab3a (Takai et al., 1996).
Rab3a expression is undetectable in trk-PC12 cells, demonstrating that it is coordinately regulated with the machinery for regulated secretion and thus possibly is involved in neuroexocytosis but apparently is dispensable for neurite outgrowth. Unexpectedly, treatment of trk-PC12 cells with antisense oligonucleotides against Rab-GDI α seems to significantly slow the initial kinetics and to reduce the final extent of the NGF-dependent neurite outgrowth. These results differ from those obtained in MDCK cells, in which apical delivery of proteins, besides being apparently SNARE independent, seems also not to require the integrity of the Rab cycle (Ikonen et al., 1995).
On the contrary, our data point to a role for a Rab protein in process formation in trk-PC12 cells, consistent with the observation that the lack of Rab-GDI α impairs axonal development in neurons in culture (D’Adamo et al., 1998). However, as in the case of neurons, it remains to be established which of the Rabs is responsible for the effects of Rab-GDI on neurite extension. Interestingly, neurite extension seems to selectively require the α isoform of Rab-GDI. In fact, in the antisense-treated samples a reduction in the levels of GDI α was accompanied by a parallel increase in GDI β, which, however, was unable to functionally compensate for the lack of GDI α.
Conclusion
These findings further strengthen the demonstration that, in the case of PC12 cells, the apparatus for regulated exocytosis is globally orchestrated. However, if a master switch for neurosecretion exists, it does not control other aspects of neuronal maturation, particularly neurite extension and cytoskeletal differentiation.
In addition, at least in regulated secretion-defective PC12 cells, neurite extension requires an intact constitutive secretory pathway and Rab cycle.
ACKNOWLEDGMENTS
We thank A. Pandiella for providing the trk-PC12 clone, C. Cortonesi for technical assistance, D.D. Dunlap for developing the computer program used for the analysis of neurite extension, and J. Meldolesi for providing the PC12-27 clone. We also thank the following persons for providing antibodies: M.K. Bennett, I. De Curtis, G. Della Valle, P. Greengard, M. Matteoli, C. Montecucco, J. Meldolesi, F. Navone, and P. Rosa, and B. Borgonovo for providing the probes used for the Northern blot analysis. This work was supported by grants from Telethon (grant 1000 to F.V. and grant 1131 to F.B.) and by the Harvard-Armenise Foundation (F.V.).
Abbreviations used:
- BotNC
botulinum neurotoxin type C
- FAK
focal adhesion kinase
- GAP-43
growth-associated protein of 43 kDa
- LDCGs
large dense-core granules
- MDCK
Madin–Darby canine kidney
- NGF
nerve growth factor
- PEI 800
polyethylenimine 800
- Rab-GDI α
Rab-guanosine diphosphate-dissociation inhibitor α
- SNAP-25
synaptosome-associated membrane protein of 25 kDa
- t-SNARE
target membrane-soluble N-ethylmaleimide-sensitive factor-associated protein receptor
- VAMP-1 and VAMP-2
vesicle-associated membrane protein-1 and -2
- v-SNARE
vesicle-soluble N-ethylmaleimide-sensitive factor-associated protein receptor
REFERENCES
- Ahnert-Hilger G, Kutay U, Chahoud I, Rapaport T, Wiedenmann B. Synaptobrevin is essential for secretion but not for the development of synaptic processes. Eur J Cell Biol. 1996;70:1–11. [PubMed] [Google Scholar]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Benfenati F, Onofri F, Giovedì S. Protein-protein interactions and protein modules in the control of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:243–258. doi: 10.1098/rstb.1999.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Borgonovo B, Racchetti G, Malosio ML, Benfante R, Podini P, Rosa P, Meldolesi J. Neurosecretion competence, an independently regulated trait of the neurosecretory cell phenotype. J Biol Chem. 1998;273:34683–34686. doi: 10.1074/jbc.273.52.34683. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr J-P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaya F, Menegon A, Menegoz M, Valtorta F, Girault J-A. Focal adhesion kinase in rat CNS. Eur J Neurosci. 1995;7:1810–1821. doi: 10.1111/j.1460-9568.1995.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clementi E, Racchetti G, Zacchetti D, Panzeri MC, Meldolesi J. Differential expression of markers and activities in a group of PC12 nerve cell clones. Eur J Neurosci. 1992;4:944–953. doi: 10.1111/j.1460-9568.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Clementi E, Raichman M, Meldolesi J. Heterogeneity of NGF-induced differentiation in PC12 cells investigated in a battery of isolated cell clones. Funct Neurol. 1993;8:109–119. [PubMed] [Google Scholar]
- Corradi N, Borgonovo B, Clementi E, Bassetti M, Racchetti G, Consalez GG, Huttner WB, Meldolesi J, Rosa P. Overall lack of regulated secretion in a PC12 variant cell clone. J Biol Chem. 1996;271:27116–27124. doi: 10.1074/jbc.271.43.27116. [DOI] [PubMed] [Google Scholar]
- Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, et al. Mutations in GDI1 are responsible for X-linked nonspecific mental retardation. Nat Genet. 1998;19:134–139. doi: 10.1038/487. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Banker GA. The economics of neurite outgrowth-addition of new membrane to growing axons. Trends Neurosci. 1996;19:144–149. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Kaplan DR. Early events in neurotrophin signaling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which responds to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Rabin SJ, Kaplan L, Reid S, Parada LF, Kaplan DR. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992;9:883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- Higgins D, Burack M, Lein P, Banker GA. Mechanisms of neuronal polarity. Curr Opin Neurobiol. 1997;7:599–604. doi: 10.1016/s0959-4388(97)80078-5. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kozaki S, Terakawa S, Kawano S, Ide C, Komiya Y. Growth cone collapse and inhibition of neurite growth by botulinum neurotoxin C1: a t-SNARE is involved in axonal growth. J Cell Biol. 1996;134:205–215. doi: 10.1083/jcb.134.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the cross-roads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- Jahn R, Hanson PI. SNAREs line up in new environment. Nature. 1998;393:14–15. doi: 10.1038/29871. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee RWH, Huttner WB. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosyl-protein sulfotransferase. J Biol Chem. 1983;258:11326–11334. [PubMed] [Google Scholar]
- Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human Trk proto-oncogene. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Südhof TC, De Camilli P. Exo-endocytic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, Catsicas S. Common and distinct fusion proteins in axonal growth and transmitter release. J Comp Neurol. 1996;367:222–234. doi: 10.1002/(SICI)1096-9861(19960401)367:2<222::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: putting Rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH, Friedman LB. Sites of plasmalemmal expansion in growth cones. Brain Res. 1993;71:181–192. doi: 10.1016/0165-3806(93)90170-f. [DOI] [PubMed] [Google Scholar]
- Posse de Chaves E, Vance DE, Campenot RB, Vance JE. Axonal synthesis of phosphatidylcholine is required for normal axonal growth in rat sympathetic neurons. J Cell Biol. 1995;128:913–918. doi: 10.1083/jcb.128.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Gatti G, Dozio N, Vicentini LM, Meldolesi J. Ca2+-dependent and independent release of neurotransmitter from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984;99:628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A. Neuronal polarity: giving neurons heads and tails. Neuron. 1995;15:743–746. doi: 10.1016/0896-6273(95)90164-7. [DOI] [PubMed] [Google Scholar]
- Rosa P, Gerdes HH. The granin protein family: markers for neuroendocrine cells and tools for the diagnosis of neuroendocrine tumors. J Endocrinol Invest. 1994;17:207–225. doi: 10.1007/BF03347721. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–492. [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Shirataki H, Nakanishi H. Rab3A small GTP-binding protein in Ca2+-regulated exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Huttner WB. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4353. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta F, Benfenati F. Membrane trafficking in nerve terminals. Adv Pharmacol. 1995;32:505–557. doi: 10.1016/s1054-3589(08)61021-2. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Jahn R, Fesce R, Greengard P, Ceccarelli B. Synaptophysin (p38) at the frog neuromuscular junction: its incorporation into the axolemma and recycling after intense quantal secretion. J Cell Biol. 1988;107:2719–2730. doi: 10.1083/jcb.107.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta F, Leoni C. Molecular mechanisms of neurite extension. Philos Trans R Soc Lond B Biol Sci. 1999;354:387–394. doi: 10.1098/rstb.1999.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Halpern JL, Montecucco C, Brown JE, Neale EA. Clostridial neurotoxins and substrate proteolysis in intact neurons. J Biol Chem. 1996;271:7694–7699. doi: 10.1074/jbc.271.13.7694. [DOI] [PubMed] [Google Scholar]
- Woodman P. Vesicle transport: more work for the Rabs? Curr Biol. 1998;8:R199–R201. doi: 10.1016/s0960-9822(98)70124-1. [DOI] [PubMed] [Google Scholar]