Abstract
Experimental determination of the free energy (ΔG) stabilizing the structure of membrane proteins (MPs) in their native environment has been hampered by the aggregation and precipitation of MPs outside the lipid bilayer. We recently demonstrated that the latter process can be prevented by the use of fluorinated surfactants, FTACs, that act as chaperones for MP insertion without partitioning in the membrane themselves. Here we combine the advantages of the chaperone-like ability of FTACs with the sensitivity of fluorescence correlation spectroscopy measurements to determine ΔG of bilayer insertion of model MPs. First, we calibrate our approach by examining the effects of chaperoned insertion on ΔG of transmembrane insertion of Annexin B12. We find that a shorter-chained surfactant, FTAC-C6, for which the working concentration range of 0.05–0.2 mM falls below CMC = 0.33 mM, has a mild effect on an apparent ΔG. In contrast, additions of a longer-chained FTAC-C8 (CMC = 0.03 mM) result in a steep and nonlinear concentration dependence of ΔG. We then apply the same methodology to the pH-triggered insertion of diphtheria toxin T-domain, which is known to be affected by nonproductive aggregation in solution. We find that the correction of the ΔG value needed to compensate for unchaperoned insertion of the T-domain exceeds 3 kcal/mole. A relatively shallow and linear dependence of the ΔG for Annexin B12 and T-domain insertion on FTAC-C6 concentration is encouraging for future applications of this surfactant in thermodynamic studies of the stability of other MPs.
Understanding the energetics of protein-membrane interactions is crucial for deciphering the problem of membrane protein (MP) stability and, ultimately, for predicting structure from sequence. One of the principal challenges for thermodynamic as well as structural characterization of MPs is their poor solubility outside the lipid bilayer. New types of nondetergent surfactants (e.g., amphipols (1) and fluorinated surfactants (2)) have been introduced to amend MP solubility. In our previous studies we have demonstrated that fluorinated surfactants, such as FTACs, can chaperone bilayer insertion of a model MP, diphtheria toxin T-domain (3,4). FTACs possess a unique combination of qualities that distinguish them from detergents and make them ideal chaperone-like compounds: they prevent MP aggregation without affecting the structure, and they dissociate from MP during bilayer insertion without partitioning into the bilayer (4). As demonstrated here, application of FTACs allows direct measurements of the free energy of membrane insertion ΔG under equilibrium conditions.
We recently demonstrated the use of fluorescence correlation spectroscopy (FCS) to characterize the thermodynamics of membrane insertion of Annexin B12 (ANX) under acidic conditions (5). Although the physiological role of transmembrane (TM) insertion of ANX is not completely understood, ANX is an invaluable model system for thermodynamic studies of insertion, owing to its reversible nature and the absence of aggregation even in the insertion-competent state (6). Thus ANX appears to be an ideal system to test the effect of FTACs on insertion energetics.
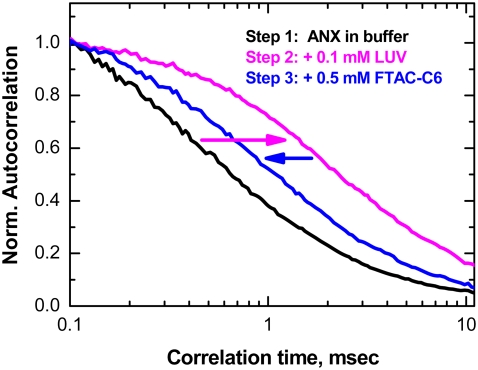
Establishing reversibility is a prerequisite for an equilibrium thermodynamic study of any transition. The general reversibility of pH-triggered ANX insertion was established previously (6). The FCS data shown in Fig. 1 establish insertion reversibility due to the addition of FTAC. Q4C ANX mutant was labeled with Alexa647 for FCS measurements as described previously (5). The mobility of ANX in solution is much faster than that of the vesicle; thus, membrane association results in a shift of the autocorrelation curve toward longer times. The addition of FTAC to the same sample results in the redistribution of ANX between large unilamellar vesicles (LUV) and surfactant, causing a reversed shift of the autocorrelation curve. (Note that the mobility of ANX or the T-domain outside the LUV does not depend significantly on the presence of surfactant, and that the surfactant itself does not bind to LUV, even in the presence of the protein (4).)
FIGURE 1.
Reversibility of membrane insertion of ANX probed by FCS. Partitioning of dye-labeled ANX into the LUV results in increased correlation time (magenta arrow), whereas the subsequent addition of FTAC reverses the binding (blue arrow). Curves are normalized for visual comparison.
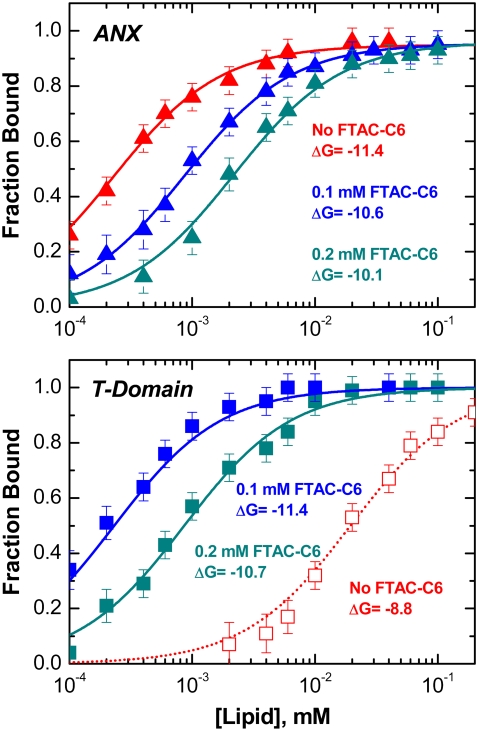
Once the reversibility of insertion is established, the ΔG of membrane partitioning can be measured in the presence of surfactants. This can be achieved by measuring amplitudes and shifts of autocorrelation curves as described in detail in our previous study (5). The resulting binding data (Fig. 2, symbols) can be fitted to a partitioning isotherm (lines) and the free energy of partitioning can be calculated from the fit (5). (Here we follow our previous designation of “partitioning”, which covers both TM insertion and interfacial (IF) association. The latter cannot be distinguished by FCS, but are established in a fluorescence quenching topology experiment (5,7)).
FIGURE 2.
Lipid titration isotherms for ANX (upper panel) and T-domain (lower panel) in the absence (red symbols) and in the presence of 0.1 and 0.2 mM FTAC-C6 (blue and cyan symbols, respectively). The LUV contained a 1:3 molar ratio of POPC/POPG. FCS data collection, binding analysis, and ΔG calculation were performed as described in Posokhov et al. (5). In the case of ANX, a progressive increase in surfactant concentration causes a gradual decrease in apparent ΔG, whereas in the case of the T-domain, it causes a sharp increase followed by a decrease in ΔG. This behavior is a result of the chaperone-like action of FTAC on membrane insertion of the T-domain (3,4) (see text).
The presence of the surfactant causes a gradual reduction in ANX partitioning from −11.4 kcal/mole in the absence of surfactant to −10.6 and −10.1 kcal/mole for 0.1 and 0.2 mM FTAC-C6, respectively. Radically different behavior is observed, however, for otherwise identical titration of the diphtheria toxin T-domain (lower panel). First, in the absence of surfactants, the apparent membrane affinity is much lower: ΔG = −8.8 kcal/mole. Although this number is consistent with our early estimates (8), it is most definitely an underestimation of the true ΔG, caused by nonproductive aggregation of the T-domain (3). The latter can be reversed by the chaperone-like action of fluorinated surfactants (3,4). Indeed, membrane partitioning of the T-domain is dramatically increased by their presence: ΔG = −11.4 and −10.7 kcal/mole for 0.1 and 0.2 mM FTAC-C6, respectively.
We summarize the effects of fluorinated surfactants on the energetics of membrane interactions of ANX and T-domain in Fig. 3. Red symbols correspond to ΔG measured in the absence of surfactants for partitioning of the T-domain (open square) and TM and IF conformations of ANX (circle and triangle, respectively). ANX conformations with different topologies were stabilized by various lipid compositions (3:1 POPC/POPG for TM, 1:3 POPC/POPG for IF (5,7)). The additions of FTAC-C6 cause relatively small changes in ΔG; however, in comparison the effects of FTAC-C8 are more pronounced and nonlinear. The latter is most likely related to the formation of the surfactant micelles (FTAC-C8 has a low critical micelle concentration (CMC = 0.03 mM)). In contrast, FTAC-C6 is a monomer in this concentration range (CMC = 0.33 mM) and demonstrates linear reduction in ΔG for both ANX(TM) and ANX(IF). The linear character of these dependences has important implications for the possibility of obtaining thermodynamic measurements of insertion of other MPs, as it will allow a true ΔG value to be estimated by an extrapolation to zero FTAC-C6 concentration.
FIGURE 3.
Thermodynamic analysis of the MP bilayer insertion chaperoned by FTACs. Red symbols correspond to no surfactant present. ANX(TM) and ANX(IF) refer to TM and IF conformations of ANX, stabilized by different lipid compositions (5,7). The addition of long-chained FTAC-C8 (CMC = 0.03 mM) causes a sharp and nonlinear decrease in ΔG, whereas the addition of short-chained FTC-C6 (CMC = 0.33 mM) results in a gradual linear decrease. The red square corresponds to a true value of ΔG of insertion of the T-domain, estimated from the linear extrapolation to zero concentration (black arrow) of the data for FTAC-C6 chaperoned insertion (black squares). ΔGcor is the free energy difference between unchaperoned (open circle) and chaperoned insertion.
Almost all MPs will be insoluble, or at least will aggregate, outside the membrane environment (membrane-competent ANX, formed at acidic pH, is a lucky exception, which makes it such a useful model for thermodynamic studies (5,6)). As a result, MPs will require some solubilizing agent, e.g., detergent, amphipol, or a nondetergent fluorinated surfactant. In our previous study we demonstrated the advantages of fluorinated surfactants as new media for handling MPs (4). The most important feature that distinguishes FTACs from detergents is their lack of interaction with membranes, caused by poor miscibility of fluorinated and hydrogenated carbons. As a result, FTACs do not disrupt or partition into LUV even at concentrations above their CMC and at overwhelming excess over lipid (4). Thus, unlike detergents, they can be used for equilibrium thermodynamic measurements, which require MPs to be distributed between the lipid bilayer and the aqueous phase, where they are chaperoned by the surfactant. We have demonstrated a principal possibility of such chaperone-like action for insertion of diphtheria toxin T-domain (3,4), and here we use T-domain N235C mutant labeled with Alexa647 to present the first example (to our knowledge) of thermodynamic measurements of chaperoned MP insertion (Figs. 2 and 3).
Black symbols in Fig. 3 correspond to the FCS-measured apparent free energy of T-domain insertion into 1:3 POPC/POPG LUV, measured in the presence of different concentrations of FTAC-C6. Similar to observations in ANX, the concentration dependence is relatively shallow and linear. Linear extrapolation of these data to zero surfactant concentration (black arrow) corresponds to the true value of free energy of insertion ΔG = −12 kcal/mole (solid red square). The difference between this number and the one measured without application of chaperones (open square) is >3 kcal/mole, indicating that the failure to use chaperones can result in substantial errors in ΔG determination.
Most of the methods available for experimental determination of protein stability are not directly applicable to MPs in their native environment. White and co-workers (9, and references therein) suggested an alternative approach that relies on the use of membrane interactions of short soluble peptides. Although the method proved extremely useful for studying the energetics of IF binding (10) and folding (11), thermodynamic measurements of TM insertion turned out to be much more elusive (12). The application of chaperoned insertion achieved by the use of fluorinated surfactants, as demonstrated here, provides new tools for systematic exploration of the thermodynamics of MP organization. The next challenge will be to apply this methodology to study the thermodynamic stability of constitutive MPs, which are normally assembled into the membranes via translocon complexes.
Acknowledgments
We are grateful to Mr. M. A. Myers for his editorial help, and to Dr. H. T. Haigler for his gift of the ANX mutant.
This work was supported by the National Institutes of Health (GM069783).
Yevgen O. Posokhov's permanent address is Institute for Chemistry, V. N. Karazin Kharkov National University, Kharkov, Ukraine.
Editor: Anthony Watts.
References
- 1.Tribet, C., R. Audebert, and J.-L. Popot. 1996. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. USA. 93:15047–15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyton, C., E. Chabaud, Y. Chaudier, B. Pucci, and J.-L. Popot. 2004. Hemifluorinated surfactants: a non-dissociating environment for handling membrane proteins in aqueous solutions? FEBS Lett. 564:312–318. [DOI] [PubMed] [Google Scholar]
- 3.Palchevskyy, S. S., Y. O. Posokhov, B. Olivier, J. L. Popot, B. Pucci, and A. S. Ladokhin. 2006. Chaperoning of insertion of membrane proteins into lipid bilayers by hemifluorinated surfactants: application to diphtheria toxin. Biochemistry. 45:2629–2635. [DOI] [PubMed] [Google Scholar]
- 4.Rodnin, M. V., Y. O. Posokhov, C. Contino-Pepin, J. Brettmann, A. Kyrychenko, S. S. Palchevskyy, B. Pucci, and A. S. Ladokhin. 2008. Interactions of fluorinated surfactants with diphtheria toxin T-domain: testing new media for studies of membrane proteins. Biophys. J. 94:4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posokhov, Y. O., M. V. Rodnin, L. Lu, and A. S. Ladokhin. 2008. Membrane insertion pathway of annexin B12: thermodynamic and kinetic characterization by fluorescence correlation spectroscopy and fluorescence quenching. Biochemistry. 47:5078–5087. [DOI] [PubMed] [Google Scholar]
- 6.Ladokhin, A. S., and H. T. Haigler. 2005. Reversible transition between the surface trimer and membrane-inserted monomer of annexin 12. Biochemistry. 44:3402–3409. [DOI] [PubMed] [Google Scholar]
- 7.Ladokhin, A. S., J. M. Isas, H. T. Haigler, and S. H. White. 2002. Determining the membrane topology of proteins: Insertion pathway of a transmembrane helix of annexin 12. Biochemistry. 41:13617–13626. [DOI] [PubMed] [Google Scholar]
- 8.Ladokhin, A. S., R. Legmann, R. J. Collier, and S. H. White. 2004. Reversible refolding of the diphtheria toxin T-domain on lipid membranes. Biochemistry. 43:7451–7458. [DOI] [PubMed] [Google Scholar]
- 9.White, S. H., A. S. Ladokhin, S. Jayasinghe, and K. Hristova. 2001. How membranes shape protein structure. J. Biol. Chem. 276:32395–32398. [DOI] [PubMed] [Google Scholar]
- 10.Wimley, W. C., and S. H. White. 1996. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3:842–848. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Vidal, M., S. Jayasinghe, A. S. Ladokhin, and S. H. White. 2007. Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity. J. Mol. Biol. 370:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladokhin, A. S., and S. H. White. 2004. Interfacial folding and membrane insertion of a designed helical peptide. Biochemistry. 43:5782–5791. [DOI] [PubMed] [Google Scholar]