Abstract
Oriented circular dichroism (OCD) was used to characterize and compare in a quantitative manner the secondary structure and concentration dependent realignment of the antimicrobial peptides PGLa and MSI-103, and of the structurally related cell-penetrating peptide MAP in aligned phospholipid bilayers. All these peptides adopt an amphiphilic α-helical conformation, and from solid-state NMR analysis they are known to bind to membranes in two distinct orientations depending on their concentration. At low peptide/lipid (P/L) ratio the helices are aligned parallel to membrane surface (S-state), but with increasing concentration they realign to a tilted orientation (T-state), getting immersed into the membrane with an oblique angle supposedly as a result of dimer-formation. In macroscopically aligned liquid crystalline 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine bilayers the two limiting states are represented by distinct OCD spectra, and all spectra at intermediate peptide concentrations can be described by a linear combination of these two line shapes. The corresponding fraction of molecules occupying the T-state was determined by fitting the intermediate spectra with a superposition of the two extreme line shapes. By plotting this fraction versus 1/(P/L), the threshold P/L* ratio for realignment was extracted for each of the three related peptides. Despite their structural similarity distinctly different thresholds were obtained, namely for MSI-103 realignment starts already at a low P/L of ∼1:236, for a MAP derivative (using a nonaggregating analog containing a D-amino acid) the transition begins at P/L ∼1:156, whereas PGLa needs the highest concentration to flip into T-state at P/L ∼1:85. Analysis of the original MAP sequence (containing only L-amino acids) gave OCD spectra compatible with β-pleated conformation, suggesting that this peptide starts to aggregate with increasing concentration, unlike the other helical peptides. All these changes in peptide conformation and membrane alignment observed here by OCD seem to be functionally relevant, as they can be correlated with the membrane perturbing activities of the three antimicrobial and cell-penetrating sequences.
INTRODUCTION
Membrane-active antimicrobial peptides with typically 12–50 amino acids are found in many organisms as part of the innate immune system to defend the host against invading bacteria and other microorganisms (1–3). There is wide evidence that pore formation and disruption of the bacterial cell membrane is one of the prevailing modes of action of these peptides, although some of them can traverse the membrane and induce killing by acting on one or more intracellular targets, or by immunostimulation (4–6). These peptides are typically cationic, and many representatives adopt an amphipathic α-helical structure when bound to lipid bilayers. It is well known that bacterial membranes contain negatively charged lipids on the outer leaflet, whereas the corresponding lipids of eukaryotic cells are predominantly neutral. Electrostatic attraction and hydrophobic peptide-lipid interactions are thus critical factors that determine the selectivity and potency of these antimicrobial agents. A correlation of primary sequence or physical properties with biological activity, however, is rather difficult, because there are no straight forward parameters that can be used to predict the efficiency of a peptide as a therapeutic agent, nor to assess any deleterious side effects on eukaryotic cells.
To examine the structures of membrane-active peptides and to deduce their modes of action, solid-state NMR analysis of macroscopically oriented membranes is a powerful approach. A comprehensive picture of membrane-bound peptides in terms of their molecular conformation and membrane alignment was gained at quasi-atomic level by collecting a number of orientational constraints (7–9). For each constraint, a specific NMR-label has to be placed into a suitable position of the peptide, e.g., 15N- and 13C-isotopes are used in the peptide backbone, and 2H- and 19F-labels in the amino acid side chains. Drawbacks of this approach are i), the intrinsically low sensitivity of NMR that necessitates relatively large amounts of material (0.1–10 mg peptide per sample), and ii), the need for synthesizing multiple peptide analogs with selective isotope labels at different positions of the molecule. Thus, a high expenditure of preparative work, spectrometer time, and costs for isotope labeled amino acids is necessary to analyze membrane-bound peptides by solid-state NMR. In view of these experimental challenges, it is difficult to precisely follow, for example, concentration dependent changes in peptide structure or membrane alignment.
Optical techniques such as oriented circular dichroism (OCD) (10–12) and polarized attenuated total internal reflection FT-IR spectroscopy (13–16), on the other hand, are convenient alternative methods for monitoring the conformation as well as the orientation of peptides embedded in lipid bilayers that are macroscopically aligned with respect to the light beam. Although both, OCD and FT-IR do not give atomic resolution, they may be regarded as complementary methods to solid-state NMR, as they are carried out under virtually the same conditions (oriented samples) and describe global features of the secondary structure and alignment of peptides in lipid membranes. Another major advantage of both techniques, compared with solid-state NMR, is the higher sensitivity (e.g., in OCD a 20- to 200-fold lower amount of peptide, i.e., typically between 0.005 and 0.05 mg of peptide are used to prepare a sample) (17), and the fact that for both techniques no isotope labeling is required. Additionally, OCD and FT-IR allow relative fast spectral acquisition times and exact control of the temperature and hydration level of the sample. Although FT-IR can be used to observe both the peptides and the lipid molecules, OCD is unable to monitor any changes in the bilayer itself. Yet, many different types of conformational characteristics and changes thereof can be directly monitored with OCD and shed light on the mechanisms of action of small peptides in lipid ensembles.
OCD structure analysis of α-helical peptides is based on Moffit's theory (18), which predicts that the transition dipole moment of the π-π* electronic transitions of the amide chromophores in a helix are polarized parallel or perpendicular to the helix axis. The OCD spectrum of a helical peptide bound to a macroscopically aligned lipid bilayer has a specific line shape, which shows its orientation with respect to the plane of the membrane, as the intensity of the corresponding CD bands depends on this orientation. Characteristic OCD spectra are obtained for peptides that are either aligned parallel to the membrane surface or inserted upright into the lipid bilayer. The distinguishing feature in the OCD spectra is the presence or absence of a negative band around 208 nm, being indicative of a surface or transmembrane helix alignment, respectively.
The two distinct types of OCD line shape have been shown in previous studies on the α-helical antimicrobial peptides alamethicin (17,19–23), melittin (23–25), and magainin (25,26). Their spectra were also found to interconvert on changing the peptide concentration (expressed as the molar peptide/lipid ratio P/L), suggesting that the peptides are able to change their alignment in the membrane. Typically, at low P/L the OCD spectrum corresponds to a helix orientation parallel to the membrane surface, i.e., the so-called S-state. Above a certain threshold concentration P/L*, whose value depends on the lipid composition of the bilayer, the OCD spectrum gradually changes to one that corresponds to a helix orientation that is perpendicular to the plane of the bilayer and has been named the inserted I-state. Any intermediate states can be fitted by a linear combination of the S- and I-state spectra, though it is not possible to distinguish between a fast time-average and a co-existence of two populations. Besides peptide concentration and lipid composition, also the level of hydration can influence the helix alignment in the membrane, as it was shown for an alamethicin/DPhPC sample with constant P/L ratio, where the OCD line shape changed reversibly and continuously from one distinct spectrum to another (17,21).
Thus, OCD is a useful technique to differentiate between distinct alignments of α-helical peptides in lipid bilayers. Even though oriented CD spectra of β-sheet structures have been sparsely discussed in the literature (27) and there exists no proper theoretical basis for interpreting OCD spectra of any other secondary structure elements yet (28), the OCD method has been applied in an empirical and comparative approach to peptides comprising of β-strands (25,29,30). Analogously to α-helical peptides, it was found that the antimicrobial 18-residue protegrin-1 (that has a β-hairpin structure), and rhesus theta defensin (an 18-residue peptide with a cyclic structure that is cross-linked by 3 disulfide bonds), also exhibited two extreme OCD spectra that correspond to two distinct alignment states in the lipid bilayer. Again, the orientational preference is strongly influenced by the lipid composition, peptide concentration and hydration conditions, and intermediate states can be fitted by linear superpositions of the two extreme spectra (29,30).
Due to these benefits of the OCD technique, and based on the pioneering work of Olah and Huang (10) and later Wu et al. (11), we have built an OCD cell to measure oriented membrane-active peptides and proteins in their native lipid environment. The cell can be easily implemented into the sample compartment of a commercially available CD spectropolarimeter. With this OCD method at hand, it is now possible to speed up the process of screening many different experimental conditions, such as P/L ratio, temperature, and humidity of the sample, under which interesting and functionally relevant orientational and secondary structural changes are expected to occur in peptide-lipid systems. After identifying the key conditions by OCD, these parameters can then be specifically adjusted in designated samples, to resolve the structural details by the more elaborate methods like solid-state NMR, electron spin resonance, x-ray diffraction, or atomic force microscopy.
As an example for taking advantage of the OCD technique, we have studied the secondary structure and orientation of three α-helical peptides in a model membrane. The 21-residue peptide PGLa (GMASKAGAIAGKIAKVALKAL-NH2), which belongs to the magainin family (31), is one of many antimicrobial peptides found in the skin of the African frog Xenopus laevis (32–34). MSI-103 ([KIAGKIA]3-NH2) is a product of rational drug design based on the sequence of PGLa as a template, with an improved antimicrobial activity (35,36). The “Model Amphipathic Peptide” MAP (KLALKLALKALKAALKLA-NH2) is also an artificial sequence, but designed to exhibit cell-penetrating properties (37). All three peptides are cationic, with a net charge of +5 to +7, and the amino acid sequence implies an amphiphilic α-helical structure with the charged lysine side chains on one side and hydrophobic residues on the opposite face. They all have a disordered structure in aqueous solution and form α-helices in contact with charged lipid membranes (34,36,38). The conformation, alignment, and dynamics of PGLa (39–42), MSI-103 (43), and MAP (44) in oriented lipid bilayers have been comprehensively characterized using solid-state NMR. Each peptide has been found to realign in the membrane from an S- to a T-state with increasing concentration, but the individual threshold P/L* ratios were not accessible and could not yet be compared in a quantitative manner nor correlated with the respective biological activities.
In this study we present OCD data of the three different peptides in oriented 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC) bilayers, verifying that they all acquire an α-helical structure in membrane-bound conditions. Additionally, systematic OCD measurements at different P/L ratios could show the realignment of these peptides from an S- to a T-state, as found in our earlier solid-state NMR studies. From spectral deconvolution of the intermediate line shapes, the precise threshold concentration at which realignment starts could be extracted for each peptide. Moreover, when studying the original MAP sequence as well as two analogs carrying a d- and l-enantiomeric CF3-Phg side chain, we were able to compare their respective helicities and reorientations. At low P/L the OCD spectra of the MAP wild-type and of the l-epimer analog showed an α-helical structure, whereas at high peptide concentration they turned into a β-stranded type. Thus, the stiff D-CF3-Phg side chain evidently prevents peptide aggregation, and has been used successfully to convert the original MAP sequence into an improved membrane-perturbing agent that does not aggregate but realigns in the lipid bilayer just like the related peptides PGLa and MSI-103.
METHODS
Materials
DMPC was purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. PGLa (GMASKAGAIAGKIAKVALKAL-NH2), MSI-103 ([KIAGKIA]3-NH2), and MAP (KLALKLALKALKAALKLA-NH2) peptides were synthesized on an Applied Biosystems 433A synthesizer (Foster City, CA), using standard solid-phase Fmoc-protocols (45). The crude material was purified by RP-HPLC on a C18 column (10 × 200 or 22 × 250 mm, GRACE) using an acetonitrile/water gradient supplemented with 5 mM HCl (46). Epimeric peptides of the MAP analog (KLALKLALKALKAA-4-CF3-Phg-KLA-NH2) were obtained by replacing Leu15 by 4-CF3-d,l-phenylglycine (CF3-Phg) followed by their HPLC separation. The configurational analysis of the d- and l-epimers was carried out using Marfey's derivatization as described previously (46). The identity of all peptides was confirmed by MALDI-TOF/ESI mass spectrometry, and purity (∼95%) by analytical RP HPLC (4.6 × 250 mm).
Oriented samples
Oriented peptide-lipid samples for OCD measurements were prepared according to the single substrate method described by Ludtke et al. (47). An appropriate peptide-lipid solution was deposited onto a circular quartz glass plate with 20-mm diameter (Suprasil QS, Hellma Optik, Jena, Germany), which served as a UV-transparent window in the OCD cell. Before use, the quartz glass window was cleaned in concentrated nitric acid, and rinsed thoroughly and repeatedly with distilled water and finally with ethanol. Afterward, the window was dried in an oven at ∼60°C.
Stock solutions with a lipid concentration of 25 mg/mL were prepared by dissolving a total amount of ∼5 mg of lipid in a 1:1 (v/v) mixture of chloroform and methanol. Similarly, peptide stock solutions with concentrations of 0.1–1.0 mg/mL were made by dissolving the lyophilized peptide in an identical solvent mixture. Next, lipid and peptide solution aliquots were mixed in a vial to obtain the desired P/L molar ratio and thoroughly vortexed. A volume of 15–80 μl (depending on P/L ratio) of the dissolved mixture was deposited onto the OCD quartz glass window using a gas tight syringe, and the solvent was allowed to evaporate until the sample appeared dry. Afterward it was placed in a desiccator under 2-mbar vacuum for at least 3 h to ensure a complete removal of organic solvent. Finally, the window with the dried sample was assembled into the OCD cell and hydrated for ∼15 h at 30°C and 97.0% relative humidity (rH) or at 40°C and 96.4% rH (obtained by using a saturated K2SO4 solution; see description of the OCD cell), on which the lipid spontaneously aligns into well-oriented bilayers. The diameter of the membrane-covered sample area was between 9 and 12 mm. The amount of lipid and peptide deposited on the quartz glass plate was kept below 0.2 mg and 30 μg, respectively, resulting in comparatively thin samples to avoid absorption flattening and linear dichroism artifacts (48).
OCD cell
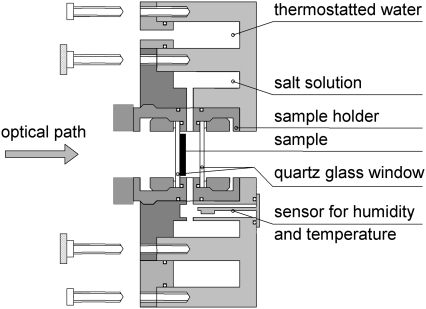
The home-built set-up used for the OCD measurements is similar to the cell described by Chen et al. (17). The optical path of the CD spectropolarimeter is parallel to the cylindrical axis of the cell and normal to the quartz glass window with the oriented sample. A cross-sectional view of the OCD cell is shown in Fig. 1. It consists of an aluminum frame with two ring-shaped cavities and a central bore for taking up the sample holder. Water from a computer-controlled external water bath thermostat (Julabo) with a thermo-electric feedback module was passed through the closed outer cavity of the cell, which allowed to control the temperature of the whole cell over a 5–50°C range with a stability of ±0.1°C. The second cavity around the central bore was filled with a small volume (∼300–500 μl) of saturated salt solution for controlling the humidity of the sample. The sample holder consists of a threaded tube with two quartz glass windows fixed vertically inside at a distance of 6 mm by two brass rings. Halfway between the windows the casing contains holes in a circular arrangement around the tube, so that the vapor from the salt solution can hydrate the sample without direct contact between the solution and the sample. The space between the two windows is sealed by four O-rings to avoid contact with ambient air. The humidity and temperature within the sample cell is monitored by a capacitive relative humidity and temperature sensor (SHT 75, Sensirion, Zurich, Switzerland), which is fixed in the immediate vicinity of the sample (within 12 mm) in a gap between the sample holder and the cavity containing the salt solution. The humidity can be measured with an absolute accuracy of ±1.8% rH by the sensor (±3% at values >90% rH). When using a saturated K2SO4 salt solution with 96–98% rH, the equilibration of temperature and humidity in the closed OCD cell takes ∼2–3 h. However, to assure proper hydration across the whole depth of the lipid bilayers, all samples were hydrated overnight (∼15 h). Sample equilibration was ensured by acquiring OCD spectra over a period of hours, until they did not change any further when taken for instance within 3–4 h.
FIGURE 1.
Cross-sectional view of the home-built OCD cell, developed to measure peptide alignment in lipid bilayers at constant temperature and humidity. Two circular reservoirs contain the thermostated water and the salt solution used to adjust the relative humidity in the central sample chamber. The cell is mounted on a rotation stage in the sample compartment of the CD spectropolarimeter and can be rotated around the cylindrical axis, which is coincident with the optical path of the polarized light.
The cylindrical OCD cell was mounted in the sample compartment of the spectropolarimeter on a rotation stage with a computer-controlled stepping motor (SKIDS-60YAW (θz); Sigma Koki Ltd., Tokyo, Japan). The OCD cell mount was attached in such a manner that the optical axis of the spectropolarimeter light beam coincides with the cylindrical axis of the cell and a symmetrical rotation around the beam axis was assured. Such rotational averaging diminishes possible spectral artifacts due to the linear dichroism (LD) that could be caused by imperfections in the sample, strain in the quartz glass plates, or imperfect alignment of the windows (12,17). To ensure that such artifacts are minimal, an LD spectrum of the sample was acquired after each series of OCD measurements. If the maximum LD was ≤0.003 dOD, the corresponding rotationally averaged OCD spectra were rated to be free of LD artifacts.
Oriented circular dichroism spectroscopy
CD measurements were carried out on a Jasco J-810 spectropolarimeter (Jasco, Tokyo, Japan). The instrument was calibrated with a 0.06% (w/v) aqueous solution of ammonium (+)-10-camphorsulfonate (ACS) at 290.5 nm. OCD spectra were acquired between 260 and 180 nm at 0.1-nm intervals. Three repeat scans at a scan rate of 10 nm min−1, 4 s response time and 1 nm bandwidth were averaged for each sample at every 45.0° of rotation of the cell, as described in Chen et al. (17) resulting in spectral acquisition times of ∼3 h per sample. The eight successive spectra were averaged. Background spectra of pure lipid bilayers were measured likewise in a separate experiment as a control, and were subtracted from the corresponding spectra of the peptide-containing samples.
OCD spectral analysis
A method for finding two extreme spectra that correlate with different alignments of an α-helix in lipid bilayers, e.g. in the S- and I-state, has been described in the literature for different peptides in various lipid environments (17,19,26,30). It was shown that intermediate OCD spectra can be described as a linear superposition of the two extreme spectra. If one can find these extreme spectra by varying the temperature or humidity of a single sample, then the amplitudes of the spectra will be intrinsically normalized to one another. However, for the peptides investigated in this study, the extreme spectra were identified by varying the P/L ratio, hence many different samples had to be prepared and the resulting OCD spectra had to be normalized according to the different peptide concentrations. In such cases one common point of intersection of the two extreme spectra and of all intermediate spectra, i.e., an isodichroic point, must exist (17). In our experiments, this isodichroic point was located in the following way: the relative amounts of peptide in the different OCD samples were known from the preparation protocol (as quantitatively confirmed by their UV spectra obtained during the measurements). To normalize the OCD spectra, in a first step each original spectrum was multiplied by an individual factor that was calculated from the ratio of the amount of peptide in the least concentrated sample and the amount of peptide in the respective sample. The resulting scaled spectra will intersect one another not exactly in one point but close to one point (given that there are always some small errors in peptide concentration, e.g., due to slightly different sample areas). This procedure was only used to locate the approximate position of the isodichroic point with an accuracy of ∼±2 nm. In the second round of the normalization procedure, each original OCD spectrum in any one peptide/lipid system was multiplied once again by a certain factor, which was now calculated from the ratio of the ellipticity of the least concentrated sample and the ellipticity of each respective sample at a single selected wavelength near the approximate position of the isodichroic point. This procedure was repeated at four or five different wavelengths close to the expected isodichroic point, resulting in a set of normalized OCD spectra intersecting in one point for each selected wavelength. Afterward, for each set the intermediate spectra were fitted by a linear combination of the extreme spectra (representing S- and T-state). Finally, the standard deviations of experimental and fitted data of each intermediate spectrum for a particular set were compared with the standard deviations obtained for the sets at other wavelengths. The set of normalized spectra with the lowest average standard deviation of all intermediate spectra was considered best, and in this way the exact location of the isodichroic point of a peptide/lipid system was identified.
RESULTS AND DISCUSSION
OCD spectra of PGLa, MSI-103, and MAP in DMPC bilayers
From solution CD experiments described in the literature it is known that PGLa, MSI-103, and MAP change from a random coil conformation in aqueous solution to an α-helical structure in the presence of lipid vesicles (34,36,38). Solid-state NMR studies have shown that interaction of these peptides with hydrated lipid bilayers not only induces an α-helical fold, but also a realignment of the helix, from an orientation parallel to the membrane surface (S-state) at low peptide concentration to a tilted orientation (T-state) at high concentration (39–44). As there was no OCD data available for these peptides to date, we have prepared, analogously to the earlier NMR experiments, oriented DMPC membranes with the peptides embedded at various P/L ratios, to i), study their conformation and alignment with an independent technique, ii), to find out whether the OCD method confirms the previous solid-state NMR results, and iii), to determine quantitatively the individual thresholds of realignment for the three peptides for the first time, which might then iv), be compared and correlated with their respective membrane-perturbing effects.
Fig. 2 A shows the normalized OCD spectra of the naturally occurring peptide PGLa in oriented DMPC bilayers in the liquid-crystalline (Lα) phase (40°C) for different P/L ratios (all at full hydration). The line shape of these OCD spectra, with a positive maximum around 192 nm and two negative bands at 209 and 223 nm, is characteristic of a peptide with a significant α-helical content. If one compares the spectra of PGLa at P/L = 1:164 and 1:109 with the S-state spectrum of the α-helical antimicrobial peptide alamethicin in the literature (17,22), the fully pronounced negative CD band at 209 nm is indicative of the S-state for PGLa at low peptide concentrations. With increasing PGLa concentration the spectral amplitude generally decreases at all wavelengths, but also the relative intensity of the 209 nm band compared to the 223 nm band is diminished, and simultaneously the maximum of the positive band is shifted from 192 to 195 nm, until at a P/L ratio of ∼1:20 a final state is obtained. These spectral changes clearly reflect the realignment of the peptide helix as a function of concentration, similar to the OCD changes of other antimicrobial peptides described in the literature, e.g., for alamethicin and melittin (17). However, in contrast to those peptides, for PGLa the extreme spectrum at high P/L ratio still has a pronounced residual negative intensity, and the band at 209 nm does not disappear completely. This observation suggests that a fully immersed I-state is not reached under the experimental conditions chosen here. Instead, realignment of PGLa stops at a tilted T-state, where the helix is oriented at an oblique angle with respect to the membrane surface. These findings are in full agreement with the solid-state NMR results on PGLa in DMPC (41), which showed that at a P/L of 1:200 the peptide lies almost flat on the bilayer surface with a tilt angle τ of 98° (S-state), whereas at a high concentration of P/L = 1:50 the tilt angle increases by ∼30° to τ ∼125° (T-state). It has to be mentioned that OCD does not allow to discriminate between the following three possibilities: i), whether the T-state is a genuine state with a stable alignment, ii), whether the T-state lineshape is an arithmetically weighted sum of the more extreme I- and S-state spectra, or iii), whether it is a time-averaged intermediate between rapidly interconverting S- and I-states. Nevertheless, we have observed previously by 2H-NMR a dynamic interconversion between the S- and T-states on a ms timescale (42). The rate of exchange was obtained from the quadrupolar splittings, which were averaged by motions on the timescale of 20 ms, thus showing that solid-state NMR is able to detect such averaging. Yet, a recent 19F-NMR analysis proved clearly that the T-state is a genuine and stable orientational state (49), rather than an average between the S- and I-states, because the 19F dipolar splittings showed plateau regions for the T-state that do not correspond to arithmetic averages. Hence we suggest that this OCD spectrum represents a genuine T-state population.
FIGURE 2.
Oriented CD spectra of peptides in DMPC bilayers, showing their predominant α-helical conformation and showing their realignment from a surface-bound S- to a tilted T-state induced by increasing the molar P/L. (A) PGLa: all spectra were acquired at 40°C and 98% rH, and normalized to cross the same isodichroic point at 199 nm (see Methods). (B) MSI-103 (modified from Strandberg et al. (43)): same conditions, spectra were normalized to an isodichroic point at 198 nm.
A comparable realignment has been observed by NMR for the related, designer-made antimicrobial peptide MSI-103 in DMPC bilayers (in the Lα phase at full hydration). Fig. 2 B shows the normalized OCD spectra of MSI-103 for varying P/L ratios, exhibiting also the characteristics of a peptide with an α-helical conformation. The spectra show that MSI-103 undergoes the same kind of S- to T-state transition as PGLa, given the distinct intensity decrease of the negative 207 nm band, the red-shift of the positive peak near 191 nm, and the overall reduction in amplitude. The spectra at a P/L ratio of 1:400 and 1:300 are almost equivalent and correspond to the S-state, whereas at 1:200 the line shape starts to change. These P/L values indicate that the MSI-103 begins to realign from the S- to the T-state at much lower concentration than PGLa. Following the gradual changes by way of a series of intermediate spectra, the final T-state is reached at P/L ratios of 1:40 and 1:20. Again, the 207-nm band does not disappear completely, which confirms that the peptide is not in the I-state. These results obtained from OCD are again consistent with our previous 19F-NMR structure analysis of MSI-103 in oriented DMPC bilayers, which showed an S-state at P/L = 1:400 with a tilt angle of τ = 101° (43). For a sample of P/L = 1:200, 2H-NMR had shown an increased tilt angle of 111°, representing a mixture of pure S- and T-state, whereas at 1:50 the pure T-state was reported with a tilt angle of 125° (43), similarly to the OCD results presented.
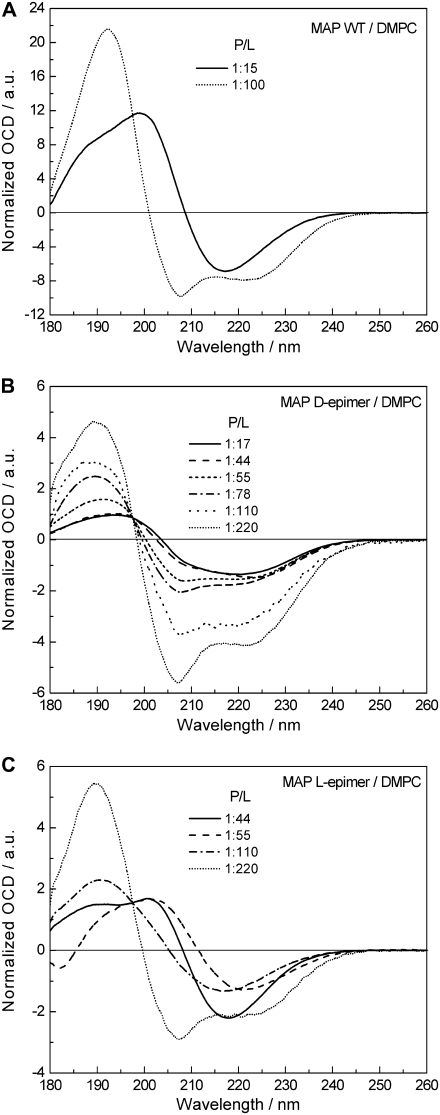
A completely different behavior is observed in Fig. 3 A in the OCD measurements of the MAP wild-type peptide in DMPC bilayers. Here, it was difficult to obtain OCD spectra in the first place, because the lipid-peptide preparations tended to slide off the vertical window in the OCD cell during the measurement at 40°C (i.e., the temperature chosen above the DMPC phase transition). In only two cases did we manage to get stable samples, and these two OCD spectra, depicted in Fig. 3 A for P/L ratios of 1:100 and 1:15, show clearly a conformational change on going from low to high peptide concentration. The spectrum at P/L = 1:100 shows a predominantly α-helical conformation with an S-state alignment (Fig. 2, A and B). At P/L = 1:15, on the other hand, the OCD spectrum exhibits a broad positive band with a shoulder at ∼189 and a maximum at 200 nm, and a single negative band around 217 nm, which points to a more β-stranded fold. To better understand this phenomenon and to emphasize the potential of the OCD technique in supporting solid-state NMR structural investigations with labeled peptides, we carried out further OCD measurements with two modified MAP analogs. In KLALKLALKALKAA-CF3-Phg-KLA-CONH2, the leucine residue at position 15 had been substituted by 4-CF3-d,l-phenylglycine, resulting in two epimeric peptide analogs containing D-CF3-Phg and L-CF3-Phg. As already mentioned above, OCD does not require any isotope labeling, but of course it allows measuring such peptides, which had originally been synthesized for the solid-state 19F-NMR analysis. A comparison of conventional CD spectra of the wild-type (WT) and CF3-Phg labeled MAP analogs in TFE/phosphate buffer (1:1 v/v) proved that a single substitution with the nonnatural 19F-labeled amino acid did not induce any significant conformational changes of the peptide in solution (44).
FIGURE 3.
OCD comparison of the cell-penetrating MAP wild-type peptide and its CF3-Phg substituted d- and l-epimeric analogs in DMPC bilayers as a function of P/L ratio. All spectra were acquired at 40°C and 98% rH, and normalized to cross the same isodichroic point at 197.5 nm. (A) MAP wild-type: showing at a low P/L ratio of 1:100 the peptide in an α-helical conformation and S-state, whereas at high P/L of 1:15 a β-stranded structure is predominant. (B) MAP d-epimer: showing an α-helical conformation throughout, and realignment from S- to T-state with increasing peptide concentration. (C) MAP l-epimer: showing the peptide in an α-helical conformation and S-state at low concentration, and β-pleated structure at high P/L ratios.
Fig. 3 B shows the normalized OCD spectra of the d-epimeric MAP analog in DMPC membranes for varying P/L ratios, using same conditions as in the experiments described above. In this case no fluidization of the samples was observed. As it had been expected for the amphiphilic MAP sequence, the line shape at low peptide concentration (P/L ∼1:220) now exhibits the typical features of an α-helix, with a positive band around 190 nm and the two negative peaks at 207 and 223 nm. The helicity of the MAP d-epimer seems to be somewhat lower than for the other peptides PGLa, MSI-103, and the MAP-WT. This is deduced from the smaller intensity ratio between the positive maximum at 190 nm and the negative minimum at 207 nm in relation to the other peptides, which implies a higher random coil contribution in the MAP d-epimer, diminishing the intensity of the positive band in the spectral range from 190 to 205 nm. The fully pronounced 207 nm band in the OCD spectrum of the MAP d-epimer at P/L ∼1:220 shows once again that also this peptide lies parallel to the membrane surface at low concentration. With increasing P/L ratio the intensity of the band at 207 nm decreases compared to 223 nm, showing that the d-epimer undergoes a realignment as an α-helix in the same way as PGLa and MSI-103, but in contrast to MAP-WT. For the d-epimeric MAP analog the extreme spectrum at high peptide concentration is reached at P/L ratios between 1:44 and 1:17. The residual negative ellipticity at 207 nm shows once again that this peptide favors a tilted state over an inserted state in liquid crystalline DMPC. Similar results had been recently obtained by solid-state 19F-NMR (44), where the same MAP d-epimer had exhibited two distinct states, and was found to be mobile (rather than immobilized by aggregation or multimerization) up to a high P/L of 1:20.
The OCD line shapes of the MAP L-epimer showed a behavior similar to that of MAP-WT. The spectra depicted in Fig. 3 C were normalized to intersect at the isodichroic point of the d-epimer at 197.5 nm, to allow for a better comparison with the data in Fig. 3, A and B. The corresponding spectra in Fig. 3 C clearly prove that at a low P/L ∼1:220 this peptide has the propensity to form an α-helix and adopt an S-state parallel to the membrane surface. However, when the concentration is raised the line shape changes drastically, as for MAP-WT. The spectrum at P/L of 1:110 compared with the spectrum at 1:220 shows a much broader positive band with a maximum at ∼191 nm, and only a single broad negative band around 217 nm. For a pure β-stranded structure one would expect the maximum of the positive band at ∼197 nm, hence this spectrum seems to represent a mixture of a large fraction of β-stranded and a lower percentage of α-helical conformations. For higher P/L ratios a shoulder around 190 and a maximum at ∼202 nm appears and again the distinct negative band at 217 nm, in full accordance with wild-type MAP (Fig. 3 A).
Only few OCD studies of peptides with a predominant β-pleated structure have been reported: the spectra of the synthetic polypeptide poly(Leu-Lys) (28), the cyclic peptide RTD-1 (30), and of protegrin-1 (29). Some of these spectra were correlated with a β-strand conformation oriented parallel to the membrane surface. Generally, the MAP l-epimer and MAP-WT spectra with P/L > 1:110 presented here, have a line shape similar to the S-state spectra of RTD-1 and poly(Leu-Lys), e.g., a broad maximum around 195 nm and one pronounced minimum at wavelengths >212 nm, however the exact position of the bands does not agree. In contrast to the RTD-1 and poly(Leu-Lys) data, the MAP spectra at high P/L ratios also possess strong positive ellipticity at wavelengths >200 nm, with an intersection of the zero baseline between 208 and 212 nm. Additionally, a similarity with solution CD spectra of Aβ-(1–42) peptide in its aggregated form (50) can be stated, which also shows a rather broad maximum with positive ellipticity in the range of 190–205 nm and a broad minimum between 212–220 nm. Due to these similarities with CD and OCD spectra of β-sheet peptides one can safely assume a predominantly β-pleated structure for the MAP wild-type and its l-epimeric analog at high P/L ratios in DMPC bilayers. Solid-state 19F-NMR studies have also reflected an immobilization of MAP at high concentration, suggesting its extensive aggregation (44). Additionally, IRRAS investigations on MAP confirm the transition from helical to β-pleated conformation at high concentrations (51). The absence of any typical β-pleated features in the OCD spectra of the d-epimeric MAP analog (Fig. 3 B) can be explained by the fact that the stiff D-CF3-phenylglycine side chain projects away from the peptide backbone in a sterically obstructive manner that hampers self-assembly of the peptides into β-sheets, as discussed in Reichert et al. (52). The incorporation of this unnatural d-amino acid has thus proven to be an effective trick to prevent the formation of β-stranded fibrils or cross-β-sheet amyloid.
Threshold values for the transition between S- and T-state
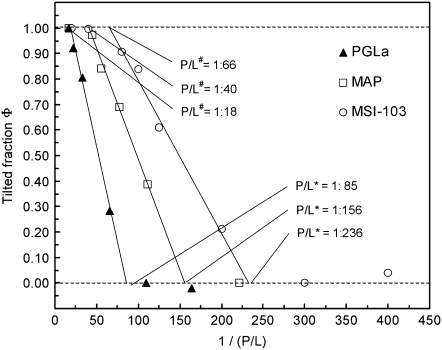
From the OCD spectra of PGLa, MSI-103, and MAP presented in Fig. 2, A and B, and Fig. 3 B, the threshold concentrations for the transition from S- to T-state were determined according to the method described in Chen et al. (17). The respective fractions of the T- and S-states were determined by deconvolution of the intermediate spectra, using the corresponding extreme spectra. The calculated fraction φ of the T-state was plotted as a function of the reciprocal P/L ratio, as described in Chen et al. (17). This resulted in characteristic curves for all three peptides (Fig. 4). At low concentration all peptides are in the S-state (φ ≈ 0), until the T-state starts to appear at a threshold concentration P/L*, and beyond some higher threshold P/L# all peptides have finally reached the full T-state. For each of the three peptides, the data points in the transition region between the two thresholds concentrations fall on a straight line. The individual threshold concentrations P/L* for PGLa, MSI-103 and MAP were assessed from the intersection of the corresponding lines of best fit with the line of φ = 0, and the P/L# values were obtained from the intersection with the line φ = 1. For all of the three peptides P/L# was ∼4 times P/L*, but remarkably the actual value of P/L* is very different for each peptide. MSI-103 starts already to realign into the T-state at a low P/L* of ∼1:236, whereas for MAP, transition starts at P/L* ∼1:156, and PGLa needs the highest concentration to get into T-state at P/L* ∼1:85. (In solid-state NMR experiments described in Tremouilhac et al. (42), the threshold concentration P/L* for PGLa realignment in DMPC was estimated to be between 1:200 and 1:100, which indicates a small discrepancy to the value of 1:85 determined here by OCD. However, this can be explained by somewhat different temperature and humidity conditions of the two measurement series.) The conclusion that MSI-103 has a much lower threshold than PGLa for realignment from the S- to the T-state, had also been observed in a qualitative manner by solid-state NMR (43).
FIGURE 4.
Fraction φ of the tilted peptide in the T-state as a function of the inverse (P/L) ratio for PGLa, MSI-103, and the MAP D-epimer in DMPC bilayers. Data in the transition regions were fitted to straight lines, whose points of intersection with the φ = 0 line give the threshold P/L* values, where the T-state starts to appear. Above some higher threshold value P/L#, which is obtained from the intersection of the fitted lines with the Φ = 1 line, all peptides have finally reached the T-state.
Table 1 lists the threshold concentration (given as P/L*), the net charge, and the hydrophobic dipole moment (μH) per residue of the three peptides PGLa, MSI-103, and d-epimeric MAP, when folded as an α-helix. The magnitude of μH is a measure of the amphiphilicity of the helix (53,54). Amphiphilic α-helical peptides, such as the ones investigated here, having mainly polar side chains protruding from one face and apolar ones protruding from the other, are characterized by large values of μH, and they tend to seek interfaces between polar and apolar phases, such as the surfaces of membranes. Hydrophobic moments were calculated according to Eq. 1 of Eisenberg et al. (53), using four different hydrophobicity scales, namely the Eisenberg normalized consensus scale (54), and three whole-residue hydrophobicity scales by White et al. (55,56). The decrease of the threshold concentration P/L* in the order PGLa > MAP > MSI-103 is correlated inversely with the net charge of the respective peptides on the one hand, and with the hydrophobic moment per residue on the other hand, regardless of which hydrophobicity scale is used for calculating μH. In other words, the higher the net charge and the amphiphilicity of a helical peptide, the lower is its threshold concentration necessary for realigning from a surface-bound to a tilted state in the DMPC lipid bilayer. The flip from the S- to the T-state has been explained by the formation of dimers in our earlier solid-state NMR investigations of these peptides (39–43), which thus seems to correlate with the membrane-perturbing activity of the respective peptide sequence. The lower threshold concentration found for MSI-103 in DMPC model membranes with both independent techniques (solid-state NMR and OCD) and the increased tendency for dimerization could be a first indication for a correlation with the differential antibacterial activities of these two peptides against seven bacterial strains, which has been tested by our group (57). Indeed, MSI-103 needs a lower minimum concentration to inhibit bacterial growth than PGLa, as was also found in the original study of the designer-made peptide sequences, where MSI-103 turned out to be more effective against bacteria with four- to eightfold lower MIC than PGLa (35,36). Nevertheless, to ascertain such a general correlation the order of the threshold values should be tested in many more different types of lipids.
TABLE 1.
Comparison of the threshold concentration P/L* for realignment, the net charge, and the hydrophobic dipole moment μH (calculated using different hydrophobicity scales) of PGLa, d-epimeric MAP, and MSI-103
| Parameter | PGLa | MAP | MSI-103 |
|---|---|---|---|
| Threshold P/L* ratio for realignment | 1:85 | 1:156 | 1:236 |
| Net charge | +5 | +6 | +7 |
| μH/residue (Eisenberg, normalized consensus scale) (53,54) | 0.411 | 0.524† | 0.631 |
| μH/residue (White, octanol interface scale) (55,56) | 0.357 | 0.377† | 0.594 |
| μH/residue (White, interface scale) (55,56) | 0.176 | 0.212† | 0.247 |
| μH/residue (White, octanol scale) (55,56) | 0.519 | 0.588† | 0.833 |
μH data for d-epimeric MAP were calculated by using the hydrophobicity of Leu at position 15 (MAP-WT) because the hydrophobicity value of the nonnatural, hydrophobic amino acid 4-CF3-phenylglycine is not listed in the scales used. However, the effect on the actual value of μH should be marginal.
MAP, which has a similar sequence as the two antimicrobial peptides a net charge of +6, and a hydrophobic moment, which lies in between the two other peptides, is known for its cell-penetrating properties rather than for its antimicrobial activity. Nevertheless, due to its comparable amphiphilic structure a concentration increase in the lipid bilayer also leads to realignment of the peptide showing an intermediate threshold concentration. This can be understood from recent findings that there seem to be common steps in certain translocation mechanisms of cell-penetrating peptides and in the lytic action of antimicrobial peptides in lipid membranes (58).
CONCLUSIONS
Oriented circular dichroism analysis of the three membrane-active peptides PGLa, MSI-103, and the d-epimer of MAP, showed that they are all α-helical when embedded in hydrated DMPC bilayers in the liquid crystalline state. The OCD technique, which gives complementary information to solid-state NMR but requires no labeling, confirms the realignment of these helices from a surface bound S-state to a tilted T-state with increasing peptide concentration, as reported earlier by NMR. Realignment into the T-state started to occur in all cases at a well-defined threshold concentration P/L*, which varies considerably from peptide to peptide (PGLa > d-epimeric MAP > MSI-103). This threshold seems to correlate with the tendency of the peptides to dimerize and perturb the lipid bilayer, which may be related to their respective antimicrobial and cell penetrating functions. Furthermore, for the wild-type sequence of MAP and an l-epimeric analog, a distinct conformational change from an α-helical to a β-stranded conformation was observed, indicative of aggregation. These findings show the ability of OCD to identify and characterize functionally relevant concentration-dependent changes of membrane-bound peptides in a quantitative manner.
Acknowledgments
We are grateful to Silvia Gehrlein, Stefanie Maurer, and Olaf Zwernemann at Forschungszentrum Karlsruhe for help with peptide synthesis and purification, Heide Mathieu for recording the OCD spectra, and Karl Krämer for helping to build the OCD cell.
Editor: Paul H. Axelsen.
References
- 1.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta. 1462:11–28. [DOI] [PubMed] [Google Scholar]
- 2.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van't Hof, W., E. C. Veerman, E. J. Helmerhorst, and A. V. Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597–619. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. L., and R. E. W. Hancock. 2006. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18:24–30. [DOI] [PubMed] [Google Scholar]
- 5.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250. [DOI] [PubMed] [Google Scholar]
- 6.Otvos, L., Jr. 2002. The short proline-rich antibacterial peptide family. Cell. Mol. Life Sci. 59:1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strandberg, E., and A. S. Ulrich. 2004. NMR methods for studying membrane-active antimicrobial peptides. Concepts Magn. Reson. 23A:89–120. [Google Scholar]
- 8.Ulrich, A. S. 2005. Solid-state 19F-NMR methods for studying biomembranes. Prog. NMR Spectr. 46:1–21. [Google Scholar]
- 9.Sternberg, U., R. Witter, and A. S. Ulrich. 2007. All-atom molecular dynamics simulations using orientational constraints from anisotropic NMR samples. J. Biomol. NMR. 38:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olah, G. A., and H. W. Huang. 1988. Circular dichroism of oriented α-helices: I. Proof of the exciton theory. J. Chem. Phys. 89:2531–2538. [Google Scholar]
- 11.Wu, Y., H. W. Huang, and G. A. Olah. 1990. Method of oriented circular dichroism. Biophys. J. 57:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel, H. 1987. Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry. 26:4562–4572. [DOI] [PubMed] [Google Scholar]
- 13.Tamm, L. K., and S. A. Tatulian. 1997. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 30:365–429. [DOI] [PubMed] [Google Scholar]
- 14.Goormaghtigh, E., V. Raussens, and J.-M. Ruysschaert. 1999. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta. 1422:105–185. [DOI] [PubMed] [Google Scholar]
- 15.Tatulian, S. A. 2003. Attenuated total reflection Fourier transform infrared spectroscopy: a method of choice for studying membrane proteins and lipids. Biochemistry. 42:11898–11907. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu, H., L. Liu, I. V. J. Murray, and P. H. Axelsen. 2007. A mechanistic link between oxidative stress and membrane mediated amyloid genesis revealed by infrared spectroscopy. Biochim. Biophys. Acta. 1768:1913–1922. [DOI] [PubMed] [Google Scholar]
- 17.Chen, F. Y., M. T. Lee, and H. W. Huang. 2002. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study on alamethicin. Biophys. J. 82:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffitt, W. 1956. Optical rotatory dispersion of helical polymers. J. Chem. Phys. 25:467–478. [Google Scholar]
- 19.Huang, H. W., and Y. Wu. 1991. Lipid-alamethicin interactions influence alamethicin orientation. Biophys. J. 60:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, K., S. J. Ludtke, W. T. Heller, and H. W. Huang. 1996. Mechanism of alamethicin insertion into lipid bilayers. Biophys. J. 71:2669–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller, W. T., K. He, S. J. Ludtke, T. A. Harroun, and H. W. Huang. 1997. Effect of changing the size of lipid headgroup on peptide insertion into membranes. Biophys. J. 73:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, M. T., F. Y. Chen, and H. W. Huang. 2004. Energetics of pore formation induced by membrane active peptides. Biochemistry. 43:3590–3599. [DOI] [PubMed] [Google Scholar]
- 24.Yang, L., T. A. Harroun, T. M. Weiss, L. Ding, and H. W. Huang. 2001. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 81:1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding, L., L. Yang, T. M. Weiss, A. J. Waring, R. J. Lehrer, and H. W. Huang. 2003. Interaction of antimicrobial peptides with lipopolysaccharides. Biochemistry. 42:12251–12259. [DOI] [PubMed] [Google Scholar]
- 26.Ludtke, S., K. He, J. Wu, and H. W. Huang. 1994. Cooperative membrane insertion of magainin correlated with its cytolytic activity. Biochim. Biophys. Acta. 1190:181–184. [DOI] [PubMed] [Google Scholar]
- 27.Woody, R. W. 1993. The circular dichroism of oriented β sheets: theoretical predictions. Tetrahedron Asymmetry. 4:529–544. [Google Scholar]
- 28.Bazzi, M., R. W. Woody, and A. Brack. 1987. Interaction of amphipathic polypeptides with phospholipids: characterization of conformations and the CD of oriented β-sheets. Biopolymers. 26:1115–1124. [DOI] [PubMed] [Google Scholar]
- 29.Heller, W. T., A. J. Waring, R. I. Lehrer, and H. W. Huang. 1998. Multiple states of β-sheet peptide protegrin in lipid bilayers. Biochemistry. 37:17331–17338. [DOI] [PubMed] [Google Scholar]
- 30.Weiss, T. M., Y. Lin, L. Ding, A. J. Waring, R. I. Lehrer, and H. W. Huang. 2002. Two states of the cyclic antimicrobial peptide RTD-1 in lipid bilayers. Biochemistry. 41:10070–10076. [DOI] [PubMed] [Google Scholar]
- 31.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 84:5449–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann, W., K. Richter, and G. Kreil. 1983. A novel peptide designated PYLa and its precursor as predicted from cloned mRNA of Xenopus laevis skin. EMBO J. 2:711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter, K., H. Aschauer, and G. Kreil. 1985. Biosynthesis of peptides in the skin of Xenopus laevis: isolation of novel peptides predicted from the sequence of cloned cDNAs. Peptides. 6:17–21. [DOI] [PubMed] [Google Scholar]
- 34.Soravia, E., G. Martini, and M. Zasloff. 1988. Antimicrobial properties of peptides from Xenopus granular gland secretions. FEBS Lett. 228:337–340. [DOI] [PubMed] [Google Scholar]
- 35.Maloy, W. L., and U. P. Kari. 1995. Structure-activity studies on magainins and other host defense peptides. Biopolymers. 37:105–122. [DOI] [PubMed] [Google Scholar]
- 36.Blazyk, J., R. Wiegand, J. Klein, J. Hammer, R. M. Epand, R. F. Epand, W. L. Maloy, and U. P. Kari. 2001. A novel linear amphipathic β-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J. Biol. Chem. 276:27899–27906. [DOI] [PubMed] [Google Scholar]
- 37.Langel, U. 2002. Cell-Penetrating Peptides: Processes and Applications. 1st ed., CRC Press, Boca Raton, FL.
- 38.Dathe, M., M. Schürmann, T. Wieprecht, A. Winkler, M. Beyermann, E. Krause, K. Matsuzaki, O. Murase, and M. Bienert. 1996. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 35:12612–12622. [DOI] [PubMed] [Google Scholar]
- 39.Glaser, R. W., C. Sachse, U. H. Dürr, P. Wadhwani, and A. S. Ulrich. 2004. Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels. J. Magn. Reson. 168:153–163. [DOI] [PubMed] [Google Scholar]
- 40.Glaser, R. W., C. Sachse, U. H. N. Dürr, P. Wadhwani, S. Afonin, E. Strandberg, and A. S. Ulrich. 2005. Concentration-dependent realignment of the antimicrobial peptide PGLa in lipid membranes observed by solid-state 19F-NMR. Biophys. J. 88:3392–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strandberg, E., P. Wadhwani, P. Tremouilhac, U. H. N. Dürr, and A. S. Ulrich. 2006. Solid state NMR analysis of the PGLa peptide orientation in DMPC bilayers: structural fidelity of 2H- versus high sensitivity 19F-NMR. Biophys. J. 90:1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremouilhac, P., E. Strandberg, P. Wadwhani, and A. S. Ulrich. 2006. Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid-state 2H-NMR. Biochim. Biophys. Acta. 1758:1330–1342. [DOI] [PubMed] [Google Scholar]
- 43.Strandberg, E., N. Kanithasen, D. Tiltak, J. Bürck, P. Wadhwani, O. Zwernemann, and A. S. Ulrich. 2008. Comparative solid-state NMR analysis of the designer-made antibiotic MSI-103 in lipid bilayers and its parent peptide PGLa. Biochemistry. 47:2601–2616. [DOI] [PubMed] [Google Scholar]
- 44.Wadhwani, P., J. Bürck, E. Strandberg, C. Mink, S. Afonin, M. Ieronimo, M. Diefenbacher, O. Kassel, and A. S. Ulrich. 2008. Preventing peptide aggregation by inhibition of β-sheet formation. J. Am. Chem. Soc. In press. [DOI] [PubMed]
- 45.Fields, G. B., and R. L. Noble. 1990. Solid-phase peptide synthesis utilizing 9-fluore-nylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161–214. [DOI] [PubMed] [Google Scholar]
- 46.Afonin, S., R. W. Glaser, M. Berditchevskaja, P. Wadhwani, K. H. Gührs, U. Möllmann, and A. S. Ulrich. 2003. 4-Fluoro-phenylglycine as a label for 19F-NMR structure analysis of membrane-associated peptides. ChemBioChem. 4:1151–1163. [DOI] [PubMed] [Google Scholar]
- 47.Ludtke, S., K. He, and H. W. Huang. 1995. Membrane thinning caused by magainin 2. Biochemistry. 34:16764–16769. [DOI] [PubMed] [Google Scholar]
- 48.Gibson, N. J., and J. Y. Cassim. 1989. Evidence for an αII-type helical conformation for bacteriorhodopsin in the purple membrane. Biochemistry. 28:2134–2139. [Google Scholar]
- 49.Afonin, S., S. L. Grage, M. Ieronimo, P. Wadhwani, and A. S. Ulrich. 2008. Temperature-dependent transmembrane insertion of the amphiphilic peptide PGLa in lipid bilayers observed by solid state 19F-NMR. J. Am. Chem. Soc. In press. [DOI] [PubMed]
- 50.Tomaselli, S., V. Esposito, P. Vangone, N. A. J. van Nuland, A. M. J. J. Bonvin, R. Guerrini, T. Tancredi, P. A. Temussi, and D. Picone. 2006. The α-to-β conformational transition of Alzheimer's Aβ-(1–42) peptide in aqueous media is reversible: a step-by-step conformational analysis suggests the location of β conformation seeding. Chembiochem. 7:257–267. [DOI] [PubMed] [Google Scholar]
- 51.Kerth, A., A. Erbe, M. Dathe, and A. Blume. 2004. Infrared reflection absorption spectroscopy of amphipathic model peptides at the air/water interface. Biophys. J. 86:3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reichert, J., D. Grasnick, S. Afonin, J. Bürck, P. Wadhwani, and A. S. Ulrich. 2007. A critical evaluation of the conformational requirements of fusogenic peptides in membranes. Eur. Biophys. J. 36:405–413. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg, D., R. M. Weiss, T. C. Terwilliger, and W. Willcox. 1982. Hydrophobic moments and protein structure. Faraday Symp. Chem. Soc. 17:109–120. [Google Scholar]
- 54.Eisenberg, D., E. Schwarz, M. Komaromy, and R. Wall. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125–142. [DOI] [PubMed] [Google Scholar]
- 55.White, S. H., and W. C. Wimley. 1998. Hydrophobic interactions of peptides with membrane interfaces. Biochim. Biophys. Acta. 1376:339–352. [DOI] [PubMed] [Google Scholar]
- 56.White, S. H. 2003. Translocons, thermodynamics, and the folding of membrane proteins. FEBS Lett. 555:116–121. [DOI] [PubMed] [Google Scholar]
- 57.Strandberg, E., D. Tiltak, M. Ieronimo, N. Kanithasen, P. Wadhwani, and A. S. Ulrich. 2007. Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl. Chem. 79:717–728. [Google Scholar]
- 58.Powers, J.-P. S., and R. E. W. Hancock. 2003. The relationship between peptide structure and antibacterial activity. Peptides. 24:1681–1691. [DOI] [PubMed] [Google Scholar]