Abstract
Purpose:
To explore the hypothesis that stent placement decreases dilator function of various arteries outside the stented segment and that angiotensin- (1-7) improves this function, and to assess the contribution of dilator signal compounds. A further objective was to test the hypothesis that on-stent delivery of Ang-(1-7) reduces neointima formation and improves endothelial function.
Methods:
Abdominal aortic stenting or sham operation was performed in the rat four weeks after stenting and treatment with intravenous saline or Ang-(1-7) infusion (24 μg/kg/h); vasomotor function in isolated thoracic aorta and brachial and iliac artery was measured in organ baths. Furthermore, Ang-(1-7)-eluting stents were designed and placed in rat abdominal aorta. Neointima formation and aortic function were tested after four weeks.
Results:
Relaxation of the thoracic aorta to metacholine was decreased after stenting compared with shams due to a decrease in nitric oxide-mediated response (67% reduction in maximal NO-dependent response). Ang-(1-7) restored the response mainly through increased prostaglandin- and possibly also endothelial-derived hyperpolarising factor-mediated relaxation. Relaxation in the brachial artery decreased after stenting (maximal response dropped by 50%), whilst contractions to phenylephrine increased. Ang-(1-7) normalised vasomotor function. Iliac artery function remained unaltered after stenting but Ang-(1-7) increased maximal relaxations by 65%. Delivery of Ang-(1-7) by means of a drug-eluting stent improved endothelial function.
Conclusion:
Stenting differentially affects dilator and contractile function in various arterial beds. Ang-(1-7) both improves dilator function and normalises contractile function. Delivery of protective peptides such as Ang-(1-7) from the stent is a new therapy option that merits further development and exploration. (Neth Heart J 2008;16:293-8.)
Keywords: Angiotensin-(1-7), endothelium, nitric oxide, prostaglandins, endothelium-dependent hyperpolarisation factor, stent
In-stent restenosis (ISR), the renarrowing of blood vessels after angioplasty with stent placement, is an ongoing clinical problem, affecting an increasing number of patients as the number of interventions increases. Recently, stents that elute cytostatic compounds have been shown to decrease restenosis, but the incidence of death or myocardial infarction after stenting has not improved, possibly due to a deficit in endothelial repair.1 Therefore, the search for less cytotoxic drugs that spare or protect the endothelium continues. In this perspective, drugs that intervene in the renin-angiotensin system are still eligible candidates.2
Recently, we observed that chronic infusion of the heptapeptide angiotensin-(1-7) (Ang-(1-7)) reduced in-stent neointima formation in the rat.3 In addition, we observed that stent placement in the rat abdominal aorta was accompanied by a loss of endotheliumdependent relaxation of the upstream thoracic aorta. This observation is in accordance with findings in patients where endothelial function in arterial segments outside the stented area shows decreased dilator function.4,5 Therefore, we hypothesise that stenting affects the function of various types of arteries throughout the body and outside the stented segment. Ang-(1-7) restored endothelial function in the rat model.3 Such beneficial effects were also observed in rat heart failure.6 We hypothesise that Ang-(1-7) restores endothelial function in all artery types outside the stented segment that is affected by stenting. Although changes in endothelial function outside the stented area have been reported several times, the contribution of the various signalling factors, such as nitric oxide (NO), vasodilating prostaglandins (PG) and endothelialderived hyperpolarising factor (EDHF), which underlie the decrease in dilator function, has not been elucidated. In addition, it is not known how this is changed by Ang-(1-7) treatment. Previous findings by other groups show that NO and PG production is increased after Ang-(1-7) in hypertensive rats.7 We hypothesise that these signal compounds are involved in the improved endothelial function after stenting.
Regardless of these matters, Ang-(1-7) is an interesting drug to test further as a treatment against neointima formation and endothelial dysfunction. We hypothesised that delivery of this peptide by means of a drug-eluting stent is an option to accomplish such beneficial effect.
In the present study we describe how stenting and chronic Ang-(1-7) infusion change endothelial function in thoracic aorta, also evaluating the contribution of NO, PG and EDHF. Furthermore, we assessed whether stenting and Ang-(1-7) infusion also changed the function of arteries downstream (iliac artery) and remote (brachial artery) of the aortic stented segment. Finally, we explored whether on-stent delivery of Ang- (1-7) has an effect on endothelial function and neointima formation.
Methods
Animals
This study was approved by the Animal Care and Use Committee of the University of Groningen and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised, 1996). Specific pathogen-free, male Wistar rats (Harlan, Horst, the Netherlands) were fed standard rat chow and water ad libitum.
Experiments in the rat stenting model
To test the effect of Ang-(1-7) infusion, bare metal stents (2.5 x 9 mm Medtronic Be-Stent coronary stents) were placed in abdominal aorta, and osmotic minipumps (Model 2004, Alzet, Charles River, Maastricht, the Netherlands) were implanted, as previously described.3 The effect of an Ang-(1-7)-eluting stent on in-stent neointima formation in abdominal aorta and on vasomotor function in thoracic aorta was studied four weeks after stent placement. Ang-(1-7)-eluting stents were compared with bare metal stents and with stents coated only with the polymer used for loading the heptapeptide.
Out of the total of 36 rats used for this study, six rats had to be excluded because of technical failure during surgery, and three stents could not be processed due to incomplete polymerisation of embedding plastic (see below), leaving n=9 for the bare metal stent group, n=10 for stents with polymer only and n=8 for stents with polymer + Ang-(1-7).
Ang-(1-7)-eluting stent details
Polyurethane-primed Medtronic Driver stents (2.5 x 8 mm) were spray-coated using a 0.5 wt% PEG-methacrylate polymer solution in methyl alcohol containing 20 wt% Ang-(1-7) (∽90 μg Ang-(1-7)). Next, a topcoat was applied by spray-coating using a polybutyl methacrylate (PBMA) solution in chloroform.
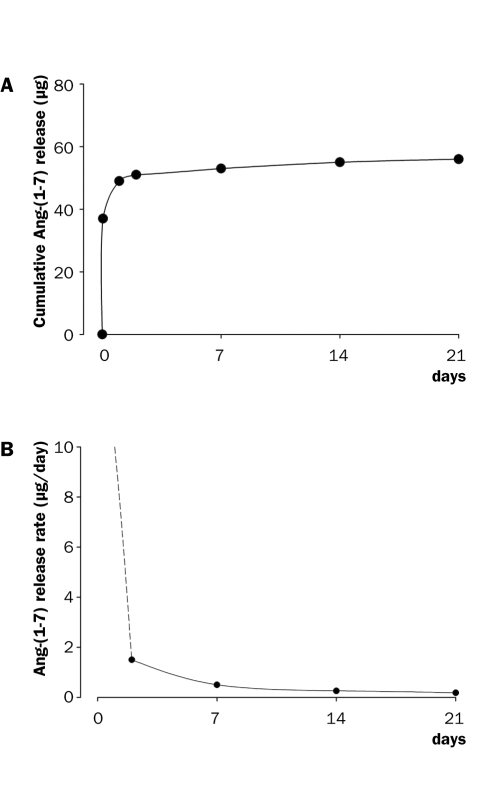
The amount of the PEG-methacrylate coating applied as a base coat on the stents was 461±43 μg, containing 93±9 μg Ang-(1-7). The weight of the PBMA topcoat was 369±50 μg. Coating durability was evaluated using internal Medtronic protocols. Smooth coatings were obtained showing good durability during crimping, ethylene oxide (EtO) sterilisation, tracking and expanding the stents, as confirmed by scanning electron microscopy. Ang-(1-7) elution from EtOsterilised stents at 1 hour, and 1, 2, 7, 14 and 21 days was determined in vitro in a buffer at 37 °C with fluorimetric quantification using fluorescamine. The stents showed an initial burst in the first 24 hours followed by a continuous slow release for up to 21 days (figure 1).
Figure 1 .
In vitro release profile of the Ang-(1-7)-eluting stent.
Stent placement and processing of tissue for analysis
Stent placement was performed when the rats were between 400 and 450 grams. The animals were anaesthetised with O2, N2O and isoflurane (Abbot B.V., Hoofddorp, the Netherlands). Stents were implanted in the abdominal aorta; animals were sacrificed 28 days after the procedure and aortic segments where isolated as previously described.8
Organ bath studies with isolated vascular rings
In this study we have defined the thoracic aorta as upstream and the iliac artery as downstream from the stented segment. Brachial artery is called remote because it is not exposed to the same blood current as the stent.
Thoracic aorta was prepared for functional measurements in Krebs solution as described previously.3 To assess the contributions of NO, vasodilator PG and EDHF to the total endothelial function, a cumulative dose of metacholine (MeCh) was administered in the presence of the NO-synthase inhibitor NG-monomethyl- L-arginine (L-NMMA) (100 μM), with or without the prostaglandin synthesis inhibitor indomethacin (10 μM). The vasodilatory response to metacholine in the presence of L-NMMA and indomethacin is EDHF-mediated; as supported by the findings in previous experiments this response can be blocked in rat aorta by charybdotoxin / apamin. All drugs were purchased from Sigma-Aldrich, Steinheim, Germany.
Iliac and brachial arteries were prepared under a dissection microscope in Krebs solution that was kept at 4 °C. Rings were cut and had an average length of 2.5±0.3 mm for iliac arteries and 2.3±0.4 mm for brachial arteries. The rings were mounted in an isometric small vessel myograph (EMKA Technologies, Paris, France), and equilibrated at zero at a diameter that is 90% of the diameter corresponding to 100 mmHg transmural pressure (0.9L100).9 PE (1 nM to 1 μM) was administered, washed out and rings were precontracted to 70% of their maximum with PE. MeCh (10 nM to 1 μM) was added to measure endothelium-dependent vasodilations, followed by 10 mM sodium nitrate (SN) to assess maximal, endothelium-independent relaxation. The response to 1 μM MeCh was identified as Emax. The responses to MeCh were expressed as % decrease in PE precontraction. The number of successfully performed functional measurements in brachial and iliac arteries are indicated in the figure legends.
Statistics
Data are expressed as mean value ± SEM. Statistical analysis of responses to single doses of PE or SN was performed by one-way ANOVA followed by Bonferroni's post-hoc test for multiple comparison between groups. Differences in dose-response curves between groups were tested by two-way ANOVA for repeated measures. Values of p≤0.05 were considered statistically significant. Numbers of observations (n) are included in the legends and indicate the number of successfully analysed rats. For statistical analysis SPSS software (Chicago, USA) was used.
Results
Effect of stenting and Ang-(1-7) infusion on upstream aortic endothelial function
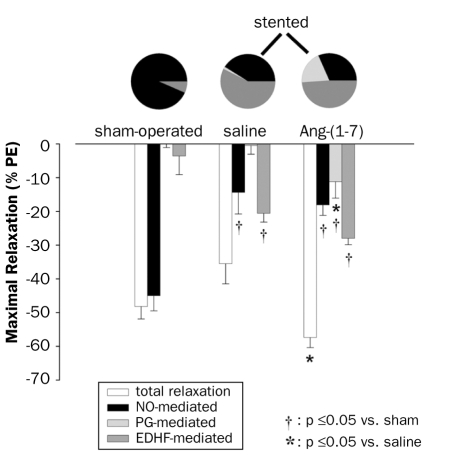
Endothelium-dependent vasodilations depend mainly on the three signalling factors: NO, PG and EDHF. The relative contribution of these factors was assessed in thoracic aorta of sham-operated and stented rats, both infused with saline by osmotic minipumps. We previously showed that total vasodilator response of thoracic aorta upstream of the stented abdominal aorta was significantly decreased in stented animals, and that the endothelium-independent relaxation to exogenously administered NO (SN response) as well as contractions to PE were not altered.3 Moreover, we showed that chronic infusion of Ang-(1-7) restored the vasodilator response. We now show that in sham rats the maximal dilator response of thoracic aorta almost exclusively depends on NO (figure 2). In stented animals NO and EDHF contributed to the vasodilations. After chronic Ang-(1-7) treatment PGmediated vasodilations emerged. There was a trend to an increase in NO- and EDHF-induced dilation of aorta as compared with saline-treated, stented rats; however, this change did not reach statistical significance (p=0.06 for EDHF). Only maximal responses differed; EC50 values were not changed.
Figure 2 .
The functional contribution of nitric oxide (NO), prostaglandins (PG) and endothelial-derived hyperpolarising factor (EDHF) to the maximal vasodilator effect of 0.1 mM metacholine (MeCh) on thoracic aortic rings, four weeks after sham operation or stenting in the abdominal aorta. Ang-(1-7) or saline was infused in stented rats. Responses are expressed as % of precontraction induced by phenylephrine (PE) 1 μM. Sham-operated animals received saline infusion. The pies indicate the percentual contribution of NO, PG or EDHF if total relaxation was set at 100%. Number of observations: stent + saline: n=10; stent + Ang-(1-7): n=6; sham: n=6.
Effect of stenting and Ang-(1-7) on vasomotor function of brachial and iliac artery
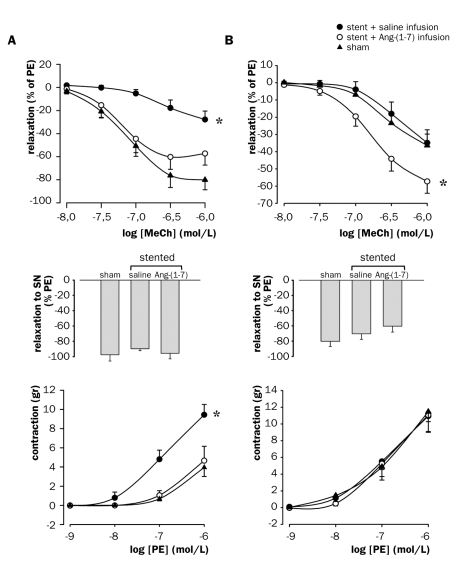
In addition to the aorta, endothelial function was assessed in the remotely situated brachial artery and the iliac artery downstream of the stent. Stenting led to a pronounced decrease in endothelium-dependent relaxation to MeCh in the brachial artery (figure 3A, top panel), whilst iliac endothelial function remained intact (figure 3B, top panel). Chronic Ang-(1-7) infusion improved endothelium-dependent vasodilation in both artery species.
Figure 3 .
Vasomotor responses of (A) brachial arteries and (B) iliac arteries to MeCh (upper panel), SN (middle panel), and PE (lower panel), four weeks after abdominal stenting in rat abdominal aorta with bare metal stents or sham operation. Ang-(1-7) or saline was infused in stented rats. Sham-operated rats received saline. PE=phenylephrine, SN=sodium nitrate, MeCh=metacholine. * p<0.05, vs. stent + Ang-(1-7) infusion and vs. sham, general linear model for repeated measures. Number of observations: stent + saline: n=8 (brachial) or 10 (iliac); stent + Ang-(1-7): n=6; sham: n=8.
Endothelium-independent relaxation to SN remained intact in both arteries (figures 3A and B, middle panels). Although iliac arteries showed a trend towards lowering after stenting with saline infusion, and a further decrease after Ang-(1-7) infusion, the differences did not reach statistical significance.
PE-induced contractions were increased in brachial arteries from stented, saline-treated animals, and were fully normalised by Ang-(1-7) to levels of sham-operated animals (figure 3A, bottom panel). In contrast, PE-induced contractions of iliac artery remained unaltered after stenting and chronic infusion of saline or Ang-(1-7) (figure 3B, bottom panel).
Effect of Ang-(1-7)-eluting stent on aortic endothelial function
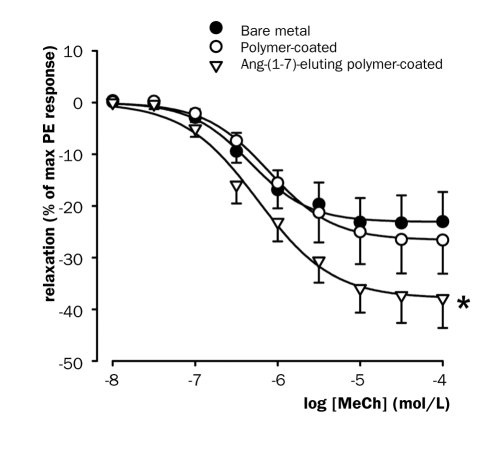
To determine whether Ang-(1-7) elution had an effect on endothelial function, the vasodilator response of isolated thoracic aorta rings to metacholine was tested in an organ bath set-up. In rats treated with the Ang-(1-7)-eluting stent endothelial function was significantly improved compared with bare stents (figure 4). The same trend was seen as compared with stents coated with polymer alone, but did not reach statistical significance. The stent coated with polymer alone did not have an effect on endothelial function.
Figure 4 .
Vasodilator function of isolated thoracic aortic rings in organ baths four weeks after stenting. MeCh=metacholine. * p<0.05 vs. bare metal, general linear model repeated measures.
Effect of Ang-(1-7)-eluting stent on neointima formation
Ang-(1-7) eluted from stents did not reduce neointima formation as compared with stents coated with polymer alone, whilst the polymer itself increased neointima formation (table 1).
Table 1.
Neointima formation in normal vs. Ang-(1-7)-eluting stent.
| Treatment protocol | Neointima area | |
|---|---|---|
| NIA (μM2) | N | |
| Bare metal stent | 0.68±0.04 | 9 |
| Polymer stent | 0.97±0.06* | 10 |
| Polymer + Ang-(1-7) | 0.98±0.08* | 8 |
* p<0.01 vs. bare metal stent.
Discussion
In our present study we assessed the effect of abdominal aortic stent placement and chronic Ang-(1-7) infusion on NO, PG and EDHF-mediated vasodilation in rat thoracic aorta. Stent placement reduced total relaxation mainly due to loss of NO-mediated vasodilation, and EDHF emerged, possibly as a compensatory reaction (further discussed below). Improvement of endothelial function after chronic Ang-(1-7) infusion was, however, mainly due to increased PG function, and possibly also EDHF. In addition, we addressed the question whether stenting and Ang-(1-7) infusion changed vascular function downstream of the stent (iliac artery) and in arteries remote from the stented area (brachial artery). Downstream of the stent, in the iliac artery, dilator function remained unaltered after stenting, but was increased by chronic Ang-(1-7) infusion. Remotely from the stent, in the brachial artery, dilator function was decreased after stenting, and was restored by Ang-(1-7) treatment. This improvement in dilator function of the brachial artery following Ang-(1-7) treatment seemed to be related to an inverse change in vasoconstrictive function, as Ang-(1-7) decreased the Emax of PE response to the level of sham-operated rats. Our third objective was to explore the effect of Ang-(1-7) (highest dose possible) when delivered from a stent. We found that neointima formation was not reduced, but endothelial function outside the stented segment was improved.
The present study shows that vascular injury by stenting in the rat abdominal aorta causes endothelial dysfunction upstream or remote from the stent. This observation is in line with a study in patients showing an inverse correlation between changes in plasma Creactive protein levels and flow-mediated brachial endothelial function 18 to 24 hours after percutaneous transluminal coronary intervention.4 This study in patients suggests that systemic endothelial function is impaired because of elevation of plasma levels of inflammatory substances. In the rat abdominal stenting model an elevation of various inflammatory cytokines was observed after stenting (H.C. Groenewegen, UMCG Groningen, personal communication), supporting the possibility that the impaired endothelial function in rats relates to inflammatory damage as well. The effects of stenting in patients was measured 18 to 24 hours after percutaneous transluminal coronary intervention, and suggests that systemic endothelial function is compromised because of the release of inflammatory substances early after intervention. Hence, early protection could counteract this detrimental event. The release of cytokines observed in the rat stent model was usually present the first day after intervention, and already decreased three days after intervention (H.C. Groenewegen, personal communication), which is in concert with a protective role of the high concentration of Ang-(1-7) shortly after stenting, and the observation in a recent study by Faria-Silva et al. that Ang-(1-7) improves the hypotensive responses to acetylcholine through NO.10 Moreover, it was recently shown that Ang-(1-7) swiftly upregulates eNOS and NO production through activation of Akt in cultured endothelial cells.11 This fast NO-mediated effect on endothelium might protect against functional decline or even apoptosis.
Hitherto it was not known which component of endothelial function would be endangered by stenting. We show here that loss of NO function is an important contributor to the decrease in endothelial function in the rat model. In contrast to NO function, EDHF function is upregulated. This upregulation of EDHF function might, however, represent a feedback mechanism to improve dilator function to a level insufficient for complete restoration. Such a compensation is also seen in the rat model for myocardial infarction,12 and might represent a general physiological feedback response on events that diminish NO function. Ang-(1-7) treatment led to improvement of endothelial dilator function by upregulation of PG and perhaps also EDHF. Increased PG levels were shown in vivo by Eatman et al. after a two-week infusion of Ang-(1-7) in male and female Dahl-sensitive rats.7 Our findings suggest that a similar effect takes place in stented animals and that improvement of PG function is not restricted to hypertensive animals. We did not observe improvement in NO-mediated dilations, as would be expected from acute studies.10,11 This might be due to differences in vessel types or chronic vs. acute experiments. However, after a two-week infusion of Ang-(1-7) in hypertensive rats NO was selectively increased in female rats, pointing at gender-specific differences.7 Whether this is also the case in stented and MI rats and what causes gender differences remains to be elucidated.
Related to this topic is the current observation that stenting and chronic Ang-(1-7) infusion differentially affect vasomotor function in upstream (aorta), remote (brachial) and downstream (iliac) arteries. In aorta and brachial artery, endothelial function was decreased in stented rats treated with saline. However, unlike in the aorta or iliac, in the brachial artery decreased dilator function was accompanied by an increased constrictor function. Ang-(1-7) resets the function to the balance observed in sham-operated rats in this artery. Therefore, a new putative mechanism of Ang-(1-7) treatment effect on vasodilator function might be introduced here, namely the inhibition of contractile function. More specific studies into this relationship will have to address whether Ang-(1-7) affects primarily dilator or contractile function.
Our study shows that Ang-(1-7) might be a relevant drug for delivery by DES as it would improve endothelial function, and thus the prognosis, in the patient. This finding becomes particularly interesting in relation to the current use of cytostatic drugs on DES as it has been suggested that release of cytostatic agents causes more pronounced endothelial dysfunction.13 Although this was observed in a very small patient group, and awaits confirmation in a larger study population, the combination of a cytostatic agent, to inhibit neointima formation, and Ang-(1-7), to improve endothelial function, is an attractive concept.
Regardless of the result obtained with Ang-(1-7), our study warrants further exploration of the stent as a platform for delivery of vasoactive peptides to protect against loss of endothelial function after stenting. The present study should only be considered as a proof-ofprinciple because it is evident that there are many options for improvement left to be explored.
Acknowledgements
We are very grateful to Hans Bartels for performing the surgical procedures. This study was financed by the Groningen University Institute of Drug Exploration (GUIDE), Groningen, and by Medtronic Bakken Research Center, Maastricht, the Netherlands.
References
- 1.Indolfi C, Pavia M, Angelillo IF. Drug-eluting stents versus bare metal stents in percutaneous coronary interventions (a metaanalysis). Am J Cardiol 2005;95:1146-52. [DOI] [PubMed] [Google Scholar]
- 2.Langeveld B, Roks AJ, Tio RA, et al. Renin-angiotensin system intervention to prevent in-stent restenosis: an unclosed chapter. J Cardiovasc Pharmacol 2005;45:88-98. [DOI] [PubMed] [Google Scholar]
- 3.Langeveld B, van Gilst WH, Tio RA, et al. Angiotensin-(1-7) Attenuates Neointimal Formation After Stent Implantation in the Rat. Hypertension 2005;45:138-41. [DOI] [PubMed] [Google Scholar]
- 4.Blum A, Schneider DJ, Sobel BE, et al. Endothelial dysfunction and inflammation after percutaneous coronary intervention. Am J Cardiol 2004;94:1420-3. [DOI] [PubMed] [Google Scholar]
- 5.Caramori PR, Lima VC, Seidelin PH, et al. Long-term endothelial dysfunction after coronary artery stenting. J Am Coll Cardiol 1999;21:1152-7. [DOI] [PubMed] [Google Scholar]
- 6.Loot AE, Roks AJ, Henning RH, et al. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 2002;105:1548-50. [DOI] [PubMed] [Google Scholar]
- 7.Eatman D, Wang M, Socci RR, et al. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1-7). Peptides 2001;22:927-33. [DOI] [PubMed] [Google Scholar]
- 8.Langeveld B, Roks AJ, Tio RA, et al. Rat abdominal aorta stenting: a new and reliable small animal model for in-stent restenosis. J Vasc Res 2004;41:377-86. [DOI] [PubMed] [Google Scholar]
- 9.Angus JA, Wright CE. Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods 2000;44:395-407. [DOI] [PubMed] [Google Scholar]
- 10.Faria-Silva R, Duarte FV, Santos RA. Short-term angiotensin(1-7) receptor MAS stimulation improves endothelial function in normotensive rats. Hypertension 2005;46:948-52. [DOI] [PubMed] [Google Scholar]
- 11.Sampaio WO, Santos RAS, Faria-Silva R, et al. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 2007;49:185-92. [DOI] [PubMed] [Google Scholar]
- 12.Malmsjö M, Bergdahl A, Zhao XH, et al. Enhanced acetylcholine and P2Y-receptor stimulated vascular EDHF-dilatation in congestive heart failure. Cardiovasc Res 1999;43:200-9. [DOI] [PubMed] [Google Scholar]
- 13.Hofma SH, van der Giessen WJ, van Dalen BM, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J 2006;27:166-70. [DOI] [PubMed] [Google Scholar]