Abstract
A dysfunction in the interaction between executive function and mood regulation has been proposed as the pathophysiology of depression. However few studies have investigated the alteration in brain systems related to executive control over emotional distraction in depression. To address this issue, 19 patients with major depressive disorder (MDD) and 20 healthy controls were scanned using functional magnetic resonance imaging. Participants performed an emotional oddball task in which infrequently presented circle targets required detection while sad and neutral pictures were irrelevant novel distractors. Hemodynamic responses were compared for targets, sad distractors, and for targets that followed sad or neutral distractors (Target-after-Sad and Target-after-Neutral). Patients with MDD revealed attenuated activation overall to targets in executive brain regions. MDD patients were also slower behaviorally in response to Target-after-Sad than Target-after-Neutral. Patients also revealed a reversed activation pattern from controls in the left anterior cingulate, insula, right inferior frontal gyrus (IFG), and bilateral middle frontal gyrus. Those patients who engaged the right IFG more during Target-after-Sad than Target-after-Neutral responded faster to targets, confirming a role of this region in coping with emotional distraction. The results provide direct evidence of the alteration in neural systems that interplay cognition with mood in MDD. (198 words)
Keywords: event-related fMRI, interaction of executive function and emotion, anterior cingulate cortex, inferior frontal cortex
1. Introduction
Emotional distraction often interferes with cognitive processing (Johnson et al., 2005, Dolcos and McCarthy, 2006). One of the cardinal features of major depressive disorder (MDD) is an inability to disengage from negative thoughts, memories and events in order to sustain attention towards on-going cognitive tasks (Lyubomirsky et al., 1998, Wenzlaff and Bates, 1998, Ellenbogen et al., 2002, Siegle et al., 2002). In turn, susceptibility to emotional distraction adversely impacts the patients’ capabilities to cope with the demands of daily living (Ottowitz et al., 2002, Rogers et al., 2004). Despite the established clinical importance of executive dysregulation of emotional processing in MDD, alterations in neural functioning associated specifically with this aspect of the disorder are not yet clear.
An influential neurobiological model proposed for mood regulation posits a failure of coordination in dorsal and ventral brain systems subserving executive control and emotional processing, respectively (Mayberg, 1997). Mayberg and colleagues propose that the rostral anterior cingulate (ACC) and related areas in the inferior and medial prefrontal cortex (PFC) may serve critical roles in balancing the relative influence of these brain systems to guide goal-directed behavior and maintain healthy mood. In healthy populations, emotional Stroop and emotional Go/NoGo tasks have been employed to investigate inhibitory cognitive control over emotional distraction by presenting task-irrelevant emotional information simultaneously with task-relevant stimulus features. The ACC, particularly its rostral and ventral aspects, is consistently activated by emotional interference on these tasks (Whalen et al., 1998, Elliott et al., 2000, Bishop et al., 2004, Etkin et al., 2006, Shafritz et al., 2006). This region has been associated with mediating conflict between competing responses (Carter et al., 1998, Botvinick et al., 2001), monitoring for the occurrence of response conflict in information processing (Carter et al., 1998, Botvinick et al., 1999, Barch et al., 2000, Carter et al., 2000, Botvinick et al., 2001), and error monitoring and detection (Rubia et al., 2003). In addition, a number of studies using Stroop, Go/No Go and other attention-demanding tasks suggest a role of the right inferior frontal gyrus (IFG) in inhibitory processes relevant for successful cognitive performance and executive function (Jonides et al., 1998, Konishi et al., 1998, D'Esposito et al., 1999, Smith and Jonides, 1999, Liddle et al., 2001, Rubia et al., 2003, Aron et al., 2004).
Of particular relevance to the present study, Dolcos and McCarthy (Johnson et al., 2005, Dolcos and McCarthy, 2006) revealed a role of the IFG in inhibiting emotional distraction in healthy adults. While subjects performed a working memory task, activation in the IFG was enhanced when the subject was distracted by negative emotional pictures relative to distracting neutral or scrambled pictures. Subjects with great activity to emotional distracters in the IFG tended to rate emotional distracters as less distracting, suggesting that activity in the IFG indexed successful inhibition of emotional distraction. However it is unknown whether recruitment of this region during emotional distraction is altered in clinical populations, such as MDD, and whether dysregulation of IFG activity has behavioral consequences on task performance.
Functional neuroimaging of MDD patients during emotional tasks has implicated dysfunction in frontolimbic regions, providing initial support for Mayberg’s neuroanatomical model of mood regulation. For instance, a sustained emotional response in the amygdala during a personal relevance rating task and decreased dorsolateral PFC activity on a digit-sorting task has been reported in MDD relative to controls (Siegle et al., 2002, Siegle et al., 2006). Siegle and colleagues also reported decreased correlation of amygdala and dorsolateral PFC activity in the MDD group (Siegle et al., 2006); however, this study did not address how emotional responses affected subsequent brain activity associated with executive control. George and colleagues (George et al., 1997) reported decreased activity in the ACC in MDD patients while they performed an emotional Stroop task while undergoing positron emission tomography (PET) scanning. Elliott and colleagues (Elliott et al., 2002) reported attenuated neural responses to emotional relative to neutral targets in ventral ACC and posterior orbitofrontal cortices during an emotional Go/No Go task in MDD. However, because the latter attentional studies used blocked designs, it is not possible to disambiguate responses to different stimulus events and time epochs during the task.
In the present event-related functional magnetic resonance imaging (fMRI) study, we extended these initial neuroimaging findings of MDD to investigate alterations in executive and emotional processing systems during an attentionally-demanding visual oddball task with intermittent emotional distraction by presentation of sad pictures (Wang et al., 2005, Wang et al., 2006). This task more closely models the disruption of ongoing task-relevant cognitive processes by sporadic mood-congruent thoughts in MDD than Stroop or Go/No Go tasks in which the emotional stimuli are themselves task-irrelevant. Furthermore, because the emotional distractors are temporally separated from presentation of the attentional targets, we could evaluate whether emotional dysregulation in MDD leads to performance decrements and differential brain activation to task-relevant attentional targets that follow emotional distractors close in time.
Our previous studies using this paradigm in healthy adults have consistently shown that the attentional targets activate dorsal frontoparietal structures and the sad distractors activate ventral frontolimbic structures, including the amygdala (Wang et al., 2005, Wang et al., 2006). In the present study, we first compared brain activation patterns in MDD and controls to attentional targets and sad distractors separately in order to address the main effect of depression on executive and emotional processing, respectively. Next, to probe the lingering impact of the sad distractors on executive function, we isolated activity to attentional target events that were preceded by the sad distractors (relative to those preceded by neutral distractors). We hypothesized that brain regions such as ACC and right IFG might be activated by this contrast and play critical roles in executive control requiring reallocation of attentional resources to task-relevant processing from task-irrelevant emotional distraction. We predicted that relative to the control group, the MDD group would have decreased activation to targets following sad distractors in these regions.
2. Methods
2.1. Subjects
Nineteen right-handed subjects (12 females and 7 males, mean age = 39.3 yr, SD = 9.0) who met full diagnostic criteria for MDD and 20 age, gender, and education-matched healthy control subjects (13 females and 7 males, mean age = 36.5 yr, SD = 10.5) participated in the study. Participants were recruited to the study via local newspaper and Duke University Medical Center web site advertisements, as well as via flyers posted in campus and medical center locations. The inclusion criteria for MDD were: (a) current major depressive disorder, as assessed by the depression module of the Diagnostic Interview Schedule (DIS) (Robins et al., 1981) and (b) a score of 14 or higher on the Hamilton Rating Scale for Depression (HAM-D, 17-item version (Hamilton, 1960, Miller et al., 1985) both on the screening day and on the day of fMRI scan. Eight out of the 19 subjects with MDD were not currently taking antidepressant medication, the remaining 11 patients were on antidepressants including Venlafaxine (n = 3), Sertraline (n = 2), Bupropion (n =2), Escitalopram (n = 2), Citalopram ( n = 1), and Mirtazapine (n = 1). Control subjects did not have MDD and were not taking any antidepressant medication. Subjects were excluded if they had: (a) past or current manic episodes or psychosis (as assessed by the Structured Clinical Interview for DSM IV Disorders – SCID (First, 1995), or (b) past or current neurological disorders. Detailed demographic and clinical assessments for the two groups are listed in Table 1. The study was approved for ethical treatment of human subjects by the Institutional Review Board at Duke University, and all subjects provided written informed consent after the procedures had been fully explained.
Table 1.
Clinical profile of subjects
| MDD (SD) | Control (SD) | P value | |
|---|---|---|---|
| N | 19 | 20 | |
| Number of males/females | 7/12 | 7/13 | |
| Age | 39.3 (9.0) | 36.5 (10.5) | 0.37 |
| Education* | 3.5 (1.1) | 3.8 (0.9) | 0.47 |
| Self-reported age of first episode | 17.7 (8.5) | n/a | |
| Number of subjects with early onset (≤18 yrs) | 12/7 | n/a | |
| HAM-D score | 19.9 (5.3) | 0.55 (0.8) | <0.0001 |
| Mini-Mental Status Examination Score | 29.1 (1.1) | 29.5 (.95) | 0.19 |
| Reaction time to attentional targets (ms) | 663.4 (78.8) | 590.0 (94.9) | 0.012 |
Note: asterisk(*) Education was calculated by categorizing highest grade completed, such that less than high school = 1, high school/GED = 2, partial college = 3, college degree = 4, advanced degree = 5. P value reflects χ2 test result.
2.2. Stimulus development for the oddball task
The stimuli and design of the emotional oddball task were identical to that described previously (Wang et al., 2005). Briefly, all of the sad pictures contained scenes of humans crying or portraying sad facial expression and depicted scenes of despair, grief, internment, incarceration, and poverty. Each sad picture was yoked to a neutral picture that was matched for the presence and number of human figures in the image, postural features, gaze direction, and gender. The attentional targets were circles of varying sizes and luminance, and the standard stimuli were phase-scrambled and luminance-matched versions of the distractors. All images were converted to grayscale.
2.3. Experimental design
All of the distractors were trial-unique. The presentation frequency for targets, sad distractors and neutral distractors was 3.33% each, with standards comprising the remaining 90% of stimuli presented. The imaging session consisted of 10 runs, each containing 150 stimuli (stimulus duration = 1500 ms, inter-stimulus interval = 2000 ms). The interval between successive rare stimuli (targets and/or distractors) was randomized between 18–20 s to allow hemodynamic responses to return to baseline.
Participants pressed a response button using their right index finger upon detection of a target oddball stimulus (circle). To verify the emotional content of the distractors, participants were asked to rate the distractors on Likert-type scales of sadness/happiness immediately after scanning (Wang et al., 2005). The analyses for the sad vs. neutral contrast were performed based on each subject’s subjective rating of the pictures. In other words, a trial was considered to contain a “sad” distractor if that individual subject rated the image as “sad” or “very sad.”
2.4. Image acquisition and analysis
Functional images were acquired on a 4.0 Tesla GE scanner and were analyzed as described previously (Wang et al., 2005). Oblique spoiled gradient-recalled acquisition images (three-dimensional, whole-brain) were acquired parallel to the anterior commissure-posterior commissure plane for high-resolution T1-weighted structural images with the following parameters: repetition time (TR) = 12.2 ms, echo time (TE) = 5.3 ms, field of view (FOV) = 24 cm, flip angle = 20°, matrix = 256×256, 68 contiguous images, slice thickness = 1.9 mm. Inward spiral gradient images were acquired with the following parameters: TR = 2000 ms, TE = 31 ms, FOV = 24 cm, flip angle = 90°, matrix = 64×64, 34 contiguous images, slice thickness = 3.75 mm, resulting in 3.75mm3 isotropic voxels.
Image pre-processing was conducted using temporal realignment for interleaved slice acquisition and spatial realignment to adjust for motion using affine transformation routines implemented in SPM99 (Wellcome Department of Cognitive Neurology, London, UK). The realigned images were co-registered to the anatomic images obtained for each participant and normalized to SPM’s template image, which conforms to the Montreal Neurologic Institute’s standardized brain space. The voxel size was 3.5×3.5×3.5 mm3 after normalization. The functional data were spatially smoothed with an 8-mm isotropic Gaussian kernel prior to statistical analysis.
The voxel-wise and region-of-interest (ROI) analyses used custom MATLAB scripts (Pelphrey et al., 2003, Dolcos and McCarthy, 2006, Wang et al., 2006).Two types of event epochs were extracted for a voxel-based event-related analysis and a functional ROI analysis. In order to investigate the brain response to attentional targets and sad distractors, the hemodynamic response was time-locked to the onset of each type of infrequent stimulus event (sad, neutral, and target). To elucidate the influence of distraction by recently-presented sad stimuli on executive function and the responses of the executive control system to emotional distraction, we further subcategorized the attentional targets into two event types: Target-after-Sad, which included all targets following sad distractors, and Target-after-Neutral, which included all targets following neutral distractors (Figure 1). The whole epoch of each event (including sad, neutral, and target events and by default the Target-after-Sad and Target-after-Neutral events) was extracted from −4 s before the onset of each stimulus to 20 s after the presence of the stimulus. Voxel-based signal percentage change at each event time point (from −4 s to +20 s) was calculated for each subject by subtracting the mean pre-stimulus baseline activity (activity to scrambled pictures presented from −4 s to 0 s prior to each event) and then averaging across all trials with the same event type. Since the scrambled pictures served as the baseline, each event was contrasted with this baseline, unless otherwise specified in the text. We validated the hemodynamic time course at each voxel by testing the correlation of the hemodynamic response across time with the canonical gamma hemodynamic response for each event in each subject. Only those voxels whose hemodynamic responses were significantly correlated with the canonical hemodynamic response (false discovery rate-corrected P < .05 with a spatial extent of five contiguous voxels) were entered into further within- and between-group analysis.
Figure 1.
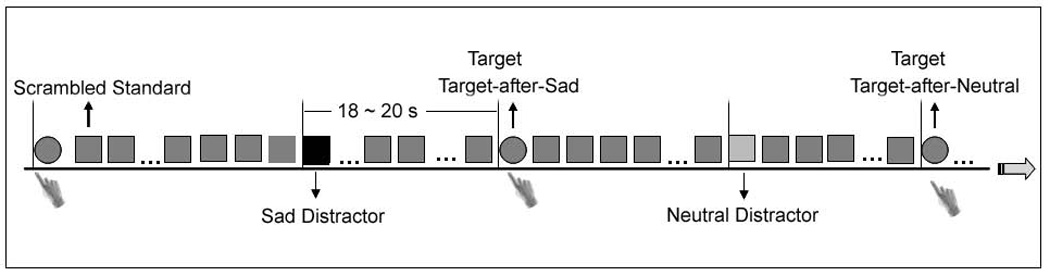
The emotional oddball task consisted of standards (90% of trials), neutral distractors (3.3%), sad distractors (3.3%), and targets (3.3%). The targets were subgrouped as targets following sad distractors (Target-after-Sad) and targets following neutral distractors (Target-after-Neutral). The task-irrelevant distractors and task-relevant targets were separated in time by 18~20 s to isolate their hemodynamic responses. A vertical line illustrates the onset of each event (0 s point of each epoch). Participants were required to detect circles (targets) and respond with a speeded button-press response.
Statistical contrasts at each time point were set up using a random-effect analysis to calculate signal differences between the conditions of interest across each group of participants. Statistical t maps at each time point were derived for the events of interest, resulting in a t statistic for every voxel. This sequential approach accounts for intersubject variability and permits generalization to the population at large. Only the results at peak time point 6 s post-stimulus are reported here. The resultant t maps were thesholded at a voxelwise false discovery rate-corrected P < 0.05 with a spatial extent of five contiguous voxels. Two-sample t-tests were conducted to compare voxel-wise signal changes at the peak time point (6 s post-stimulus) between MDD and controls at each stimulus condition (sad, neutral, and target), thresholded at P < 0.001 uncorrected with a spatial extent of five contiguous voxels. Voxel-based ANOVA analysis at the peak time point (6 s post-stimulus) was computed using stimulus type (Target-after-Sad, Target-after-Neutral) as a within-subjects factor and clinical diagnosis (MDD, control) as the between-subjects factor. ANOVAs were thresholded at P < 0.001 uncorrected with a spatial extent of five contiguous voxels for statistical significance.
To visualize the hemodynamic response profile for each functional region, an ROI analysis was performed. Those independent clusters which showed a significant difference in the ANOVA analysis were identified as ROIs. The mean signal change within each ROI was computed for each time point for each event. To confirm the voxel-based findings, a statistical analysis using ANOVA was conducted on the ROIs, focused on the mean percent signal change by hemisphere at the peak time point (6 s post-stimulus). An alpha level of P < 0.05 (two-tailed) was used for the ROI analyses. The Bonferroni test was used for the post-hoc analyses. Linear regression analysis was used to correlate peak signal changes in each ROI with reaction times (RTs) to attentional targets or with HAM-D score with a statistical threshold of r > 0.5 and P < 0.05 (two-tailed).
3. Results
3.1. Behavioral data: RT and emotional ratings
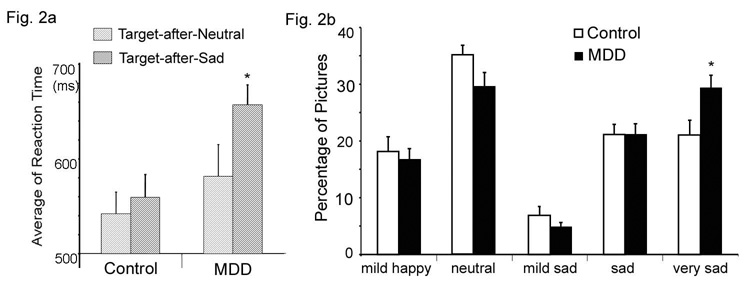
MDD patients had slower RTs to attentional targets during the scan, t(37) = 2.69, P < 0.05, regardless of the valence of the previous distractor. The mean (SD) RT was 663.4 (78.8) ms for the MDD group and 590.0 (94.9) ms for the control group. A repeated-measures ANOVA on target subtype (Targets-after-Sad, Target-after-Neutral) using group (MDD, control) as the between-subjects factor revealed a significant main effect of group, F(1,38) = 5.01, P < 0.031, with MDD patients showing slower responses. The main effect of target subtype was also significant, F(1,37) = 5.67, P = 0.023, with sad distractors showing slower responses. Although there was no significant interaction effect, the RT slowing was exaggerated in MDD patients when the targets were preceded by sad distractors (Figure 2a). The MDD group was significantly slower in response to the Target-after-Sad event than the control group, t(37) = 3.05, P = 0.004, and within the MDD group, RTs to Target-after-Sad events were significantly slower than RT to Target-after-Neutral events, t(37) = 2.31, P = 0.027.
Figure 2.
2a) The influence of emotional distraction on reaction time (RT) to the presentation of subsequent targets. The MDD group revealed significantly slower RT when a target was preceded by a sad distractor (Target-after-Sad) than when a target was preceded by a neutral distractor (Target-after-Neutral), * P <0.05. 2b) Comparison of the percentage of pictures out of the set of 100 that were rated as ‘mildly happy’, ‘neutral’, ‘mildly sad’, ‘sad’ and ‘very sad’ by controls and MDD subjects. Results indicate a bimodal distribution. The MDD group rated significantly more pictures as ‘very sad’ (*P < .05), indicating a negative emotional processing bias.
Subjective ratings for the picture distractors were analyzed using student’s t tests. The MDD group rated more pictures as ‘very sad’ than controls, t(37) = 2.51, P = 0.018, suggesting a negative emotional processing bias (Figure 2b).
3.2. fMRI results
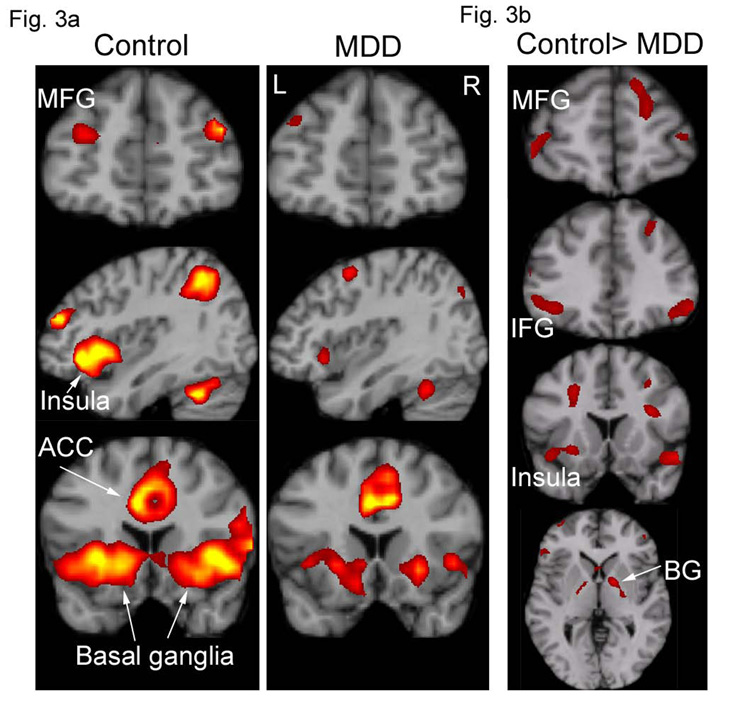
Random-effect within-group analysis revealed that the control group showed activation in response to attentional targets in the dorsal executive system, including bilateral middle frontal gyrus (MFG), IFG (BA45 and 47), dorsal ACC, posterior cingulate, supramarginal gyrus, superior parietal lobule, precuneus, thalamus and striatum (Figure 3a). The control group showed activation in response to the sad distractors (relative to neutral distractors) in ventral posterior and frontolimbic regions, including bilateral extrastriate cortex, fusiform gyrus, amygdala, anterior insula, IFG (BA47), and middle and superior temporal sulci. These results are consistent with our previous findings in healthy adults using the same paradigm (Wang et al., 2005). The depressed group activated similar regions as the controls (Figure 3a). For clarity, we only report below the results which show significant differences between the two groups rather than separate main effects within the two groups.
Figure 3.
3a) Voxel-based random effect analysis for the MDD and control groups on the activation to attentional targets at the peak time point. Both of the groups activated a dorsal executive system, although the MDD had a lesser extent of activation (false discovery rate corrected P < 0.05, spatial extent of five contiguous voxels). 3b) Significantly decreased activation in the MDD group compared with the control group from a two-sample t test on activation to attentional targets at the peak time point (P < 0.001, uncorrected, spatial extent of five contiguous voxels). ACC = anterior cingulate cortex; BG = basal ganglia; IFG = inferior frontal gyrus; INS = insula; MFG = middle frontal gyrus; L = left hemisphere; R = right hemisphere.
3.2.1. Alterations in executive and emotional processing systems in MDD
A direct statistical comparison between the MDD and control groups revealed activity reductions in the MDD group to attentional targets in the executive system (Figure 3b, Table 2) including superior frontal gyrus, middle frontal gyrus, supramarginal gyrus, insula, basal ganglia, and bilateral IFG (left BA47 and right BA45). Activation patterns to the sad distractors relative to neutral ones were comparable between the groups. However, MDD patients showed greater deactivation than controls to sad stimuli relative to baseline in dorsal frontoparietal regions, including the middle frontal gyrus, supramarginal gyrus, precuneus, postcentral gyrus and temporal parietal conjunction area (Table 3, Figure 4). Note that most of these regions showed activation in response to attentional targets (Figure 3 and Figure 4). The task-induced deactivation usually occurs during an active task relative to a "resting" or "passive" baseline in a default-mode network. It is postulated to result from a reallocation of processing resources (McKiernan et al., 2003). The magnitude of deactivation increases with task difficulty (McKiernan et al., 2003). The enhanced deactivation for emotional distractors in the regions associated with cognitive function may reflect reallocation of attentional resources to sad distractors (Posner and Dehaene, 1994, Drevets, 1998). Thus the increased deactivation might suggest a strong emotional distraction effect in MDD.
Table 2.
Brain regions showing significant group differences (threshold P < 0.001, five contiguous voxels) in activation to attentional targets (MDD < control).
| Region | BA area | Hemisphere | MNI coordinates |
T value | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | Y | z | |||||
| middle frontal gyrus | BA 10 | L | −42 | 53 | 0 | 3.33 | 28 |
| R | 46 | 42 | 4 | 3.23 | 26 | ||
| inferior frontal gyrus | BA 47 | L | 49 | 25 | −11 | 4.21 | 42 |
| BA 45 | R | 32 | 14 | 25 | 4.03 | 25 | |
| precentral gyrus | L | −28 | 4 | 39 | 4.63 | 132 | |
| insula | L | −42 | 11 | −11 | 3.31 | 36 | |
| superior temporal gyrus | BA 38 | R | 49 | 11 | −11 | 3.80 | 29 |
| precuneus | BA 7 | L | −7 | −67 | 60 | 4.09 | 18 |
| basal ganglia | L | −28 | 4 | −4 | 3.43 | 53 | |
| R | 25 | −11 | 18 | 3.89 | 69 | ||
| middle occipital gyrus | BA 19 | L | −32 | −77 | 21 | 3.89 | 26 |
| cerebellum | L | −25 | −81 | −35 | 3.92 | 91 | |
Note: BA = Brodmann area, MNI = Montreal Neurologic Institute coordinate system
Table 3.
Brain regions showing significant group differences (threshold P < 0.001, five contiguous voxels) in deactivation to sad relative to neutral distractors (MDD > control).
| Region | BA area | Hemisphere | MNI coordinates |
Cluster size | |||
|---|---|---|---|---|---|---|---|
| x | y | z | T value | ||||
| middle frontal gyrus | BA 9 | L | −28 | 28 | 35 | 3.41 | 9 |
| supramarginal gyrus | BA 40 | R | 56 | −32 | 25 | 3.44 | 26 |
| precuneus | BA 7 | L | −7 | −63 | 46 | 4.50 | 55 |
| postcentral gyrus | BA 43 | L | −49 | −18 | 21 | 4.00 | 68 |
| superior temporal gyrus | BA 41 | L | −35 | −32 | 11 | 4.06 | 24 |
Note: BA = Brodmann area. MNI = Montreal Neurologic Institute coordinate system.
Figure 4.
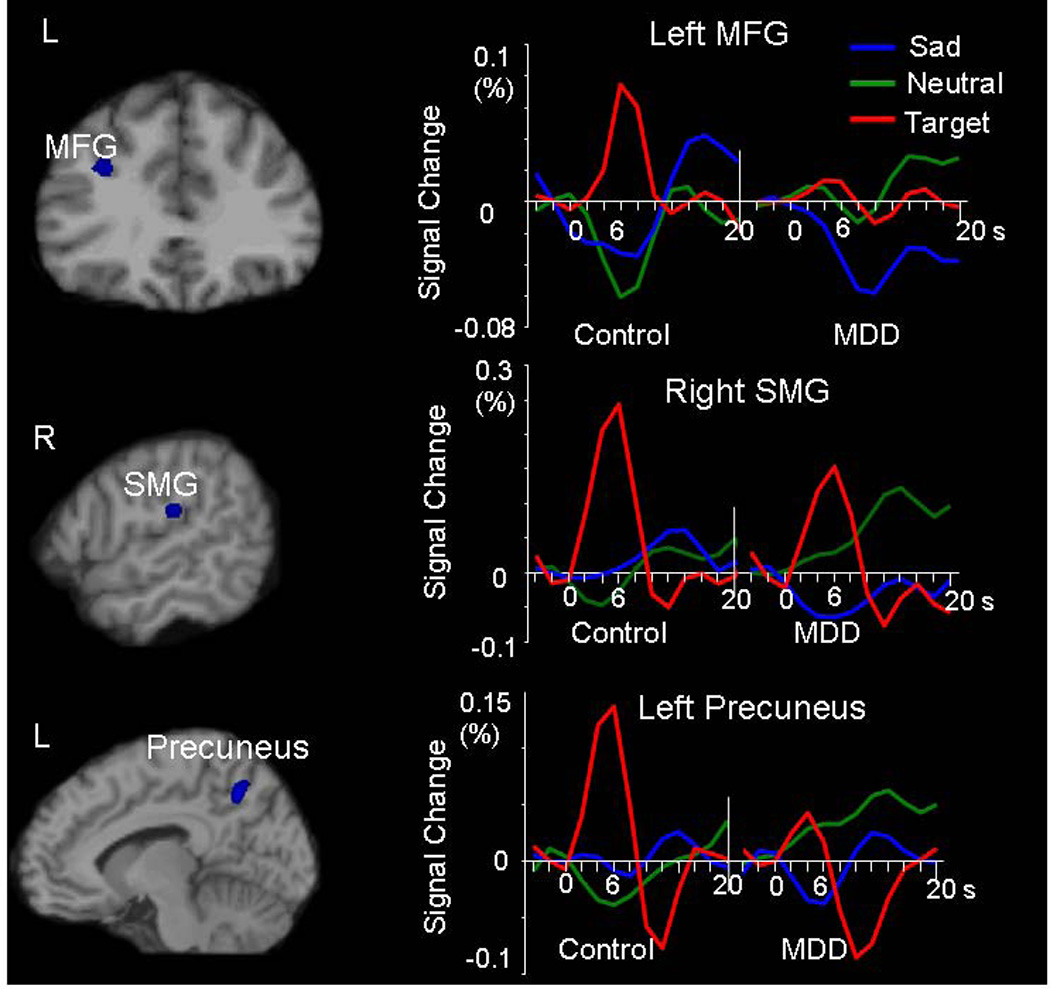
Significantly increased deactivation in response to sad vs. neutral contrast in the MDD group relative to the control group. Brain maps, voxel-based two-sample t test analysis at the peak time point (P < 0.001, uncorrected, spatial extent of five contiguous voxels); Waveforms, ROI analysis showing the hemodynamic response with time course. The MDD group revealed increased deactivation in response to sad relative to neutral distractors and decreased activation in response to targets compared with the control group. MFG = middle frontal gyrus, SMG = supramarginal gyrus
Unlike other studies that reported significantly stronger activation to negative emotional stimuli in the emotional system in MDD (Sheline et al., 2001, Siegle et al., 2002, Fu et al., 2004, Canli et al., 2005, Siegle et al., 2006), the MDD group only showed increased activation than the control group using a less stringent statistical threshold (p < .05 uncorrected, spatial extent of 5 voxels) in the emotional system including the left IFG (BA 47), hypothalamus, inferior temporal gyrus as well as right uncus, ventral basal ganglia, and ventral extrastriate cortex. We further investigated whether antidepressant medication weakened the activation to emotional stimuli in the MDD group. Relative to the medicated group (n = 11), the medication-free group (n = 8) revealed stronger activation in the left amygdala in response to sad distractors when compared to neutral distractors (P<0.001 uncorrected, spatial extent of 5 voxels).
3.2.2. Influence of emotional distraction on the processing of subsequent attentional targets
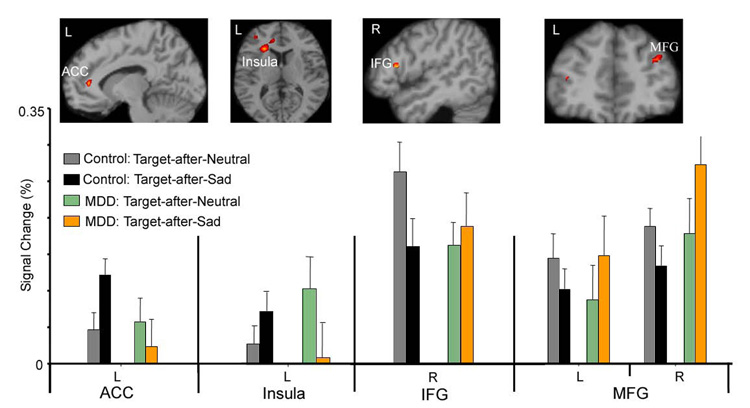
To investigate the influence of emotional distraction on the processing of subsequent attentional targets and the responses of the executive control system to emotional distraction, we first conducted an ANOVA to compare activation to the Target-after-Sad and Target-after-Neutral events in MDD patients relative to controls. The group × stimulus type interaction (Table 4 and Figure 5) revealed significant effects in the left rostral ACC (BA 32) and anterior insula (BA 31), right IFG (BA 44), and bilateral middle frontal gyrus (BA 10). Follow-up ANOVA and Bonferroni post hoc tests were conducted on functional ROIs extracted from these regions and were correlated with the behavioral RT effects. Findings from each of these regions are discussed, in turn, below.
Table 4.
Brain regions that show an interaction effect of group × target subtype
| Contrast | Region | BAarea | Hemisphere | Cluster size | Peak Voxel MNI coordinates |
ROI F value |
ROI P value |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Control > MDD | MFG | BA10 | L | 6 | −39 | 42 | 14 | 6.348 | 0.016 |
| Target-after-Neutral >Target-after Sad | BA 10 | R | 40 | 35 | 42 | 28 | 9.580 | 0.004 | |
| BA 9 | R | 7 | 39 | 18 | 35 | ||||
| IFG | BA 44 | R | 13 | 49 | 14 | 18 | 6.966 | 0.012 | |
| Control > MDD | |||||||||
| Target-after-Sad >Target-after-Neutral | Insula | BA13 | L | 25 | −28 | 18 | 14 | 28.801 | 0.000 |
| ACC | BA 32 | L | 46 | −11 | 35 | 11 | 7.233 | 0.011 | |
Note: the coordinates represent the location of the voxel which showed peak activation in the region using voxel-based ANOVA analysis (threshold P < 0.001, five contiguous voxels); the F and P value were from ROI-based ANOVA analysis
Figure 5.
Brain regions which revealed significant interaction effect of group (control, MDD) × target subtype (Target-after-Sad, Target-after-Neutral) in the voxel-based analysis (top), and the mean signal percentage change in these regions in the ROI analysis (bottom) at the peak time point.
The ACC region showed stronger activation to the Target-after-Sad events relative to the Target-after-Neutral events in controls, but not in MDD patients. Post-hoc tests confirmed a significant decline in engagement of this region during the Target-after-Sad events in MDD patients relative to controls, t(37) = 2.29, P = 0.028. Stronger activation to the Target-after-Sad relative to Target-after-Neutral in controls is consistent with the rostral ACC’s putative role in mediating conflict between prepotent distractors and task-relevant stimulus processing. The reduced activation to Target-after-Sad in MDD patients suggests possible attenuated function of the ACC in conflict control or monitoring. Similar to the left ACC, the left insula also showed stronger activation to the Target-after-Sad events relative to the Target-after-Neutral events in controls, but the pattern was reversed in MDD patients.
In contrast to the rostral ACC and insula, the right IFG (BA 44) revealed stronger activation in controls for the Target-after-Neutral events relative to the Target-after-Sad events (Figure 5). Relative to controls, the MDD group had significantly attenuated activation for the Target-after-Neutral vs. Target-after-Sad contrast, t(37) = 2.23, P = 0.034. The MDD group had relatively stronger activation to Target-after-Sad relative to Target-after-Neutral. Similar to the right IFG, bilateral MFG revealed significantly stronger activation in MDD patients for the Target-after-Sad events relative to the Target-after-Neutral events. Consistent with Elliott and colleagues (Elliott et al., 2002), we found that controls had the opposite pattern - relatively stronger activation in this region to the Target-after-Neutral events (Figure 5). Post-hoc t tests showed that the interaction effect in the right MFG was driven by the increased activation to the Target-after-Sad events in the MDD group relative to controls (t(37) = 2.30, P = 0.027).
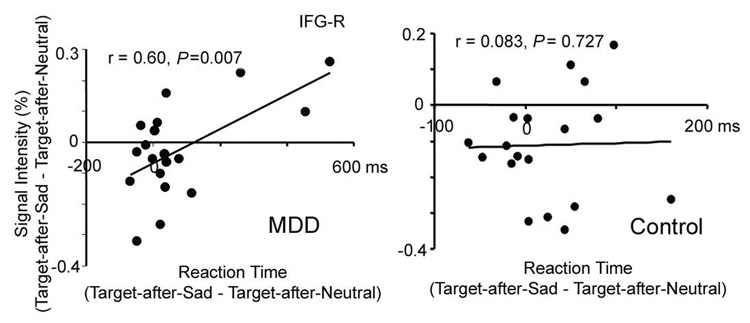
To identify brain regions which were directly associated with slower RT to Target-after-Sad relative to Target-after-Neutral in MDD, linear regression analyses were conducted within the MDD group to correlate the RT of Target-after-Sad vs. Target-after-Neutral with the activations to Target-after-Sad vs. Target-after-Neutral in these frontal regions. Among the four regions which showed a significant interaction effect, only the activation of the right IFG in MDD revealed a significant correlation with the RT difference (left ACC, r(37) = −0.38, P =0.11, left insula, r(37) = 0.23, P = 0.35, right IFG, r(37) = −0.60, P = 0.007, left MFG, r(37) = 0.10, P = 0.69, and right MFG r(37) = 0.10, P = 0.68). As shown in Figure 6, MDD patients who had stronger activation to the Target-after-Sad vs. Target-after-Neutral contrast in the right IFG showed faster RT when comparing Target-after-Sad vs. Target-after-Neutral events. Thus, the relatively high activation to Target-after-Sad vs. Target-after-Neutral event in these MDD patients might reflect an effortful inhibition processing as a compensatory effect due to the dysfunction of the ACC. Of note stronger activation of control subjects in the right IFG in response to targets (collapsed across the Target-after-Sad and Target-after-Neutral subtypes), but not to the subtypes of targets, was correlated with faster RT in target detection (rRT, target = − 0.65, P = 0.002). Given that the control group had a reverse pattern from the MDD patients, with stronger activation to Target-after-Neutral vs. Target-after-Sad, the role of the right IFG may be different between the two groups. Nevertheless, both enhanced activation to targets in controls and enhanced activation to Target-after-Sad vs. Target-after-Neutral were directly correlated with behavior outcome, the speed of target detection, indicating a role of this region in successful reallocation of attention from task-irrelevant stimuli to targets. Overall, the MDD subjects as a group had decreased activation in this region and slower RT. Combined, these data implicate altered function of the right IFG in MDD in executive control over emotional distraction and reallocation of attention on task-relevant stimuli.
Figure 6.
Emotional distraction activity in right inferior frontal gyrus (IFG-R) correlates with reaction time (RT) to detect subsequent targets in MDD. The percent signal change difference in IFG-R (Target-after-Sad minus Target-after-Neutral) was correlated with the RT difference to the same stimuli in the MDD patients but not in controls.
4. Discussion
There are three major findings in our current study: 1) relative to controls, slower performance concurrent with hypoactivity of the executive system during target detection in MDD including bilateral MFG, IFG, supramarginal gyrus, insula, and basal ganglia; 2) relative to controls, depressed patients showed increased deactivation in the executive system while processing emotional distractors; and 3) relative to controls, the MDD group revealed slower reaction time and a reverse activation pattern from controls in prefrontal regions in response to targets following sad distraction vs. targets following neutral distractors.
Executive dysfunction is one of the major cognitive deficits in MDD. The slow reaction time to targets, together with decreased activation to attentional targets and increased deactivation to sad relative to neutral distractors in the dorsal executive regions (including MFG, supramarginal gyrus and precuneus) suggest a profound executive dysfunction in our MDD group. This result is consistent with previously reported hypometabolism of dorsolateral PFC in PET (Dolan et al., 1993, Mayberg et al., 1994, Drevets, 1999) and some fMRI studies in MDD (Elliott et al., 1997, Davidson et al., 2003, Siegle et al., 2006).
Importantly, both behavioral response and fMRI signal intensity change in our study showed direct evidence of how emotional distraction impacts on executive function. Our current study extends existing findings showing that recently-presented emotional stimuli could interfere with on-going task processing. For instance, Johnson and colleagues (Johnson et al., 2005) have extensively studied the effect of cognitively “refreshing” a just-activated representation (2 or 4 s before) and have shown that emotional stimuli can disrupt refreshing representations and impair feature binding (Mather et al., 2006). Siegel and colleagues (Lyubomirsky et al., 1998, Wenzlaff and Bates, 1998, Ellenbogen et al., 2002, Siegle et al., 2002) showed a lasting effect of emotional processing on amygdala activation in MDD up to 25 sec after stimulus presentation. The current study shows that prolonged processing of sad stimuli in MDD can adversely impact attentional processing for 18 s or longer.
A novel finding of the current study was the distinct roles of left ACC and insula, right IFG, and bilateral MFG in inhibiting emotional distraction and the alteration in activation of these regions in MDD. In healthy controls, the left ACC and left insula revealed different activation patterns from the MFG and right IFG. The left ACC and insula showed increased activation to the Target-after-Sad relative to Target-after-Neutral stimuli, consistent with the literature suggesting that the ACC is associated with conflict control and monitoring (Elliott et al., 2002, Shafritz et al., 2006). In our study, competition for executive resources might have occurred due to residual processing of the prepotent emotional distractors and the subsequent reallocation of attentional resources required to perform the target detection task. The insula is closely connected to the PFC and ACC and forms part of a frontal-striatal attentional network (Schmitz et al., 2006). The hyperactivation in the left ACC along with the insula to the Target-after-Sad stimuli in controls supports a role of conflict control or conflict monitoring in the task. The decreased ACC to Target-after-Sad in the MDD group is consistent with the literature suggesting a dysfunction of the left ACC in emotional regulation in MDD (Elliott et al., 2002, Shafritz et al., 2006).
The ACC system has been implicated in relatively faster and urgent inhibition, whereas the frontal-parietal system is involved in more deliberate and controlled inhibition (Garavan et al., 2002). Our results in the healthy controls support the segregation of these prefrontal regions in executive control by showing regional specificity in activation patterns to targets following sad distraction across the left ACC/insula and the right IFG/bilateral MFG. Different from the role of effortful control over conflicts in the ACC, Dolcos and McCarthy (Dolcos and McCarthy, 2006) revealed a role of IFG as an index of successful inhibition of emotional distraction. Stronger activation to Target-after-Neutral vs. Target-after-Sad found in our current study supports this role of IFG in healthy controls. Our results also extended the findings of Dolcos and McCarthy (Dolcos and McCarthy, 2006) to depressed population and found altered function in inhibition of emotional distraction in MDD (as reflected by decreased activation to Target-after-Neutral vs. Target-after-Sad contrast).
Similarly, the MDD group had significantly stronger activation to the Target-after-Sad stimuli in bilateral MFG, particularly in the right MFG. This result could be due to the failure of conflict control in the left ACC, which releases the representation of the previous sad image from prolonged processing. Based on the model proposed by Cohen and colleagues (Cohen et al., 2000), multiple representations of stimuli would increase the activity in dorsolateral PFC. Alternatively, the increased activation to the Target-after-Sad stimuli in MDD might be a compensatory effect for the insufficient conflict control and inhibition in the left ACC and right IFG (Harvey et al., 2005, Wagner et al., 2006). Nevertheless, the increased activation in the MDD group is limited to the targets following sad stimuli. Decreased activation was observed in response to targets overall collapsing the target subtypes in bilateral MFG, indicating the task importance in influencing fMRI results in MDD. Two recent studies in medication-free MDD patients revealed increased prefrontal activation during effortful cognitive tasks compared to the controls (Matsuo et al., 2006, Wagner et al., 2006). Although antidepressant medication use may influence the fMRI findings in MDD (Mayberg et al., 2000, Davidson et al., 2003, Fu et al., 2004), task differences may also greatly contribute to the discrepancy in findings across studies as evident by our current study (discrepancy of activation in the MFG to overall targets and target subtypes). A future study with pure unmedicated subjects or subgroups with medication responder vs. non-responders would explicate which clinical profiles are more closely associated with alteration in the cognitive control over emotional distraction in MDD.
Unlike some studies that found increased or prolonged activation in the amygdala to emotional stimuli (Sheline et al., 2001, Siegle et al., 2002, Fu et al., 2004, Canli et al., 2005, Siegle et al., 2006), we did not find a significant change in amygdala activation in our MDD patients relative to controls. However, we found an increase in activation to sad vs. neutral distractors in the cortex surrounding the amygdala (i.e., in the uncus), IFG (BA 47) and hypothalamus at a reduced threshold, suggesting a hyperactivated emotional system in MDD. Nevertheless, the most significant finding in response to emotional distraction was the increased deactivation in the dorsal executive system, not hyperactivity in the emotional system, suggesting that, in some MDD samples, dorsal executive dysfunction may be a stronger feature of neurobiological impairment compared to ventral emotional dysfunction. The less significant hyperactivity in the emotional system in current study was partially associated with anti-depressant medication use in 11 of the 19 MDD subjects as revealed by the difference in activation of amygdala between medicated and the nonmedicated group. Thus, the significant findings in the executive system might indicate a slower recovery of the executive control system than the emotional processing system following antidepressant treatment.
The present results contrast with the effects of transient sad mood in healthy individuals using the same task (Wang et al., 2006). In our prior study in healthy subjects, transient sad mood induction evoked stronger activation in the amygdala and ventromedial PFC than happy mood in response to sad distractors without affecting executive function (Wang et al., 2006). Thus, across studies using an identical paradigm, we have identified neurobiological markers that distinguish healthy transient mood effects from pathological depressive mood on executive function. A couple of studies (Keedwell et al., 2005, Surguladze et al., 2005, Fu et al., 2007) have reported different patterns of neural response to sad and happy facial expressions in MDD. Given the features of negative attentional bias and anhedonia (lack of pleasure) of MDD, one might predict that a happy stimulus might produce a less distractive effect on the performance of attentional targets detection in the MDD group relative to controls. Patients with MDD could be an important model for understanding the differential impact of emotional valence on executive function.
In summary, consistent with the model by Mayberg (Mayberg, 1997), we found profoundly decreased activity in the executive system during target detection and mildly increased activation during emotional distraction. Importantly, we found altered activation in PFC and ACC brain regions associated with executive control over emotional distraction in MDD. This study has potentially important implications for understanding PFC function in affective and mood disorders. Further studies of the effect of overt top-down control over emotional distraction are warranted to help understand the effects of treatment interventions in MDD on brain regions that interface executive and emotional processing.
Acknowledgements
This research was supported by the Duke Silvio O. Conte Center for the Neuroscience of Depression (P50-MH60451). GM was supported by a DVA Senior Research Career Scientist Award and by the DVA VISN6 Mental Illness Research, Education, and Clinical Center (MIRECC).
Competing interests statement
The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who's in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RS. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? Journal of Neurology, Neurosurgery & Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annual of the New York Academy of Sciences of the United States of America. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal Suppression of Regional Cerebral Blood Flow during Emotional versus Higher Cognitive Processes: Implications for Interactions between Emotion and Cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Stress and selective attention: the interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. NeuroReport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Biometrics Research Department. New York State Psychiatric Institute. 1995 [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, Mitterschiffthaler MT, Andrew CM, Pich EM, Bullmore ET. Neural responses to happy facial expressions in major depression following antidepressant treatment. American Journal of Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulated activation in mood disorder subjects during a response interference task (the Stroop) Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehericy S, Allilaire JF, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cognitive, Affective & Behavioral Neuroscience. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Urchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nature Neuroscience. 1998;1:80–94. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality & Social Psychology. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Greene EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18:614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry. 2006;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN., Jr Paralimbic hypoperfusion in unipolar depression. Journal of Nuclear Medicine. 1994;35:929–934. [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. The Journal of Neuroscience. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and Executive Processes in the Frontal Lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, McCarthy G. Mood Alters Amygdala Activation to Sad Distractors During an Attentional Task. Biological Psychiatry. 2006;15:1139–1146. doi: 10.1016/j.biopsych.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, LaBar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5:12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Bates DE. Unmasking a cognitive vulnerability to depression: how lapses in mental control reveal depressive thinking. Journal of Personality & Social Psychology. 1998;75:1559–1571. doi: 10.1037//0022-3514.75.6.1559. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]