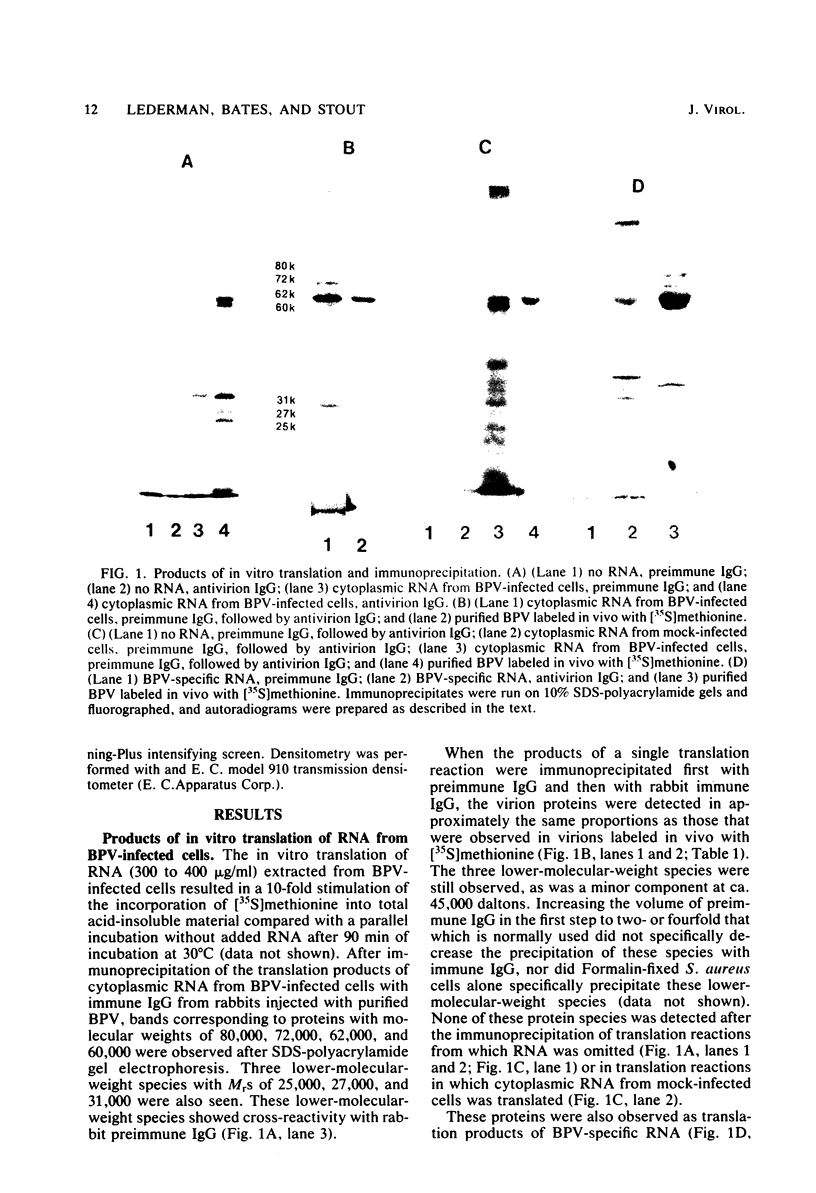
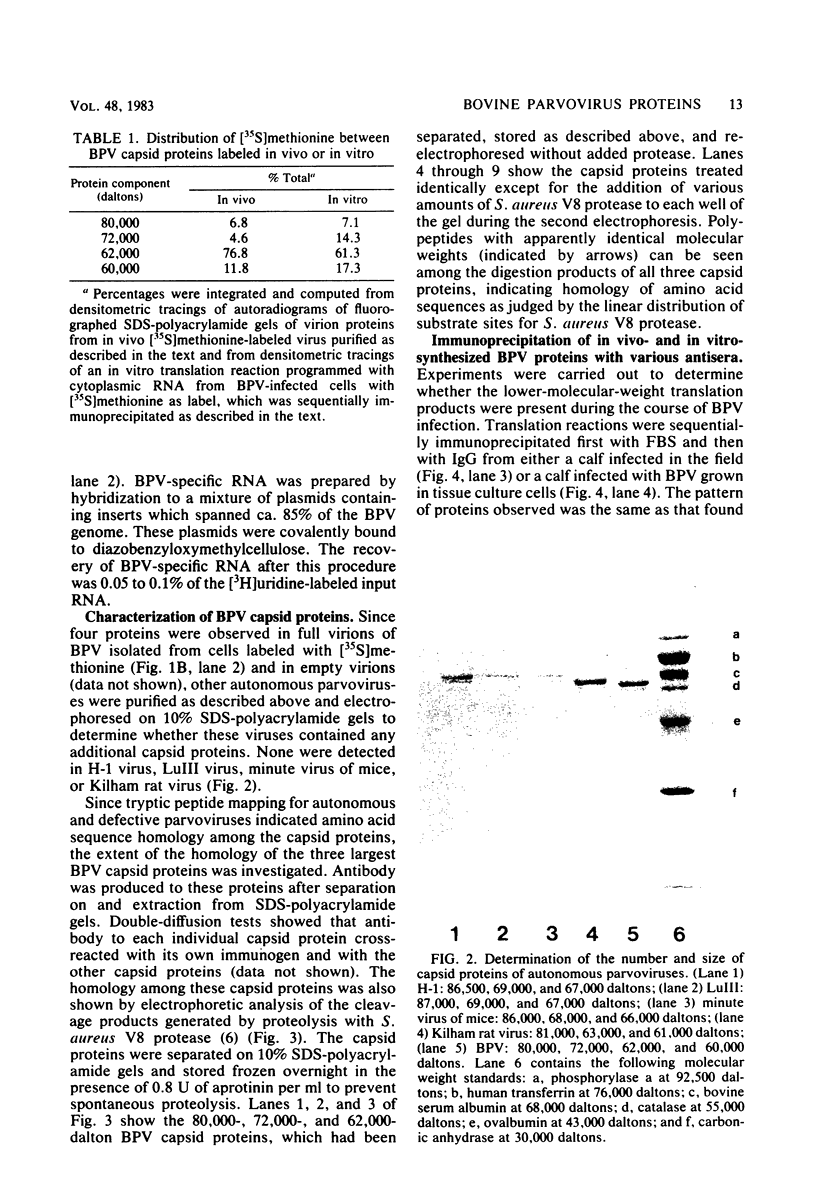
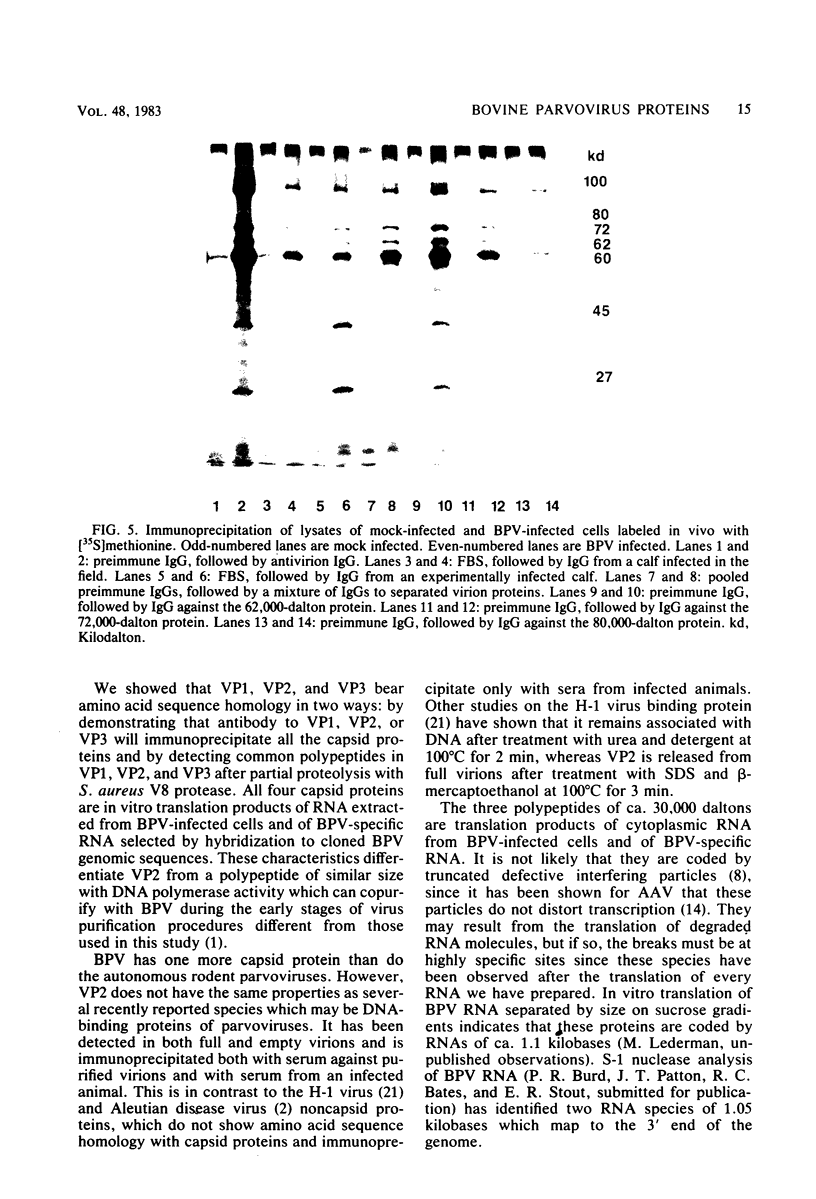
Abstract
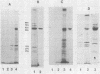
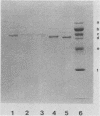
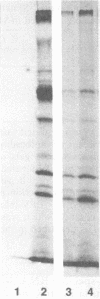
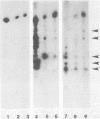
Total cytoplasmic RNA from bovine parvovirus (BPV)-infected cells or BPV-specific RNA selected by hybridization to cloned BPV genomic sequences were translated in a message-dependent rabbit reticulocyte lysate. Immunoprecipitation, using immunoglobulin G from rabbits injected with purified BPV, resulted in the detection of [35S]methionine-labeled polypeptides with MrS of 80,000, 72,000, 62,000, and 60,000. These in vitro translation products had the same mobility on sodium dodecyl sulfate-polyacrylamide gels as that of the four proteins found in purified virions. The three largest polypeptides had amino acid sequence homology, as judged by serological methods and partial proteolysis with Staphylococcus aureus V8 protease. Additional noncapsid proteins with MrS of 25,000, 27,000, and 31,000 were also detected as translation products of these RNAs. All of the above species were immunoprecipitated by immunoglobulin G from a calf which was naturally infected with BPV. All four capsid proteins but only one of the lower-molecular-weight polypeptides were detected after the immunoprecipitation of BPV-infected cells. The results presented here indicate that the BPV genome codes for four capsid proteins and a noncapsid protein which may be structurally related to the capsid proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Hansen U., Handa H., Sharp P. A. Sequential transcription-translation of simian virus 40 by using mammalian cell extracts. Mol Cell Biol. 1981 Oct;1(10):919–931. doi: 10.1128/mcb.1.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Conboy J. G., Rosenberg L. E. Posttranslational uptake and processing of in vitro synthesized ornithine transcarbamoylase precursor by isolated rat liver mitochondria. Proc Natl Acad Sci U S A. 1981 May;78(5):3073–3077. doi: 10.1073/pnas.78.5.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Johnson F. B., Blacklow N. R., Hoggan M. D. Immunological reactivity of antisera prepared against the sodium dodecyl sulfate-treated structural polypeptides of adenovirus-associated virus. J Virol. 1972 Jun;9(6):1017–1026. doi: 10.1128/jvi.9.6.1017-1026.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Hoggan M. D. Structural proteins of HADEN virus. Virology. 1973 Jan;51(1):129–137. doi: 10.1016/0042-6822(73)90373-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Lee H. M., Hoggan M. D., Johnson F. B. Adenovirus-associated virus structural protein sequence homology. J Gen Virol. 1979 Oct;45(1):209–216. doi: 10.1099/0022-1317-45-1-209. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Laughlin C. A., Carter B. J. Adeno-associated virus RNA transcription in vivo. Eur J Biochem. 1981 Dec;121(1):147–154. doi: 10.1111/j.1432-1033.1981.tb06443.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S., Mukoyama J. Isolation and characterization of a parvovirus of rabbits. Infect Immun. 1977 Nov;18(2):495–500. doi: 10.1128/iai.18.2.495-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S. Structural and nonstructural proteins of a rabbit parvovirus. J Virol. 1983 Feb;45(2):627–633. doi: 10.1128/jvi.45.2.627-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Bates R. C. Effect of bovine parvovirus replication on DNA, RNA, and protein synthesis in S phase cells. Virology. 1976 Aug;73(1):72–78. doi: 10.1016/0042-6822(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: preparation of efficient protein synthesis lysates and the purification and characterization of the heme-regulated translational inhibitory protein kinase. Methods Enzymol. 1979;60:459–484. doi: 10.1016/s0076-6879(79)60045-9. [DOI] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I. Definition of subclasses of nucleoplasmic RNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5011–5015. doi: 10.1073/pnas.74.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]