Abstract
The purpose of this study was to evaluate non-contrast magnetic resonance imaging (MRI) findings of adhesive capsulitis and correlate them with clinical stages of adhesive capsulitis. This will hopefully define a role for shoulder MR imaging in the diagnosis of adhesive capsulitis as well as in potentially directing appropriate treatment. Forty-seven consecutive non-contrast magnetic resonance imaging examinations of 46 patients with a clinical diagnosis of adhesive capsulitis were retrospectively reviewed and correlated with clinical staging. Specific MRI criteria correlated with the clinical stage of adhesive capsulitis, including the thickness and signal intensity of the joint capsule and synovium as well as the presence and severity of scarring in the rotator interval. Routine MRI of the shoulder without intraarticular administration of gadolinium can be used to diagnose all stages of adhesive capsulitis, including stage 1, where findings may be subtle on clinical examination. We believe that future studies assessing the role of MRI in guiding the initiation of appropriate treatment should be undertaken.
Keywords: adhesive capsulitis, shoulder, magnetic resonance imaging
Introduction
Primary adhesive capsulitis (AC) is a clinical condition of progressive shoulder pain and decreased passive and active range of motion [1–3]. Four stages of the disease were initially described by Neviaser and subsequently modified using arthroscopic criteria [3–5]. These publications underscored the importance of appropriate clinical staging in the determination of optimum treatment [3–5].
The determination of the stage of adhesive capsulitis has traditionally been made utilizing clinical criteria, described in detail by Neviaser [6] (Table 1). This approach is effective in the hands of a skilled orthopedic surgeon or musculoskeletal physician. However, many patients with adhesive capsulitis are treated by their primary care physicians, and in this circumstance, magnetic resonance imaging (MRI) is used as an adjunct to clinical diagnosis. The clinical presentation of adhesive capsulitis can mimic other conditions such as calcific tendinitis, early glenohumeral joint arthrosis, or subacromial impingement. In the case of secondary AC, the diagnosis can be obscured by the primary disease. Diagnostic imaging studies can aid in defining the status of the capsule with regards to its structural integrity and MRI signal characteristics, the latter of which reflect their histopathologic state [7].
Table 1.
Stages of adhesive capsulitis (adapted from Hannafin and Chiaia CORR [2])
| Description | |
|---|---|
| Stage 1 | Duration of symptoms 0–3 months |
| Pain with active and passive ROM | |
| Limitation of forward flexion, abduction, internal rotation, external rotation | |
| EUA: normal or minimal loss of ROM | |
| Arthroscopy: diffuse glenohumeral synovitis | |
| Pathology: hypertrophic, hypervascular synovitis; rare inflammatory cell infiltrates, normal capsule | |
| Stage 2 | Duration of symptoms 3 to 9 months |
| Chronic pain with active and passive ROM | |
| Significant limitation of forward flexion, abduction, internal rotation, external rotation | |
| EUA: no change in ROM compared with when patient is awake | |
| Arthroscopy: diffuse, pedunculated synovitis | |
| Pathology: hypertrophic, hypervascular synovitis with perivascular and subsynovial scar, fibroplasias, and scar formation in the underlying capsule | |
| Stage 3 | Duration of symptoms 9 to 15 months |
| Minimal pain except at end ROM | |
| Significant limitation of ROM with rigid “end feel” | |
| EUA: no change in ROM compared with when patient is awake | |
| Arthroscopy: no hypervascularity seen; remnants of fibrotic synovium. Diminished capsular volume | |
| Pathology: “burned out” synovitis without significant hypertrophy or hypervascularity. Dense scar formation of the capsule | |
| Stage 4 | Duration of symptoms: 15 to 24 months |
| Minimal pain | |
| Progressive improvement in ROM |
ROM Range of motion, EUA examination under anesthesia
The imaging findings of adhesive capsulitis have been described and have traditionally been limited to conventional arthrography. The arthrographic criteria of adhesive capsulitis include the following: limited injectable fluid capacity of the glenohumeral joint (7–10cc), a small dependent axillary fold, and irregularity of the anterior capsular insertion at the anatomic neck of the humerus [8, 9]. More recently, MRI features helpful in the diagnosis of AC have been described in the literature, with relatively disappointing overall specificity for the disease [10–13]. Most of these reports have included MRI images obtained with either indirect (intravenous) or direct (intraarticular) arthrography [14–16].
To our knowledge, specific non-contrast MRI findings that correspond to the clinical stage of AC have not been established and reported in the literature. The primary aim of this retrospective review of patients was to determine if the MRI findings indicative of AC could be correlated to their clinical function and stage of disease. In addition, we aimed to assess the ability of MRI to detect the morphology of the synovium and capsule in early stages of AC.
Materials and methods
This protocol was approved by our Institutional Review Board. All patients at our institution between 1992 and 2005 who were evaluated by the orthopedic surgery department with either the presumptive clinical diagnosis of AC or MRI findings suggestive of adhesive capsulitis were eligible for this study. Patients with MRIs and detailed clinical information that permitted determination of stage were included. Of the 46 patients included in this study (47 exams, one patient underwent bilateral studies), there were 33 women and 13 men. Subjects’ age ranged from 32 to 74years, with a mean age of 53.
The MRI examinations were read by two experienced musculoskeletal radiologists who were blinded to the patient’s clinical stage of adhesive capsulitis The specific MRI findings evaluated included those previously reported in the MR arthrography as well as the orthopedic literature as diagnostic of adhesive capsulitis such as scarring in the rotator interval, synovitis, and capsular thickening [16, 17]. Moreover, the morphology of the anterior capsule was specifically studied, including its thickness and signal intensity. The shoulder MR exams were performed in the setting of routine clinical care; thus the contralateral shoulder was not imaged as a control. Normal reference ranges, however, for asymptomatic joint capsule and synovial thickness, have been previously determined to be 2.9mm or less [11]. Signal intensity was judged in reference to the normal anterior capsule, which is intermediate in signal intensity on proton density fast spin-echo images. Combined capsular and synovial thickness was measured at the level of the mid-axillary pouch on three consecutive non-contrast fast spin-echo proton density oblique coronal images on a GE Advantage Workstation (Milwaukee, WI, USA) utilizing digital calipers as the synovial and capsular contributions to total thickness could not be separately measured. Measurements were averaged for the final value used for data analysis. Scarring in the rotator interval was graded subjectively as mild, moderate, or severe. Signal intensity of the capsule was graded as hypointense, hyperintense, or isointense relative to normal low signal intensity capsule.
MRI examinations were performed on a 1.5-Telsa scanner (Signa Horizon LX, General Electric Medical Systems, Milwaukee, WI, USA), with a dedicated shoulder coil (Med Rad multipurpose array, Indianola, PA, USA). Pulse sequence parameters included an oblique coronal fast spin-echo (FSE) fat-suppressed sequence fast spin-echo [repetition time (TR) 4,000ms, echo time (TE) 80ms, echo train length (ETL) 8–10, matrix 256 × 224, slice thickness (THK) 3mm (no interslice gap), field of view (FOV) 16cm, fat suppression; oblique coronal proton density FSE (TR 4,000ms, TE 34ms, ETL 8–14, matrix 512 × 384, THK 3mm (no interslice gap), FOV 16cm]; oblique sagittal proton density FSE [TR 3,500–4,000ms, TE 34ms, ETL 8–12, matrix 512 × 224, THK 4mm (skip 0.5mm), FOV 16cm]; and axial proton density FSE [TR 4,000ms, TE 34ms, ETL 8–14, matrix 512 × 384, THK 3.5mm (no interslice gap), FOV 15–16cm; Figs. 1, 2, 3, and 4].
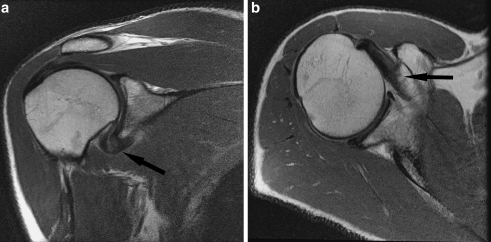
Fig. 1.
a Oblique coronal proton density FSE image of a 55-year-old man with clinical stage 1 adhesive capsulitis. There is thickening of the axillary pouch, which is only mildly hyperintense (arrow). b Axial proton density FSE image of the same patient demonstrates moderate scarring of the rotator interval (arrow)
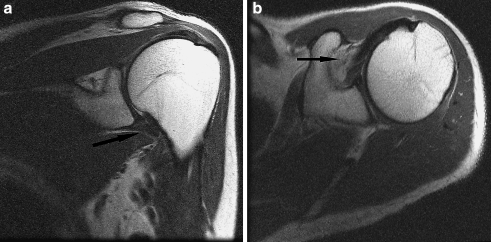
Fig. 2.
a Oblique coronal proton density FSE image of a 57-year-old woman with clinical stage 2 adhesive capsulitis. Note the moderately thickened, hyperintense capsule at the level of the axillary pouch (arrow). b Axial proton density FSE image of the same patient demonstrates mild hyperintensity in the rotator interval (arrow)
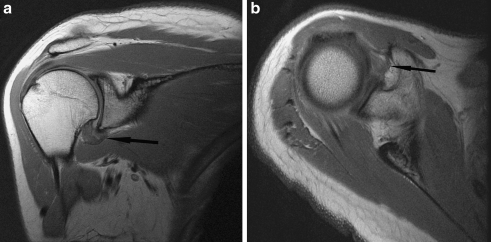
Fig. 3.
a Oblique coronal proton density FSE image of a 48-year-old woman with clinical stage 3 adhesive capsulitis. There is mild thickening of the capsule, which is hypointense (arrow). b Axial proton density FSE image of the same patient demonstrates mild scarring in the rotator interval (arrow)
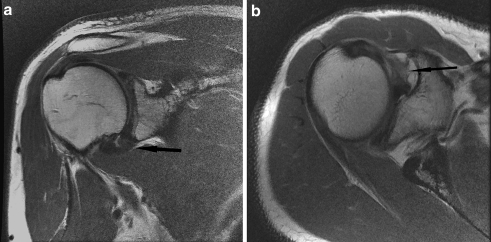
Fig. 4.
a Oblique coronal proton density FSE image of a 62-year-old man with clinical stage 4 adhesive capsulitis. The capsule is mildly thickened, redundant, and hypointense (arrow). b Axial proton density FSE image of the same patient shows mild scarring in the rotator interval (arrow)
The clinical assessment and staging of adhesive capsulitis have been described (see Table 1). In stage 1, the patient has had symptoms for approximately 1 to 3months. There is usually pain with active and passive motion; however, motion can be normal or nearly normal. Arthroscopic and histologic examination reveals a hypertrophic, hypervascular synovitis with a normal underlying capsule. In stage 2, patients report symptoms for a longer time, generally 3 to 9months. There is chronic pain with active and passive motion and significant limitations in forward flexion, abduction, internal rotation, and external rotation. Arthroscopic inspection and pathologic examination demonstrate both a considerable inflammatory component and a component of fibrosis with a hypertrophic, hypervascular glenohumeral joint synovitis, perivascular scar formation, and significant capsular thickening and scar [2]. In stage 3, the patient has had symptoms for 9 to 15months and has minimal pain except at the end ranges of motion. There is significant loss of motion with a solid endpoint. Arthroscopic and histologic evaluations demonstrate atrophic synovitis and dense capsular scar. Stage 4 is the final stage; the patient has had symptoms for 15 to 24months, has minimal pain, and experiences progressive improvement in motion. The clinical diagnosis of stage 1 was present in eight subjects; stage 2 in 23 subjects; stage 3 in eight subjects; and stage 4 in eight subjects.
Descriptive analysis consisted of means, standard deviations, and ranges for continuous data and frequencies and percentages for discrete data. Inferential analysis consisted of a one-way ANOVA model for comparison of disease stage with pouch size in millimeter. A post hoc test comparing each disease stage was calculated using a Dunnett T3 comparison because the standard deviations were not equivalent. Comparisons of discrete measures across stage categories were performed using a Fisher’s exact test due to sparse cell sizes. A critical p value of 0.05 was used for all hypothesis tests. All analyses were performed using SPSS for Windows 13.0 (Chicago, IL, USA) and The SAS System 9.1 (Cary, NC, USA).
Results
Thickening of the axillary pouch ranged from 2 to 13mm, with an average of 7mm. All subjects demonstrated scarring of the rotator interval, including 16 subjects with mild, 26 with moderate, and five with severe. Analysis of signal intensity of the capsule included five subjects with isointensity, 13 with hypointensity, and 29 with hyperintensity relative to the normal signal of shoulder capsule.
Capsular and synovial thickening as measured in the axillary pouch correlated with clinical stage of AC with a mean axillary pouch thickness (7.5mm) for stage 2 as compared to stage 1 (4.1mm), stage 3 (5.5mm), and stage 4 (4.1mm; p < 0.05]. Values for stages 1, 3, and 4 when compared to each other were not statistically significant. Evaluation of capsular signal was significant (p = 0.02), with hyperintense signal correlating with stage 2. Although all subjects demonstrated scarring in the rotator interval, the degree of scarring was not statistically significant between patient groups.
Discussion
AC is a clinical condition of progressive pain and decreased range of motion of the glenohumeral joint [6]. Primary AC is an idiopathic condition characterized by the insidious onset of symptoms, most commonly seen in women over 40years, generally without an antecedent inciting event or medical/surgical condition, although diabetes mellitus and hypothyroidism are risk factors for primary AC. Secondary causes of AC include history of severe trauma and prior surgery [2].
Although the exact etiology of AC is controversial, this disease is thought to be a cytokine-mediated synovial inflammation with subsequent capsular fibrosis [18]. The initial stages of AC have a predominance of pain, with gradually increasing joint stiffness brought on by ongoing synovial inflammation and capsular fibrosis. In the later stages, as the inflammatory phase subsides, capsular fibrosis is at its peak [18, 19] (see Table 1).
AC has been described as self-limiting, but complete resolution may take up to 2years. Treatment options for AC have included but are not limited to physical therapy, corticosteroid injections, closed manipulations, and capsular release [2, 20]. The suggested treatment protocol for AC is dependent on the clinical stage; therefore, correct diagnosis of AC as well as correct identification of the stage of AC may affect treatment and ultimately shorten the clinical course [2, 19, 20]. The goal of the treatment of patients with stage 1 adhesive capsulitis is to essentially perform a “chemical synovectomy” and interrupt the inflammatory cycle, which can be accomplished with an intraarticular corticosteroid injection. One can then institute rehabilitation exercises focusing on range of motion (ROM) more effectively. The treatment of patients in stage 2 is primarily corticosteroid injection and physical therapy, with recalcitrant cases necessitating arthroscopic capsular release and manipulation.
While assigning stages to adhesive capsulitis is traditionally made on clinical grounds, we speculate that imaging-based staging may assist in determination of potential response to treatment. In addition, we propose that signal abnormalities in the capsule diagnosed non-invasively by MRI correlate with the histopathology. Distinguishing between stage 1 and stage 2 on clinical grounds, for example, is often based on patient response to a single intraarticular corticosteroid/local anesthetic injection. We propose that the degree of capsular thickening and hyperintensity on MRI corresponds to the hypertrophic, hypervascular synovitis and inflammatory changes seen in cases of stages 1 and 2.
The use of magnetic resonance imaging to diagnose adhesive capsulitis has previously been described. Connell et al. described a series of 24 patients, all of whom underwent arthroscopic capsulotomy [10]. All patients were evaluated with indirect MRI arthrography. These authors found soft tissue intensity demonstrating variable post-contrast enhancement in the rotator interval as well as partially encasing the biceps anchor as useful imaging findings suggestive of adhesive capsulitis. All MRI images in that series were obtained after the administration of intravenous gadolinium and findings were not correlated with the clinical stages of adhesive capsulitis. Of note, all patients went on to subsequent surgical intervention.
Some authors have found post-contrast enhancement of the joint capsule and synovial membrane in the rotator interval helpful in diagnosing adhesive capsulitis [15, 21]. In contrast, other authors have found intraarticular gadolinium administration not to be useful in diagnosing adhesive capsulitis [11]. Manton et al. found no significant difference in a series of 28 patients between capsular and synovial thickness, intraarticular fluid volume, and irregularities of the capsule with MRI arthrography between symptomatic and asymptomatic groups [12].
In our series, we used similar criteria to Emig et al., including capsular and synovial thickness, thickness of the coracohumeral ligament, and volume of intraarticular fluid [11]. Emig et al. found a correlation between joint capsule and synovium thickness greater than 4mm and clinical diagnosis of adhesive capsulitis; however, these authors did not correlate their MRI findings to clinical stage [11].
We speculate that the presence of increased signal intensity of the joint capsule and synovium in patients with stage 2 AC in our series likely reflects the active synovial and capsular response in this stage of the disease. The signal characteristics of collagen correlate with the degree of cellularity at histologic inspection, with areas of low cellularity and dense collagen being lower signal intensity on all pulse sequences [22, 23]. The appearance of indolent fibrosis and scarring on MRI has been described as being low signal intensity on all pulse sequences, with areas of hypercellularity and increased free water resulting in higher signal intensity [24]. Histologic studies have confirmed the presence of neovascularization and disruption of the normal collagen architecture in cases of lateral epicondylitis, correlating to areas of increased signal intensity on MRI examinations [7]. We propose that the high signal intensity seen in the glenohumeral joint capsule, specifically in stage 2, may reflect the hypervascular synovitis seen at histopathology.
In conclusion, adhesive capsulitis is a syndrome of shoulder pain and limited range of motion, the cause of which may be idiopathic or secondary to a variety of clinical conditions. Early diagnosis and establishment of clinical stage is important in the prompt and effective treatment of this disorder. MRI of the shoulder is an effective and non-invasive means of diagnosing suspecting cases and also provides information that may assist the clinician in differentiating between the early and late stages. Capsule and synovial thickness, as measured in the axillary pouch, demonstrates the greatest correlation with clinical stage of adhesive capsulitis. Earlier, more hypervascular stages exhibit greater combined synovial and capsular thickening, while later more fibrotic stages demonstrate only capsular thickening. Hyperintensity of capsular signal was most closely associated with stage 2 disease. Rotator interval scarring is a non-specific signs of AC and was not found to correlate with clinical stage.
References
- 1.Duplay S (1872) De la peri-arthrite scapulo-humerale et des raideurs de lepaule qui en sont la consequence. Arch Gen Med 20:513–542
- 2.Hannafin JA, Chiaia TA (2000) Adhesive capsulitis: a treatment approach. Clin Orthop 372:95–109 [DOI] [PubMed]
- 3.Neviaser JS (1945) Adhesive capsulitis of the shoulder. A study of the pathological findings in periarthritis of the shoulder. J Bone Joint Surg 27:211–222
- 4.Neviaser RJ (1983) Painful conditions affecting the shoulder. Clin Orthop 173:63–69 [PubMed]
- 5.Neviaser RJ, Neviaser TJ (1987) The frozen shoulder. Diagnosis and management. Clin Orthop 223:59–64 [PubMed]
- 6.Neviaser TJ (1996) Adhesive capsulitis. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG (eds) Operative arthroscopy, 2nd edn. Lippincott-Raven, Philadelphia
- 7.Potter HG, Hannafin JA, Morwessel RM, DiCarlo EF, O’Brien SJ, Altchek DW (1995) Lateral epicondylitis: correlation of MRI imaging, surgical, and histopathological findings. Radiology 196:43–46 [DOI] [PubMed]
- 8.Loyd JA, Loyd HM (1983) Adhesive capsulitis of the shoulder: arthrographic diagnosis and treatment. South Med J 76:879–883 [DOI] [PubMed]
- 9.Neviaser TJ (1980) Arthrography of the shoulder. Orthop Clin North Am 11:205–217 [PubMed]
- 10.Connell D, Padmanabhan R, Buchbinder R (2002) Adhesive capsulitis: role of MRI imaging differential diagnosis. Eur Radiol 12(8):2100–2106 [DOI] [PubMed]
- 11.Emig EW, Schweitzer ME, Karasick D, Lubowitz J (1995) Adhesive capsulitis of the shoulder: MRI diagnosis. Am J Roentgenol 164:1457–1459 [DOI] [PubMed]
- 12.Manton GL, Schweitzer ME, Weishpaut D, Karasick D (2001) Utility of MRI arthrography in the diagnosis of adhesive capsulitis. Skeletal Radiol 30(6):326–330 [DOI] [PubMed]
- 13.Tamai K, Yamato M (1997) Abnormal synovium in frozen shoulder: a preliminary report with dynamic magnetic resonance imaging. J Shoulder Elbow Surg 6(6):534–543 [DOI] [PubMed]
- 14.Jung JY, Jee WH, Chun HJ, Kim YS, Chung YG, Kim JM (2006) Adhesive capsulitis of the shoulder: evaluation with MR arthrography. Eur Radiol 16(4):791–796 [DOI] [PubMed]
- 15.Lefevre-Colau MM, Drape JL, Fayad F et al (2005) Magnetic resonance imaging of shoulders with idiopathic adhesive capsulitis: reliability of measures. Eur Radiol 15(12):2415–2422 [DOI] [PubMed]
- 16.Mengiardi B, Pfirrmann CW, Gerber C, Hodler J, Zanetti M (2004) Frozen shoulder: MR arthrographic findings. Radiology 233(2):486–492 [DOI] [PubMed]
- 17.Ekelund AL, Rydell N (1992) Combination treatment for adhesive capsulitis of the shoulder. Clin Orthop 282:105–109 [PubMed]
- 18.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL (1997) Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res 15:427–436 [DOI] [PubMed]
- 19.Grey RG (1978) Brief note. The natural history of “idiopathic” frozen shoulder. J Bone Joint Surg Am 60-A:564 [PubMed]
- 20.Griggs SM, Ahn A, Green A (2000) Idiopathic adhesive capsulitis. A prospective functional outcome study of nonoperative treatment. J Bone Joint Surg Am 82-A:1398–1407 [PubMed]
- 21.Carrillon Y, Noel E, Fantino O, Perrin-Fayolle O, Tran-Minh VA (1999) Magnetic resonance imaging findings in idiopathic adhesive capsulitis of the shoulder. Rev Rhum Engl Ed 66(4):201–206 [PubMed]
- 22.Munshi M, Pretterklieber ML, Chung CB et al (2004) Anterior bundle of ulnar collateral ligament: evaluation of anatomic relationships by using MRI imaging, MRI arthrography and gross anatomic and histologic analysis. Radiology 231:797–803 [DOI] [PubMed]
- 23.Yacoe ME, Bergman AG, Ladd AL, Hellman BH (1993) Dupuytren’s contracture: MRI imaging findings and correlation between MRI signal intensity and cellularity of lesions. Am J Roentgenol 160:813–817 [DOI] [PubMed]
- 24.Amis ES Jr (1991) Retroperitoneal fibrosis. Am J Roentgenol 157:321–329 [DOI] [PubMed]