Abstract
Hybrid cells generated by fusing dendritic cells with tumor cells (DC-TC) are currently being evaluated as cancer vaccines in preclinical models and human immunization trials. In this study, we evaluated the production of human DC-TC hybrids using an electrofusion protocol previously defined for murine cells. Human DCs were electrically fused with allogeneic melanoma cells (888mel) and were subsequently analyzed for coexpression of unique DC and TC markers using FACS and fluorescence microscopy. Dually fluorescent cells were clearly observed using both techniques after staining with Abs against distinct surface molecules suggesting that true cell fusion had occurred. We also evaluated the ability of human DC-TC hybrids to present tumor-associated epitopes in the context of both MHC class I and class II molecules. Allogeneic DCs expressing HLA-A*0201, HLA-DRβ1*0401, and HLA-DRβ1*0701 were fused with 888mel cells that do not express any of these MHC molecules, but do express multiple melanoma-associated Ags. DC-888mel hybrids efficiently presented HLA-A*0201-restricted epitopes from the melanoma Ags MART-1, gp100, tyrosinase, and tyrosinase-related protein 2 as evaluated by specific cytokine secretion from six distinct CTL lines. In contrast, DCs could not cross-present MHC class I-restricted epitopes after exogenously loading with gp100 protein. DC-888mel hybrids also presented HLA-DRβ1*0401- and HLA-DRβ1*0701-restricted peptides from gp100 to CD4+ T cell populations. Therefore, fusions of DCs and tumor cells express both MHC class I- and class II-restricted tumor-associated epitopes and may be useful for the induction of tumor-reactive CD8+ and CD4+ T cells in vitro and in human vaccination trials.
Dendritic cells (DCs)3 are potent APCs that are capable of stimulating both naive CD4+ Th cells and CD8+ CTLs (1). Therefore, DCs are being extensively evaluated as vehicles for Ag delivery in immunotherapies for the treatment of patients with cancer. Many techniques have been used to load DCs with tumor-associated Ags, including pulsing with synthetic peptides that represent T cell epitopes (2, 3). This strategy has been used in several human clinical vaccination trials (4, 5); however, it is limited to patients who express the particular peptide MHC-restricting molecule. Alternatively, DCs have been pulsed with recombinant proteins (6) or transduced with recombinant viruses (7-12). These approaches circumvent the MHC restrictions associated with peptides but are generally limited to individual proteins. Because many tumors display heterogenous expression of target Ags (13), strategies that induce T cell responses against multiple proteins may be more efficacious. Another concern is that some of these Ag loading techniques facilitate the presentation of immunogenic viral or bacterial epitopes in addition to those from the tumor-associated protein.
Other Ag presentation systems have been developed to stimulate polyclonal immune responses against multiple tumor-associated proteins, including those that are known, undefined, shared, or unique. For example, DCs have been loaded with killed tumor cells (14) and tumor lysates (15, 16). These approaches may be inefficient for stimulating polyclonal CTL responses because some proteins are not efficiently cross-presented even by DCs that have mechanisms which allow exogenously loaded proteins to enter MHC class I-processing pathways (1). Alternatively, DCs have been pulsed with peptides eluted from tumor cells (17) and have been transfected with tumor-derived RNA (18, 19). In murine models, these approaches have induced protective and therapeutic immune responses against tumors and are currently being evaluated in human clinical vaccination trials.
Another evolving strategy for inducing immunotherapeutic responses against cancer is the use of hybrid cells generated by fusing DCs with tumor cells (DC-TC) (20-22). Theoretically, DC-TC hybrids possess properties of both parental cell types necessary for inducing primary CD4+ and CD8+ T cell responses. Namely, fusions of DCs and tumor cells should express shared and unique tumor-associated Ags, high levels of MHC class I and class II molecules, and adhesion and costimulatory molecules. Vaccinations with DC-TC hybrids have stimulated protective and therapeutic immune responses in several rodent tumor models including carcinomas, lymphomas, melanomas, and gliomas (23-29). In addition, several in vitro studies with human cells have suggested that DC-TC hybrids can present relevant tumor-associated Ags in the context of class I HLA molecules and induce tumor-reactive CTL (30-33). However, in some of these studies, the production of multinucleated hybrids with fused cell membranes was not clearly documented. Because previous investigations have demonstrated that physical mixtures of DCs and tumor cells can sometimes induce effective CTL responses (34), some results pertaining to the enhanced immunogenicity of DC-TC hybrids have been difficult to interpret.
Two human clinical trials have been reported in which patients with melanoma (35) or malignant glioma (36) were vaccinated with autologous DC-TC hybrids made with the fusogenic agent polyethylene glycol (PEG). Although the treatments were safe and well-tolerated, few clinical responses were observed. An alternate technique for generating DC-TC hybrids is to expose cells to electric fields (37). A cell mixture is first treated with an alternating nonuniform electric current of low strength that causes cells to align, forming tight membrane contacts. Subsequently, a direct current pulse with high intensity but short duration disrupts cell membranes reversibly. After cessation of this pulse, membrane resealing occurs between cells in close contact. Results from several investigations have demonstrated that electrofusion can generate genuine DC-TC hybrids (22, 28, 33). In FACS profiles and confocal micrographs, fused cells appear as a distinct population that are dually fluorescent for individual cell-specific markers and are clearly distinguishable from DCs or tumor cells alone that remain in the mixture. Furthermore, hybrids can be seen as individual multinucleated cells on Giemza-stained cytocentrifuge slides.
Vaccinations with DC-TC hybrids generated by electrofusion have been shown to be therapeutic in murine tumor models (28). Namely, intranodal injection of DC-TC fusion cells with IL-12 as an adjuvant eradicated established s.c. GL261 gliomas and D5LacZ3 pulmonary metastases. To adapt this technology to human clinical trials, we evaluated the ability of electrofused human DC-TC hybrids to present tumor-associated Ags in the context of both MHC class I and class II molecules. Allogeneic DC-TC hybrids were produced by fusing DCs expressing HLA-A*0201, -DRβ1*0401, and -DRβ1*0701 with melanoma cells (888mel) that express none of these HLA molecules. The generation of membrane-fused DC-888mel hybrids was verified using a combination of FACS and fluorescence microscopy. Based on specific IFN-γ secretion by six unique tumor-reactive CTL lines, DC-888mel hybrids presented immunodominant epitopes from the melanoma Ags, MART-1, gp100, tyrosinase, and an alternate isoform of tyrosinase-related protein (TRP) 2 (TRP2-6b) in the context of HLA-A*0201. Furthermore, these hybrids were specifically recognized by CD4+ T cell lines reactive with gp100 epitopes in the context of HLA-DRβ1*0401 and -DRβ1*0701. These results suggest that electrofused human DC-TC hybrids may elicit both CD4+ and CD8+ T cell responses, and hence, may be useful in human cancer vaccine trials. They may also be useful for the identification of new human tumor-associated Ags, particularly for cancers for which it has been difficult to establish tumor-reactive T cell populations by traditional means. In addition, human DC-TC hybrids may enable the ex vivo generation of tumor-reactive T lymphocytes for use in adoptive therapy protocols.
Materials and Methods
Cell lines and reagents
Human melanoma cell lines and EBV-transformed B cells were routinely cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS and 2 mM l-glutamine (Invitrogen, Carlsbad, CA). Human lymphocytes were cultured in complete medium consisting of RPMI 1640, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (Invitrogen) and 10% heat-inactivated human AB serum (Gemini Bio-Products, Woodland, CA; Valley Biomedical, Winchester, VA). Multiple melanoma-reactive T cell lines and clones (38-45) were used to evaluate the presentation of MHC-restricted epitopes by DC-TC hybrids as presented in Table I.
Table I.
Characteristics of Ag-reactive T cell lines and clones
| T Cell Clone/Line | CD4/CD8 | HLA Restriction Element | Peptide Specificity | Reference |
|---|---|---|---|---|
| TIL 1200 | CD8 | HLA-A*0201 | gp100:154–162 | 38, 39 |
| CK3H6 | CD8 | HLA-A*0201 | gp100:209–217 | 40 |
| JR1E2 | CD8 | HLA-A*0201 | gp100:280–288 | 40 |
| MB4 | CD8 | HLA-A*0201 | TRP2-6b:403–411 | 41 |
| JB2F4 | CD8 | HLA-A*0201 | MART-1:27–35 | 40 |
| GA3D | CD8 | HLA-A*0201 | tyrosinase:368–377(370D) | 42 |
| TH1F2L | CD8 | HLA-A*0201 | NY-ESO-1:157–165 | 43 |
| B104 | CD4 | HLA-DRβ1*0401 | gp100:170–190 | 44 |
| BR-B8 | CD4 | HLA-DRβ1*0701 | gp100:44–59 | 45 |
Expression of MART-1 and gp100 in melanoma cell lines was assessed by FACS using mAbs (M2-7 C10 from Dr. Y. Kawakami (Keio University School of Medicine, Tokyo, Japan; Ref. 46; HMB45 from Enzo Diagnostics (Farmingdale, NY)), and the presence of tyrosinase was assessed by immunohistochemistry (13). Expression of TRP2-6b and NY-ESO-1 was previously evaluated on the basis of specific IFN-γ secretion by two T cell clones, MB4 and M8, respectively, that specifically recognize peptides from these proteins in the context of HLA-A*0201 (41). Although the 888 melanoma cell line (888mel) did not express HLA-A*0201, recognition of this cell line, and hence, expression of TRP2-6b and NY-ESO-1, was evaluated after stable transfection with cDNA encoding HLA-A*0201. The F002R melanoma cell line (F002Rmel) did not naturally express gp100, but it had previously been transduced with a vesicular stomatitis virus (VSV)-pseudotyped retroviral vector encoding either gp100 or green fluorescence protein (GFP) (44). Expression of HLA class II molecules on the surfaces of melanoma cells was up-regulated by transduction with a retroviral construct encoding the HLA class II transactivator (CIITA) gene as previously described (47). The expression of cell surface HLA class I and class II molecules on melanoma cells was confirmed by FACS using mAbs against HLA-A2 (One Lambda, Conestoga, CA), HLA-DR (L243; BD Biosciences, Franklin Lakes, NJ), HLA-DR4 (Accurate Chemical and Scientific, Westbury, NY), and HLA-DR7 (Pel-Freez Biologicals, Rogers, AR). In addition, HLA haplotypes of cell lines were determined by DNA sequencing in the HLA Laboratory (National Institutes of Health). The expression of MART-1, gp100, tyrosinase, TRP2-6b, NY-ESO-1, HLA-A*0201, HLA-DRβ1*0401, and HLA-DRβ1*0701 in melanoma cell lines was as follows: 888mel (MART-1+, gp100+, tyrosinase+, TRP2-6b weak, NY-ESO-1−, HLA-A*0201−, HLA-DRβ1*0401−, HLA-DRβ1*0701−), 624mel CIITA (MART-1+, gp100+, tyrosinase+, TRP2-6b+, NY-ESO-1+, HLA-A*0201+, HLA-DRβ1*0401+, HLA-DRβ1*0701+), 526mel CIITA (MART-1+, gp100+, tyrosinase+, TRP2-6b+, NY-ESO-1−, HLA-A*0201+, HLA-DRβ1*0401+, HLA-DRβ1*0701−), F002Rmel CIITA-gp100 (gp100+, HLA-A*0201+, HLA-DRβ1*0401−, HLA-DRβ1*0701+), F002Rmel CIITA-GFP (gp100−, HLA-A*0201+, HLA-DRβ1*0401−, HLA-DRβ1*0701+), 1088mel CIITA (gp100+, HLA-DRβ1*0401+, HLA-DRβ1*0701−), 1978mel CIITA (gp100+, HLA-DRβ1*0401−, HLA-DRβ1*0701+), and 1861mel CIITA (gp100+, HLA-DRβ1*0401+, HLA-DRβ1*0701−).
Electrofusion of DCs and tumor cells
Immature DCs were prepared as previously described (48). Briefly, adherent PBMC were cultured in RPMI 1640 with 10% heat-inactivated FBS containing 2000 IU/ml GM-CSF (Amgen-Immunex, Seattle, WA) and 1000 IU/ml IL-4 (R&D Systems, Minneapolis, MN). Five days later, DC maturation was induced with 10 ng/ml TNF-α (Sigma-Aldrich, St. Louis, MO) and 1 μg/ml PGE2 (Sigma-Aldrich). On day 7, electrofusion of the DCs and 888mel cells was performed as previously described with slight modification (28). In most experiments, 888mel cells were labeled with CFSE (Molecular Probes, Eugene, OR) before electrofusion: 107 cells/ml in HBSS were labeled in the presence of 5 μM dye for 10 min at 37°C, and labeling was terminated by dilution with ice-cold HBSS. In all experiments, 888mel cells were irradiated with 10,000 cGy before electrofusion. To induce fusion, DCs and irradiated tumor cells were mixed at a ratio of 1:1 and were suspended in a 5% glucose solution containing 0.1 mM Ca(CH3COO)2, 0.5 mM Mg(CH3COO)2, and 0.3% BSA. The pH of the fusion medium was adjusted to 7.2–7.4 with l-histidine (all chemicals from Sigma-Aldrich). After centrifugation, the cells were resuspended in the same medium in the absence of BSA. Routinely, 1 ml of cell suspension containing 1 × 107 cells were processed using a specially designed concentric fusion chamber. For electrofusion, a pulse generator (model ECM 2001; BTX Instruments, Genetronics, San Diego, CA) was used for application of the field pulses. Cell alignment was first induced by dielectrophoresis with an alternating current (ac) pulse of 150 V/cm for 10 s. Subsequently, cell fusion was triggered by application of a single square wave direct current (dc) pulse of 1200 V/cm for 25 μs. The fusion mixture was allowed to rest for 5 min before suspension in RPMI 1640 with 10% heat-inactivated FBS. After overnight incubation at 37°C, cells were separated on the basis of adherence. The nonadherent population was aspirated and consisted primarily of residual DCs. The adherent population was harvested using a solution of 0.05% trypsin and 0.02% EDTA (Trypsinversene; BioWhittaker, Walkersville, MD) and contained predominantly fusion hybrids and tumor cells.
FACS analyses and fluorescence microscopy
In some experiments, 888mel cells were labeled with CFSE before electrofusion to enable visualization with FACS or fluorescence microscopy. After fusion, DC-888mel hybrids were detected by staining with PE-conjugated mAbs against DC cell surface markers including CD11c, CD40, CD80, CD86, CD83, and HLA-DR (BD PharMingen, San Diego, CA). Alternatively, in other experiments, 888mel cells were not labeled before fusion, and DC-TC hybrids were detected by costaining with mAbs against markers expressed on either cell type. Namely, FITC-conjugated anti-HLA-DR was used as a DC marker concomitantly with mAbs against gp100 (Enzo Diagnostics) or HLA-A24 (One Lambda), both of which were only expressed in the 888mel cells. These Abs were visualized using a secondary PE-conjugated goat anti-mouse IgG (Caltag Laboratories, Burlingame, CA).
Cytokine release assays
Recognition of target cells by melanoma-reactive CD8+ and CD4+ T cell lines and clones was evaluated on the basis of specific IFN-γ secretion. In one set of experiments, cytokine secretion was measured in response to immature DCs loaded with recombinant gp100 protein or melanoma cell lysates, as well as DCs transduced with an adenoviral vector encoding gp100. Recombinant proteins were commercially produced and purified (Novavax, Columbia, MD). DCs expressing HLA-A*0201 and HLA-DRβ1*0701 were incubated with 10 μg/ml gp100 protein (or NY-ESO-1 as a negative control), overnight at 37°C in 96-well plates (1 × 105 cells/well; 100 μl/well). Alternatively, lysates of F002Rmel CIITA-gp100 cells (or F002Rmel CIITA-GFP as a negative control) were prepared by exposing the cells to five freeze-thaw cycles. Lysates were incubated with DCs at a ratio of one DC to one melanoma cell equivalent overnight at 37°C in 96-well plates (1 × 105 cells/well; 100 μl/well). DCs were also transduced with recombinant adenoviruses encoding gp100 (or MART-1 as a negative control) (Genzyme, Cambridge, MA) as previously described (10) and were incubated 24–48 h at 37°C in 96-well plates (1 × 105 cells/well; 100 μl/well). As positive controls for T cell function, specific IFN-γ secretion was measured in response to peptide-loaded DCs and melanomas. For HLA-A*0201-restricted CD8+ T cell populations, DCs were incubated with 1 μg/ml of the appropriate peptide 1–3 h at 37°C. For HLA class II-restricted CD4+ T cells, DCs were incubated with 50 μg/ml of the appropriate peptide ∼3 h at 37°C. In addition, the melanoma cell lines, F002Rmel CIITA-gp100 and -GFP, were harvested. Responder T cells (105) were coincubated with 105 stimulator cells (250 μl total) ∼20 h at 37°C, and the concentration of human IFN-γ in coculture supernatants was measured by ELISA (Pierce-Endogen, Cambridge, MA).
Recognition of DC-888mel hybrid cells by melanoma-reactive CD8+ and CD4+ T cell lines and clones was also evaluated on the basis of specific IFN-γ secretion. Responder T cells (105) were coincubated with 1–4 × 104 fusion cells in 96-well plates ∼20 h at 37°C, and the concentration of human IFN-γ in coculture supernatants was measured by ELISA (Pierce-Endogen). As positive controls for T cell function, specific IFN-γ secretion was measured in response to peptide-loaded target cells and melanomas. For HLA-A*0201-restricted CD8+ T cell populations, T2 cells were incubated with 1 μM of the appropriate peptide 1–3 h at 37°C. For class II HLA-restricted CD4+ T cells, EBV-transformed B cells expressing HLA-DRβ1*0401 or HLA-DRβ1*0701 were incubated with 50 μM of the appropriate peptide ∼3 h at 37°C. In addition, melanoma cell lines expressing various combinations of HLA-A*0201, HLA-DRβ1*0401, and HLA-DRβ1*0701, were harvested. Responder T cells (105) were coincubated with 105 stimulator cells, and the concentration of human IFN-γ in coculture supernatants was measured by ELISA (Pierce-Endogen).
Results
Characterization of human DC-melanoma cell hybrids
Phenotypic analysis of human DCs and hybrids of DCs fused with 888 melanoma cells (DC-888mel) was performed by FACS. Before electrofusion, DCs displayed markers consistent with those of mature DCs (Fig. 1); they expressed CD11c but not the monocyte/macrophage marker CD14 or the NK cell marker CD57. In addition, they expressed HLA class I and class II molecules, costimulatory molecules B7.1 and B7.2, and maturation markers CD40 and CD83. In >50 experiments in which entire viable cell populations were gated, >90% of cells simultaneously expressed these DC markers while <4% expressed the T cell marker CD3, and <1% expressed the B cell marker CD19 or the monocyte/macrophage marker CD14.
FIGURE 1.

DC phenotype before electrofusion. DCs from donor 4 were stained with FITC- (x-axis; FL1-H) and PE- (y-axis; FL2-H) conjugated mAbs as indicated and analyzed by FACS.
Electrofusion of DCs and 888mel cells generated heterokaryons that expressed both green fluorescence (CFSE) from the tumor cells and several DC markers (Fig. 2). After electrofusion, cells were incubated overnight at 37°C and were subsequently separated on the basis of adherence. The majority of dually fluorescent fusion cells were detected in the adherent cell population. Also contained in the adherent fraction was a significant population of melanoma cells that were only positive for CFSE. As estimated by FACS analyses characteristic of those presented in Fig. 2, the percentages of DC-TC fusion cells in adherent populations ranged from 12 to 52%. In contrast, the nonadherent population primarily contained DCs, with smaller populations of hybrid cells and melanoma cells.
FIGURE 2.

FACS analyses of DC-TC hybrid cells generated by electrofusion. 888 melanoma cells were labeled with CFSE (x-axis; FL1-H) and were fused with DCs from donor 4. After overnight culture, both adherent and nonadherent cell populations were stained with PE- (y-axis; FL2-H) conjugated mAbs as indicated.
FACS analyses are useful for estimating fusion efficiencies, but they may be misleading because dually fluorescent events could represent cell aggregates or DCs that have engulfed tumor cell debris as opposed to membrane-fused hybrids. To evaluate whether true cell fusions were produced in the experiments presented in this study, fluorescence microscopy was used to evaluate the quality of dual fluorescence on individual cells. CFSE-labeled 888mel cells were fused with DCs, and the adherent population was stained with PE-conjugated anti-HLA-DR as a DC marker (Fig. 3). As a negative control, adherent 888mel cells that had undergone the electrofusion process were also stained with PE-conjugated anti-HLA-DR. In the DC-888mel fusion population, individual cells were clearly positive for both CFSE and HLA-DR. Also, some cells were observed that were only positive for green fluorescence, as expected from the FACS analyses. However, in the preparation consisting only of tumor cells, no HLA-DR expression was observed. Close examination of these cytospin slides also revealed that dually positive fusion cells contained multiple nuclei further suggesting that true cell fusion had occurred.
FIGURE 3.
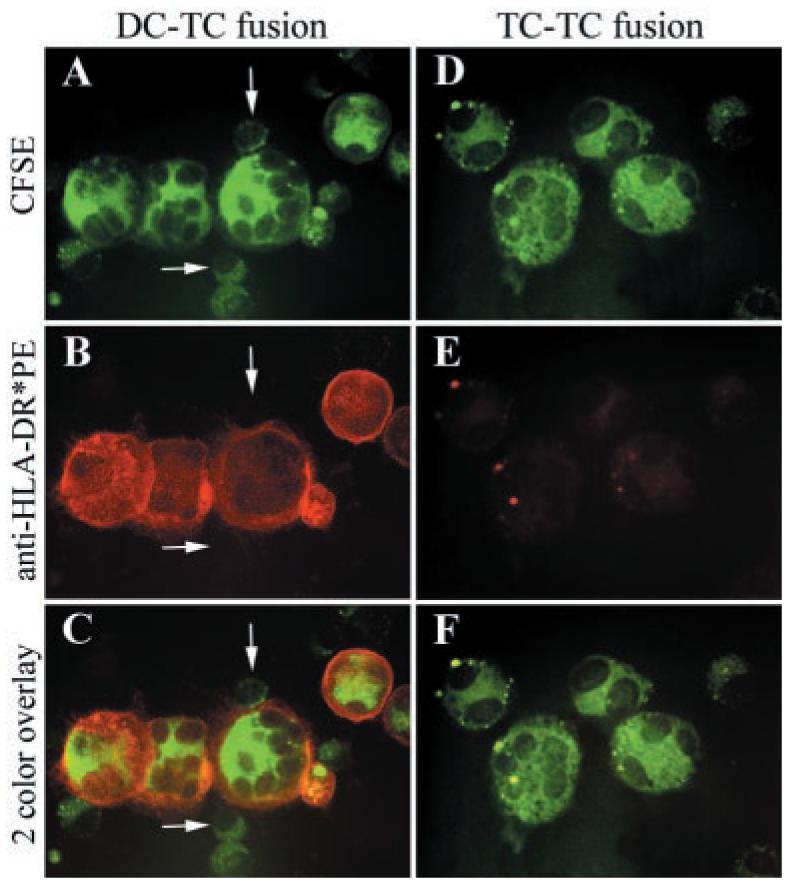
Fluorescence micrographs of hybrid cells generated by electrofusion. A–C, 888 melanoma cells were labeled with CFSE (A) and were fused with DCs from donor 4. After overnight culture, the adherent cell population was stained with PE-conjugated anti-HLA-DR (B). Finally, a two-color overlay was prepared to enable visualization of double-positive cells (C). As estimated by FACS analysis, 29% of cells in the adherent fraction of DC-888mel fusion cells from donor 4 in this experiment were positive for both CFSE and HLA-DR. White arrows indicate melanoma cells that were only positive for CFSE. D–F, As a control, 888 melanoma cells were labeled with CFSE (D) and were fused in the absence of DCs. After overnight culture, the adherent cell population was stained with PE-conjugated anti-HLA-DR (E). Finally, a two-color overlay was prepared to enable visualization of double-positive cells (F).
Although the fluorescence micrographs suggested that cell fusion had, in fact, occurred, the argument could still be made that dually fluorescent cells represent DCs that had engulfed tumor cell debris because CFSE is an intracellular stain. Therefore, to evaluate whether fusion of cell membranes had occurred, we costained DC-888mel fusion cells with Abs against two distinct surface markers (Fig. 4). In these experiments, 888mel cells were not prelabeled with CFSE. 888mel cells expressed HLA-A24 on their surfaces whereas DCs from donor 7 did not. After electrofusion of either DCs and unlabeled 888mel cells or of 888mel cells alone, the adherent populations were stained with anti-HLA-A24 that was indirectly coupled to PE through a labeled secondary Ab. Subsequently, the cells were counterstained with FITC-conjugated anti-HLA-DR. In the DC-888mel fusion population, individual cells were clearly positive for both HLA-A24 and HLA-DR. Also, some cells were observed that only expressed individual cell markers. However, only HLA-A24+ cells were found in the tumor cell alone preparation.
FIGURE 4.

Fluorescence micrographs of hybrid cells generated by electrofusion. A–C, 888 melanoma cells (without prior CFSE labeling) were fused with DCs from donor 7. After overnight culture, the adherent cell population was concomitantly stained with FITC-conjugated anti-HLA-DR (A) and anti-HLA-A24 that was indirectly coupled to PE (B). Finally, a two-color overlay was prepared to enable visualization of double-positive cells (C). The white arrow indicates an individual tumor cell that only expressed HLA-A24, and the blue arrow indicates a single DC that only expressed HLA-DR. D–F, As a control, 888 melanoma cells (without prior CFSE staining) were fused in the absence of DCs. After overnight culture, the adherent cell population was concomitantly stained with FITC-conjugated anti-HLA-DR (D) and anti-HLA-A24 that was indirectly coupled to PE (E). Finally, a two-color overlay was prepared to enable visualization of double-positive cells (F).
Lack of cross-presentation of gp100 by DCs
DCs are potent APCs that are sometimes able to cross-present MHC class I-restricted epitopes from exogenously loaded Ags (1, 6). To determine whether gp100 protein could be cross-presented by DCs, IFN-γ secretion by two CTL lines (CK3H6 and tumor-infiltrating lymphocyte (TIL) 1200) that recognize gp100 epitopes in the context of HLA-A*0201 was measured in response to three different HLA-A*0201+ DCs loaded with recombinant gp100 protein or lysates of melanoma cells expressing gp100 after retroviral transduction (F002Rmel CIITA + VSV-gp100) (Table II). DCs loaded with recombinant NY-ESO-1 protein and lysates of gp100− melanoma cells expressing GFP after retroviral transduction (F002Rmel CIITA + VSV-GFP) were included as negative controls. In addition, whole F002Rmel CIITA cells that naturally expressed HLA-A*0201 were included as controls for T cell function after transduction with VSV-gp1000 or VSV-GFP. Furthermore, DCs were pulsed with relevant peptides (gp100:209-217 for CK3H76 and gp100:154-162 for TIL 1200) and were transduced with an adenoviral vector encoding gp100 as positive control for Ag presentation by the DCs in the context of HLA-A*0201. CK3H6 specifically recognized each of the three DC populations after pulsing with gp100:209-217 and after transduction with adenovirus encoding gp100. However, no specific IFN-γ secretion was observed in response to DCs loaded with whole gp100 protein or melanoma cell lysates. The same trend was observed for TIL 1200 that specifically recognized gp100:154-162; however, the total amounts of IFN-γ secreted were significantly lower.
Table II.
Recognition of DCs loaded with gp100 proteina
| Target Cells | Preloaded Peptideb | Genetic Modification | Preloaded Proteinc | CK3H6 (CD8) | TIL 1200 (CD8) | B104 (CD4) |
|---|---|---|---|---|---|---|
| Media | —d | — | — | 151 | 61 | 39 |
| F002Rmel CIITA | — | VSV-GFP | — | 144 | 57 | 42 |
| F002Rmel CIITA | — | VSV-gp100 | — | 10,989e | 8,258 | >20,000 |
| Donor 1 DC | — | — | — | 102 | 44 | 38 |
| gp100:209–217 | — | — | >20,000 | nd | nd | |
| gp100:154–162 | — | — | nd | 328 | nd | |
| gp100:170–190 | — | — | nd | nd | >20,000 | |
| — | Ad-MART-1 | — | 157 | 50 | 41 | |
| — | Ad-gp100 | — | 7,053 | 371 | >20,000 | |
| — | — | NY-ESO-1 | 801 | 65 | 624 | |
| — | — | gp100 | 392 | 53 | >20,000 | |
| — | — | gp100− lysatef | 134 | 47 | 41 | |
| — | — | gp100+ lysateg | 112 | 49 | >20,000 | |
| Donor 2 DC | — | — | — | 116 | 43 | 43 |
| gp100:209–217 | — | — | >20,000 | nd | nd | |
| gp100:154–162 | — | — | nd | 350 | nd | |
| gp100:170–190 | — | — | nd | nd | >20,000 | |
| — | Ad-MART-1 | — | 166 | 52 | 41 | |
| — | Ad-gp100 | — | 5,981 | 198 | 8,088 | |
| — | — | NY-ESO-1 | 142 | 51 | 160 | |
| — | — | gp100 | 134 | 48 | >20,000 | |
| — | — | gp100− lysate | 120 | 44 | 40 | |
| — | — | gp100+ lysate | 122 | 52 | >20,000 | |
| Donor 3 DC | — | — | — | 113 | 46 | 38 |
| gp100:209–217 | — | — | >20,000 | nd | nd | |
| gp100:154–162 | — | — | nd | 292 | nd | |
| gp100:170–190 | — | — | nd | nd | >20,000 | |
| — | Ad-MART-1 | — | 255 | 70 | 41 | |
| — | Ad-gp100 | — | 7,489 | 219 | 6,413 | |
| — | — | NY-ESO-1 | 426 | 81 | 423 | |
| — | — | gp100 | 148 | 53 | >20,000 | |
| — | — | gp100− lysate | 274 | 64 | 50 | |
| — | — | gp100+ lysate | 142 | 56 | >20,000 |
Recognition of DCs pulsed with recombinant gp100 protein or melanoma cell lysates or transduced with recombinant adenovirus encoding gp100 by HLA-A*0201 and HLA-DRβ1*0701-restricted, gp100-reactive T cell populations. IFN-γ secretion (picograms per milliliter) in 20 h coculture supernatants of target cells expressing both HLA-A*0201 and HLA-DRβ1*0701 with T lymphocytes. nd, not done.
DCs were preincubated with 50 μg/ml gp100:170–190, 1 μg/ml gp100:209–217, or 1 μg/ml gp100:154–162.
DCs were preincubated with recombinant proteins at 10 μg/ml or melanoma cell lysates at a ratio of 1 DC to 1 melanoma cell equivalent overnight at 37°C.
— indicates none.
Underlined values indicate that IFN-γ secretion in response to DCs loaded with protein, peptide, or melanoma lysates, or adenovirally transduced DCs, or melanoma cell lines was ≥50 pg/ml and at least twice background with relevant control target cells.
The gp100− lysate was prepared from F002Rmel CIITA cells transduced with VSV-GFP.
The gp100+ lysate was prepared from F002Rmel CIITA cells transduced with VSV-gp100.
We also evaluated MHC class II-restricted Ag presentation by these same DC populations. IFN-γ secretion was measured by a CD4+ T cell clone (B104) that specifically recognized gp100:170-190 in the context of HLA-DRβ1*0701, which was concomitantly expressed on all three DCs. B104 specifically recognized DCs that had been pulsed with gp100:170-190 and those that had been adenovirally transduced with gp100. In addition, B104 specifically released IFN-γ in response to DCs loaded with recombinant gp100 protein and lysates of melanoma cells expressing gp100. These results demonstrated the integrity of the gp100 protein and lysate preparations in that DCs were capable of processing exogenously loaded Ags and presenting relevant epitopes in the context of MHC class II molecules.
Presentation of MHC class I- and class II-restricted epitopes by DC-888mel hybrids
To determine whether DC-TC hybrids presented relevant MHC class I-restricted tumor-associated epitopes, IFN-γ secretion by multiple HLA-A*0201-restricted, melanoma-reactive CTLs was measured in response to allogeneic DC-888mel hybrids consisting of HLA-A*0201+ DCs and HLA-A*0201− 888mel cells (Table III). Three gp100-reactive CTL lines, each of which recognized a unique peptide, specifically released IFN-γ in response to DC-888mel fusions prepared from two different donors. These T cells did not recognize a physical mixture of DCs and irradiated 888mel cells, nor did they secrete IFN-γ in response to 888mel cells that had undergone the electrofusion procedure alone. Furthermore, in a separate experiment, these T cells were not stimulated by fusions of HLA-A*0201− DCs and 888mel cells. Similar results were also observed using HLA-A*0201-restricted CTLs that specifically recognized epitopes from MART-1, tyrosinase, and TRP2-6b. The amounts of IFN-γ secreted by several of these CTLs in response to cells from the adherent fraction of the DC-888mel fusion preparations were comparable to that released in response to HLA-A*0201+ melanoma cells. This suggested that DC-888mel hybrids were potent stimulators because significantly fewer cells were used in these samples compared with melanoma cells. For example, in the first experiment described in Table III, 3.75 × 104 total fusion cells were used as targets as opposed to 105 tumor cells. However, only 29% of the adherent DC-888mel fusion population from donor 4 were hybrid cells as estimated by FACS analysis. Because heterokaryons were not separated from tumor cells or DCs, 104 DC-888mel hybrids from donor 4 stimulated secretion of comparable amounts of IFN-γ by several CTL lines as 105 melanoma cells. Two CTL populations, CK3H6 and GA3D, also secreted cytokine in response to the nonadherent fraction of the DC-888mel fusion samples. This was probably due to the small numbers of hybrids present in these fractions as observed by FACS (Fig. 2). As an additional negative control, an HLA-A*0201-restricted, NY-ESO-1-reactive CTL line was included because 888mel cells do not efficiently express this protein. These T cells did not recognize HLA-A*0201+ DC-888mel hybrids.
Table III.
Recognition of DC-TC hybrids by HLA-A*0201-restricted, tumor-reactive T cell populationsa
| Expt. | Target Cell | HLA-A2 Expression |
Cell Description | TIL 1200 | CK3H6 | JR1E2 | MB4 | JB2F4 | GA3D | TH1F2L |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Media | − | None | 254 | 8 | 0 | 33 | 0 | 178 | 14 |
| T2 + HBV:18–26(23Y) | + | Nonfused | 180 | 2 | 0 | 14 | 20 | 93 | 312 | |
| T2 + relevant peptide | + | Nonfused | 4,355b | >20,000 | 13,496 | >20,000 | 5,572 | 6,049 | >20,000 | |
| 888mel | − | Nonfused | 156 | 0 | 6 | 34 | 8 | 33 | 21 | |
| 526mel CIITA | + | Nonfused | 5,899 | 4,348 | 2,493 | 12,731 | 7,014 | 2,546 | 21 | |
| 624mel CIITA | + | Nonfused | 5,708 | 8,918 | 9,581 | >20,000 | 18,734 | 9,591 | 8,592 | |
| Donor 2 DC-888melc | DC+-mel− | Fused adherentd | >2,000 | 3,323 | 1,553 | 266 | 1,577 | 5,773 | 68 | |
| Donor 2 DC-888melc | DC+-mel− | Fused nonadherent | 328 | 258 | 21 | 63 | 60 | 1767 | 136 | |
| Donor 2 DC-888melc | DC+-mel− | Physically mixed | 249 | 32 | 2 | 59 | 0 | 516 | 100 | |
| Donor 4 DC-888melc | DC+-mel− | Fused adherente | >2,000 | 4,148 | 1,252 | 190 | 775 | 6,050 | 60 | |
| Donor 4 DC-888melc | DC+-mel− | Fused nonadherent | 270 | 121 | 13 | 44 | 6 | 2,399 | 99 | |
| 888melc | − | Fused adherent | 238 | 14 | 18 | 37 | 0 | 22 | 31 | |
| 2f | Media | − | None | 245 | 13 | 8 | ||||
| Donor 2 DC-888melg | DC+-mel− | Fused adherent | 1,407 | 1,873 | 253 | |||||
| Donor 5 DC-888melh | DC−-mel− | Fused adherent | 276 | 16 | 19 |
IFN-γ secretion (picograms per milliliter) in 20 h coculture supernatants of target cells with T lymphocytes. The data in each of the two panels were generated in separate experiments.
Underlined values indicate that IFN-γ secretion in response to peptide-loaded T2 cells, HLA-A*0201+ melanoma cell lines, or hybrid cell populations was 50 pg/ml and at least twice background with any HLA-A*0201− or Ag− target cell (including a physical mixture of 888 melanoma cells and DCs).
For these fused cell populations, 1 × 105 T cells were coincubated with 3.75 × 104 target cells.
In the adherent fraction of DC-888mel fusion cells from donor 2 in this experiment, 48% of cells were positive for both CFSE and HLA-DR by FACS.
In the adherent fraction of DC-888mel fusion cells from donor 4 in this experiment, 29% of cells were positive for both CFSE and HLA-DR by FACS.
In the second experiment, 1 × 105 T cells were coincubated with 1.5 × 104 target cells.
In the adherent fraction of DC-888mel fusion cells from donor 2 in this experiment, 19% of cells were positive for both CFSE and HLA-DR by FACS.
In the adherent fraction of DC-888mel fusion cells from donor 5 in this experiment, 25% of cells were positive for both CFSE and HLA-DR by FACS.
Presentation of MHC class II-restricted tumor-associated epitopes by DC-888mel hybrids was also evaluated. IFN-γ secretion by a gp100-reactive HLA-DRβ1*0401-restricted CD4+ T cell line and by an HLA-DRβ1*0701-restricted T cell clone was measured in response to allogeneic DC-888mel hybrids consisting of DCs expressing both of these MHC class II molecules and 888mel cells that express neither (Table IV). Both of these T cell populations specifically released cytokine in response to DC-888mel fusions prepared from two different donors. These T cells did not recognize a physical mixture of DCs and irradiated 888mel cells, nor did they secrete IFN-γ in response to 888mel cells that had undergone the electrofusion procedure alone. Furthermore, in a separate experiment, these T cells were not stimulated by fusions of 888mel cells with DCs that did not express HLA-DRβ1*0401 or -DRβ1*0701.
Table IV.
Recognition of DC-TC hybrids by HLA-DRβ1*0401- and -DRβ1*0701-restricted, gp100-reactive T cell populationsa
| Expt. | Target Cell | HLA-DR4/DR7 Expression | Cell Description | BR-B8 | B104 |
|---|---|---|---|---|---|
| 1 | Media | DR4−DR7− | None | 11 | 0 |
| Donor 2 DC-888melb | DC++-mel−− | Fused adherentc | 1,475d | 725 | |
| Donor 2 DC-888melb | DC++-mel−− | Fused nonadherent | 399 | 38 | |
| Donor 2 DC-888melb | DC++-mel−− | Physical mixture | 0 | 7 | |
| Donor 4 DC-888melb | DC++-mel−− | Fused adherente | 1,475 | 255 | |
| Donor 4 DC-888melb | DC++-mel−− | Fused nonadherent | 243 | 29 | |
| 888melb | DR4−DR7− | Fused adherent | 0 | 0 | |
| 2f | Media | DR4−DR7− | None | 0 | 1 |
| Donor 2 DC-888melg | DC++-mel−− | Fused adherent | 2,460 | 68 | |
| Donor 5 DC-888melh | DC−−-mel−− | Fused adherent | 8 | 25 | |
| 3 | Media | DR4−DR7− | None | 0 | 10 |
| 1088 EBV-B + Flu HA:306–324 | DR4+DR7− | Nonfused | 4 | ||
| 1088 EBV-B + gp100:44–59 | DR4+DR7− | Nonfused | 1,664 | ||
| 1978 EBV-B + IgK:188–201 | DR4−DR7+ | Nonfused | 19 | ||
| 1978 EBV-B + gp100:170–190 | DR4−DR7+ | Nonfused | 7,541 | ||
| 888mel | DR4−DR7− | Nonfused | 0 | 0 | |
| F002Rmel CIITA-GFP | DR4−DR7+ | Nonfused | 0 | 8 | |
| F002Rmel CIITA-gp100 | DR4−DR7+ | Nonfused | 10 | >20,000 | |
| 624mel CIITA | DR4+DR7+ | Nonfused | 1,946 | 9,673 | |
| 1088mel CIITA | DR4+DR7− | Nonfused | 3,519 | 25 | |
| Donor 2 DC-888melb | DC++-mel−− | Fused adherentc | 5,338 | 883 | |
| Donor 2 DC-888melb | DC++-mel−− | Fused nonadherent | 937 | 66 | |
| Donor 2 DC-888melb | DC++-mel−− | Physical mixture | 31 | 29 | |
| 888melb | DR4−DR7− | Fused adherent | 0 | 0 |
IFN-γ secretion (picograms per milliliter) in 20 h coculture supernatants of target cells with T lymphocytes. The data in each of the three panels were generated in separate experiments.
For these fused cell populations, 1 × 105 T cells were coincubated with 3.75 × 104 target cells.
In the adherent fraction of DC-888mel fusion cells from donor 2 in this experiment, 48% of cells were positive for both CFSE and HLA-DR by FACS.
Underlined values indicate that IFN-γ secretion in response to peptide-loaded EBV-B cells, melanoma cell lines, or hybrid cell populations was 50 pg/ml and at least twice background with relevant control target cells (including a physical mixture of 888 melanoma cells and DCs).
In the adherent fraction of DC-888mel fusion cells from donor 4 in this experiment, 29% of cells were positive for both CFSE and HLA-DR by FACS.
In the second experiment, 1 × 105 T cells were coincubated with 1.5 × 104 target cells.
In the adherent fraction of DC-888mel fusion cells from donor 2 in this experiment, 19% of cells were positive for both CFSE and HLA-DR by FACS.
In the adherent fraction of DC-888mel fusion cells from donor 5 in this experiment, 25% of cells were positive for both CFSE and HLA-DR by FACS.
Discussion
The absence of an effective T cell-mediated immune response to cancer in many patients may be the result of inefficient Ag processing and presentation by tumor cells (49, 50). Down-regulation of MHC molecules as well as defective Ag processing has been observed in neoplastic cells (51, 52). In addition, tumor cells do not generally express costimulatory and/or adhesion molecules that may be necessary to mount a primary immune response (53). To overcome this unresponsiveness, a variety of vaccination strategies have been developed to stimulate T lymphocytes that recognize Ags expressed in tumor cells. Sporadic clinical responses have been observed in trials in which Ags or peptide epitopes have been used directly to immunize patients (54). However, these approaches have largely been unsuccessful especially when the target Ags are nonmutated self proteins (55). Therefore, the use of DCs as an adjuvant in cancer vaccine trials is currently being intensively studied because DCs are potent APCs capable of initiating primary immune responses.
Many methods have been developed for loading DCs with Ags including transfection with tumor-derived RNA or DNA (18, 19), transduction with recombinant viruses (7-12), and loading with killed tumor cells (14), tumor lysates (15, 16), recombinant protein (6), or synthetic peptides (3, 55). Alternatively, hybrids of DCs and tumor cells have been produced using PEG or electrofusion (20-22). Theoretically, DC-TC hybrids are appealing because they should induce a polyclonal immune response, including both CD4+ Th cells and CD8+ CTL, against a multitude of shared and unique tumor-associated Ags. In preclinical murine models, DC-TC hybrids have stimulated potent protective and therapeutic immune responses to carcinomas, lymphomas, melanomas, and gliomas (23-29). However, in two recent human clinical trials, fusions of DCs and autologous tumor cells were primarily ineffective for the treatment of patients with melanoma and glioma (35, 36). In both of these protocols, PEG was used as the fusogenic reagent. In one previous investigation, electrofusion was preferred over PEG-mediated fusion specifically because fusion efficiency was very low with PEG, ranging from only 0.5 to 4.5% (33). In another investigation, a direct comparison was made between DC-TC fusions made using PEG and electrofusion in a prophylactic DA3 mammary carcinoma model (25). In that study, DCs were labeled with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) before fusion and tumors cells were prelabeled with CFSE such that fusion efficiency was monitored by FACS for dual fluorescent events. The conclusion was drawn that no significant differences were observed in either the quality of the fusion cells or in the ability of those cells to mediate protective immunotherapy. However, the FACS profile for PEG-fused cells appeared nearly identical to that for DCs and tumor cells that had simply been cocultured. Namely, both profiles indicated that these cell populations predominantly contained two separate cell types, one green and one red, with very few dual fluorescent cells. Alternatively, cells generated by electrofusion appeared to contain a much more obvious population of dual fluorescent cells. Furthermore, we have previously attempted to generate DC-TC hybrids using PEG but could not demonstrate effective fusion, partly because Abs against DC markers bound nonspecifically to tumor cells alone after PEG treatment (data not shown). Therefore, we have focused our analysis on DC-TC hybrids generated by electrofusion and have verified the occurrence of chimeric hybrids using a series of stringent criteria.
Prelabeling of DCs and tumor cells coupled with FACS analysis is a convenient means of assessing fusion efficiency. However, FACS data can be misleading because dual fluorescent events may represent cell aggregates or DCs that have engulfed tumor cell debris. Results from some previous investigations have been difficult to interpret because it was unclear whether immune responses were mediated by true membrane-fused cells or by DCs presenting Ag through uptake of tumor-associated material. In fact, several investigations have demonstrated that physical mixtures of DCs and tumor cells can elicit potent immune responses (34). To evaluate whether true fusion was achieved in the current study, we used a combination of FACS analysis and fluorescence microscopy. In the adherent fraction of the fusion population, there was clearly a distinct population of cells that were positive for CFSE from the prelabeled melanoma cells and for several DC markers by both FACS (Fig. 2) and microscopic analyses (Fig. 3). Fluorescence micrographs also revealed that dually colored cells were multinucleated because CFSE is a cytoplasmic dye that does not stain cell nuclei. However, the argument could still be made that these cells were DCs that had engulfed tumor cell debris. Therefore, we evaluated expression of two distinct cell surface markers, namely HLA-A24 that was only expressed on 888mel and HLA-DR that was only present on the DCs before fusion. In the adherent fraction of the fusion population, there were cells that expressed both of these markers suggesting that we had, in fact, generated membrane-fused hybrids (Fig. 4).
The primary theoretical advantages of DC-TC hybrids over DCs loaded with Ags by other means are 2-fold: 1) they should be able to present epitopes from multiple tumor-associated Ags simultaneously, and 2) they should be able to present relevant epitopes in the context of both MHC class I and class II molecules. DCs have unique Ag processing pathways that enable exogenously loaded proteins to be presented in the context of MHC class I molecules (1). However, cross-presentation is, generally, not very efficient in the absence of carrier proteins or particles (6). As an example, we have never observed cross-presentation of the melanoma Ag gp100, either in the form of a recombinant protein or tumor cell lysate (Table II). In contrast, hybrids of HLA-A*0201+ DCs and HLA-A*0201− gp100+ melanoma cells were clearly recognized by three CTL lines, each of which was reactive with a unique HLA-A*0201-restricted gp100 epitope (Table III). In addition, DC-888mel hybrids presented MHC class I-restricted epitopes from other melanoma Ags including MART-1, tyrosinase, and TRP2-6b. Furthermore, DC-888mel hybrids presented class II MHC-restricted epitopes from gp100. These results suggest that DC-TC hybrids may be able to induce both CTL and Th cell responses against a variety of different tumor-associated Ags.
Several in vitro and in vivo applications can be envisioned for the use of electrofused DC-TC hybrids as APCs. The immuno-therapeutic potential of these hybrid cells can be evaluated in human clinical vaccination trials. In addition, they may be useful for the identification of new tumor-associated Ags. If DC-TC hybrids can stimulate tumor-reactive CD8+ and CD4+ T lymphocytes, the antigenic specificities of those T cells could be ascertained by-screening tumor-derived cDNA libraries (56, 57) or peptides eluted from tumor cells for recognition (58, 59). Furthermore, fusions of DCs and tumor cells may be useful in vitro for generating populations of tumor-reactive T cells for use in adoptive therapy protocols. In several investigations, significant objective clinical response rates have been achieved by the adoptive transfer of autologous tumor-reactive T cells (60). For patients with melanoma, it has been comparatively easy to raise these T cell populations from TIL. In addition, melanoma cells seem to be uniquely immunogenic in that they are often directly able to stimulate tumor-reactive CTL in vitro without manipulation such as the introduction of costimulatory molecules or cytokine adjuvants. In contrast, it has been significantly more difficult to raise such T cell populations for other cancers. Even in the context of renal cell carcinoma, which is often responsive to immunotherapy with IL-2 (61), it has been difficult to raise tumor-reactive T cells in vitro, either from TIL or from PBMC stimulated with whole tumor cells. In addition, mounting evidence suggests that CD4+ T lymphocytes may play a critical role in the induction and persistence of tumor-reactive CD8+ T cells (60, 62-64). Therefore, the use of DC-TC hybrids as APCs may be a viable means for raising heterogeneous populations of tumor-reactive CD8+ and CD4+ T cells for adoptive transfer for patients with cancer.
Acknowledgments
We thank Dr. Mark Dudley for providing the CK3H6, JR1E2, and JB2F4 T cell clones; Dr. Hung Khong for the MB4 T cell clone; and Dr. Rejean LaPointe for the B104 T cell clone.
Footnotes
This work was supported in part by National Cancer Institute Grant PO1 CA12582.
Abbreviations used in this paper: DC, dendritic cell; DC-TC, DC fused with tumor cell; PEG, polyethylene glycol; TRP, tyrosinase-related protein; VSV, vesicular stomatitis virus; GFP, green fluorescent protein; CIITA, class II transactivator; TIL, tumor-infiltrating lymphocyte.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 3.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 1996;183:283. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat. Med. 1996;2:52. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 5.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, Von Den DP, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999;190:1669. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997;158:2723. [PubMed] [Google Scholar]
- 7.Szabolcs P, Gallardo HF, Ciocon DH, Sadelain M, Young JW. Retrovirally transduced human dendritic cells express a normal phenotype and potent T-cell stimulatory capacity. Blood. 1997;90:7. [PubMed] [Google Scholar]
- 8.Strobel I, Krumbholz M, Menke A, Hoffmann E, Dunbar PR, Bender A, Hobom G, Steinkasserer A, Schuler G, Grassmann R. Efficient expression of the tumor-associated antigen MAGE-3 in human dendritic cells, using an avian influenza virus vector. Hum. Gene Ther. 2000;11:2207. doi: 10.1089/104303400750035735. [DOI] [PubMed] [Google Scholar]
- 9.Reeves ME, Royal RE, Lam JS, Rosenberg SA, Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996;56:5672. [PubMed] [Google Scholar]
- 10.Linette GP, Shankara S, Longerich S, Yang S, Doll R, Nicolette C, Preffer FI, Roberts BL, Haluska FG. In vitro priming with adenovirus/gp100 antigen-transduced dendritic cells reveals the epitope specificity of HLA-A*0201-restricted CD8+ T cells in patients with melanoma. J. Immunol. 2000;164:3402. doi: 10.4049/jimmunol.164.6.3402. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield LH, Jilani SM, Chakraborty NG, Bui LA, Ribas A, Dissette VB, Lau R, Gamradt SC, Glaspy JA, McBride WH, et al. Generation of melanoma-specific cytotoxic T lymphocytes by dendritic cells transduced with a MART-1 adenovirus. J. Immunol. 1998;161:5607. [PubMed] [Google Scholar]
- 12.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from α-fetoprotein. Cancer Res. 1999;59:3134. [PubMed] [Google Scholar]
- 13.Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL. Comparative analysis of the in vivo expression of tyrosinase, MART- 1/Melan-A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. J. Immunother. 1998;21:27. doi: 10.1097/00002371-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 2000;192:1535. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 16.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 17.Zitvogel L, Mayordomo JI, Tjandrawan T, Deleo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J. Exp. Med. 1996;183:87. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J. Exp. Med. 1997;186:1177. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell DA, Nair SK. RNA-transfected dendritic cells in cancer immunotherapy. J. Clin. Invest. 2000;106:1065. doi: 10.1172/JCI11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart I, Colaco C. Immunotherapy: fusion induces tumour rejection. Nature. 1997;388:626. doi: 10.1038/41662. [DOI] [PubMed] [Google Scholar]
- 21.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat. Med. 1997;3:558. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 22.Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, Dalgleish AG. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim. Biophys. Acta. 2000;1500:265. doi: 10.1016/s0925-4439(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Koido S, Tanaka Y, Chen D, Kufe D, Gong J. The kinetics of in vivo priming of CD4 and CD8 T cells by dendritic/tumor fusion cells in MUC1-transgenic mice. J. Immunol. 2002;168:2111. doi: 10.4049/jimmunol.168.5.2111. [DOI] [PubMed] [Google Scholar]
- 24.Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc. Natl. Acad. Sci. USA. 2000;97:2715. doi: 10.1073/pnas.050587197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindner M, Schirrmacher V. Tumour cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylen-glycol versus electro-fusion protocols. Eur. J. Clin. Invest. 2002;32:207. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J. Immunol. 2000;165:1705. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J. Immunol. 1998;161:5516. [PubMed] [Google Scholar]
- 28.Hayashi T, Tanaka H, Tanaka J, Wang R, Averbook BJ, Cohen PA, Shu S. Immunogenicity and therapeutic efficacy of dendritic-tumor hybrid cells generated by electrofusion. Clin. Immunol. 2002;104:14. doi: 10.1006/clim.2002.5224. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Holmes LM, Franek KJ, Burgin KE, Wagner TE, Wei Y. Purified hybrid cells from dendritic cell and tumor cell fusions are superior activators of antitumor immunity. Cancer Immunol. Immunother. 2001;50:456. doi: 10.1007/s002620100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes LM, Li J, Sticca RP, Wagner TE, Wei Y. A rapid, novel strategy to induce tumor cell-specific cytotoxic T lymphoctye responses using instant dentritomas. J. Immunother. 2001;24:122. [PubMed] [Google Scholar]
- 31.Soruri A, Fayyazi A, Neumann C, Schlott T, Jung T, Matthes C, Zwirner J, Riggert J, Peters JH. Ex vivo generation of human anti-melanoma autologous cytolytic T cells by dendritic cell/melanoma cell hybridomas. Cancer Immunol. Immunother. 2001;50:307. doi: 10.1007/s002620100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol. Immunother. 2002;51:299. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jantscheff P, Spagnoli G, Zajac P, Rochlitz CF. Cell fusion: an approach to generating constitutively proliferating human tumor antigen-presenting cells. Cancer Immunol. Immunother. 2002;51:367. doi: 10.1007/s00262-002-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melcher A, Todryk S, Bateman A, Chong H, Lemoine NR, Vile RG. Adoptive transfer of immature dendritic cells with autologous or allogeneic tumor cells generates systemic antitumor immunity. Cancer Res. 1999;59:2802. [PubMed] [Google Scholar]
- 35.Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J. Immunother. 2002;25:421. doi: 10.1097/00002371-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol. Immunother. 2001;50:337. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neil GA, Zimmermann U. Electrofusion. Methods Enzymol. 1993;220:174. doi: 10.1016/0076-6879(93)20082-e. [DOI] [PubMed] [Google Scholar]
- 38.Bakker AB, Schreurs MW, Tafazzul G, de Boer AJ, Kawakami Y, Adema GJ, Figdor CG. Identification of a novel peptide derived from the melanocyte-specific gp100 antigen as the dominant epitope recognized by an HLA-A2.1-restricted anti-melanoma CTL line. Int. J. Cancer. 1995;62:97. doi: 10.1002/ijc.2910620118. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961. [PubMed] [Google Scholar]
- 40.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother. 2002;25:243. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP)-2, a new TRP- 2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J. Immunol. 2002;168:951. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley JP, Rosenberg SA, Parkhurst MR. Identification of a new shared HLA-A2.1 restricted epitope from the melanoma antigen tyrosinase. J. Immunother. 2001;24:212. [PubMed] [Google Scholar]
- 43.Bownds S, Tong-On P, Rosenberg SA, Parkhurst M. Induction of tumor-reactive cytotoxic T-lymphocytes using a peptide from NY-ESO-1 modified at the carboxy-terminus to enhance HLA-A2.1 binding affinity and stability in solution. J. Immunother. 2001;24:1. doi: 10.1097/00002371-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Lapointe R, Royal RE, Reeves ME, Altomare I, Robbins PF, Hwu P. Retrovirally transduced human dendritic cells can generate T cells recognizing multiple MHC class I and class II epitopes from the melanoma antigen glycoprotein 100. J. Immunol. 2001;167:4758. doi: 10.4049/jimmunol.167.8.4758. [DOI] [PubMed] [Google Scholar]
- 45.Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, Restifo NP. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J. Immunol. 2000;164:3535. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakami Y, Battles JK, Kobayashi T, Ennis W, Wang X, Tupesis JP, Marincola FM, Robbins PF, Hearing VJ, Gonda MA, Rosenberg SA. Production of recombinant MART-1 proteins and specific antiMART-1 polyclonal and monoclonal antibodies: use in the characterization of the human melanoma antigen MART-1. J. Immunol. Methods. 1997;202:13. doi: 10.1016/s0022-1759(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Boss JM, Hu SX, Xu HJ, Blanck G. Apoptosis-independent retinoblastoma protein rescue of HLA class II messenger RNA IFN-γ inducibility in non-small cell lung carcinoma cells: lack of surface class II expression associated with a specific defect in HLA-DRA induction. J. Immunol. 1996;156:2495. [PubMed] [Google Scholar]
- 48.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J. Exp. Med. 1993;177:265. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seliger B, Hohne A, Knuth A, Bernhard H, Meyer T, Tampe R, Momburg F, Huber C. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res. 1996;56:1756. [PubMed] [Google Scholar]
- 51.Marincola FM, Shamamian P, Alexander RB, Gnarra JR, Turetskaya RL, Nedospasov SA, Simonis TB, Taubenberger JK, Yannelli J, Mixon A. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J. Immunol. 1994;153:1225. [PubMed] [Google Scholar]
- 52.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional β2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl. Cancer Inst. 1996;88:100. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved: where are we going? J. Natl. Cancer Inst. 2002;94:805. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 56.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol. Today. 1997;18:267. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 58.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 59.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 60.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995;13:688. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 62.Goedegebuure PS, Eberlein TJ. The role of CD4+ tumor-infiltrating lymphocytes in human solid tumors. Immunol. Res. 1995;14:119. doi: 10.1007/BF02918172. [DOI] [PubMed] [Google Scholar]
- 63.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J. Immunol. 2000;164:562. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333:1038. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]


