Summary
Flt3 ligand (Flt3L) stimulates the proliferation and differentiation of hematopoietic cells. Subcutaneous Flt3L administration has been shown to effectively manage some murine cancers and in humans, to lead to an increase in peripheral blood monocyte and dendritic cell (DC) counts. In the current study, we determined the effects of Flt3L therapy on patients with melanoma and renal cancer, and in particular, if Flt3L could be used either by enhancing the immunization of patients with melanoma to tumor antigen peptides in vivo, or by mobilizing DC precursors to allow the production of larger numbers of cultured DC. Flt3 ligand administration resulted in a 19-fold increase in DC counts in the peripheral blood of patients. The DC generated in vivo appeared only partially activated, expressing increased levels of CD86, CD33, and major histocompatibility complex class II, but no or low levels of CD80 and CD83. This partial activation may account for the lack of enhanced immune responses to melanoma antigens and absence of clinical responses in the patients even in combination with antigen immunization. Flt3 ligand administration did result, however, in a 7-fold increased yield of monocytes per liter of blood from leukapheresed patients. Dendritic cells were as readily generated from monocytes collected before and after Flt3L therapy, and they stimulated allogeneic T-cell proliferation in a mixed leukocyte reaction to a similar magnitude. Thus, the use of Flt3L may be an important method to mobilize DC precursors to allow patient therapy with larger numbers of cultured DC.
Keywords: Dendritic cells, Flt3 ligand, Melanoma cancer, Renal cancer
Dendritic cells (DC), the most effective antigen presenting cells, are potent activators of specific immune responses via their ability to process and present specific antigens in the context of the major histocompatibility complex and costimulatory molecules (1). The low number of DC in the circulation and the difficulty in isolating them from tissues has limited their clinical use. Although DC potentially available for in vitro manipulation and clinical use can be found in peripheral blood, they are in low numbers, estimated to be < 1% of peripheral blood mononuclear cells (PBMC) (2). Dendritic cells can be generated by harvesting monocytes from peripheral blood and culturing them in vitro with interleukin (IL)-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) (3). These monocytes can then be activated ex vivo and either transduced with specific antigens or pulsed with antigen, followed by infusion in vivo. Cultured DC pulsed with tumor antigens can induce tumor regression in some murine models (4,5). Clinical trials utilizing cultured DC have reported objective clinical responses in some patients; Kugler et al. (6) reported complete responses in 4 of 17 patients, and Nestle et al. (7) reported complete responses in 2 of 16 patients. The low percentage of complete responders may possibly be a result of the limited numbers of DC that have been administered. For instance, Dhodapkar et al. (8) reported administering 2.8 × 106 antigen-pulsed DC, Kugler et al. (6) reported administering 5 × 107 DC fused with autologous tumor, and Nestle et al. (7) reported administration of 1 × 106 DC pulsed with tumor lysates or a cocktail of peptides. Therefore, methods are needed to increase the number of DC that can be administered.
One potential agent that appears to increase DC precursors in vivo is Flt3 ligand (Flt3L), which is a naturally occurring glycoprotein that stimulates the proliferation and differentiation of hematopoietic cells (9). Its receptor is Fms-like tyrosine kinase 3 (Flt3), which is a member of the type III receptor tyrosine kinase family and is expressed exclusively on primitive hematopoietic progenitor cells in mice (10,11) and in CD34+ cells from bone marrow in humans (12). Flt3 ligand administration may provide a means of increasing in vivo DC, which then may act in vivo to mediate a therapeutic response. Alternatively, Flt3L may be useful in obtaining larger numbers of DC, either directly or after in vitro culturing of precursors, which could be used for generating tumor vaccines.
Flt3 ligand has been shown in preclinical models to increase the number of cells expressing a DC phenotype peripherally in mice (13,14). Moreover, Flt3L has been shown to slow tumor growth and induce regression of a methylcholanthrene-induced murine fibrosarcoma (15). In a separate study, Flt3L effectively managed murine melanoma and murine lymphoma (16).
When administered to healthy human volunteers, Flt3L increased by 30-fold the number of cells in the circulation with a DC phenotype and increased white blood cell (WBC) counts (17). Recently, Morse et al. (18) reported that Flt3L administered to patients with colon cancer doubled the WBC counts and increased circulating DC by nine fold. No clinical responses were seen, but because no native immunogenicity of colon cancer has been described, this may explain the lack of response. In both studies, Flt3L was found to be safe and well tolerated at the doses tested.
In the current study, we determined the effects of Flt3L on patients with melanoma and renal cancer, tumors that appear to be immunogenic as evidenced by their ability to respond to cytokine therapy. A number of antigens are associated with melanoma and renal cancer and have been identified (19,20). For instance, the melanoma antigen gp100 is recognized by tumor-infiltrating lymphocytes that are associated with tumor regression after adoptive immunotherapy in patients with metastatic melanoma (21). Previously, we have immunized patients with peptide vaccines based on HLA-A2 restricted epitopes of melanoma antigens (21,22). The patients developed measurable increases in their T-cell reactivity against melanoma when tested in vitro, but clinical responses using vaccines alone have been rare (21,23). In the current study, a cohort of patients with melanoma cancer were administered Flt3L and a vaccine consisting of four tumor antigen peptides, to determine if Flt3L could increase the ability to immunize against these tumor antigens.
The goal of this study was to determine if Flt3L could be used either by enhancing the immunization of patients to tumor antigen peptides in vivo, or by mobilizing DC precursors, which could be exploited to increase yields of cultured DC for clinical therapy.
PATIENTS AND METHODS
Patient Criteria
In an Institutional Review Board approved protocol at the National Cancer Institute, 31 patients with a history of metastatic melanoma and renal cell cancer received systemic therapy with Flt3L. All patients had a history of metastatic melanoma or renal cell cancer with evaluable disease by either physical examination or radiographic workup consisting of computed tomography of the chest, abdomen, and pelvis. Their Eastern Cooperative Oncology Group performance status was 0 (90% of patients) or 1 (10%). Other eligibility requirements included serum creatinine of 2.0 mg/dl or less, bilirubin of 1.6 mg/dl or less, WBC count of 3,000 cells/mm3 or greater, platelet count greater than 90,000/mm3, and serum aspartate amino transferase and amino alanine transferase less than two times normal. Patients were excluded if they had any form of therapy except surgery during the month before starting therapy with Flt3L. In addition, patients had to be free of active systemic infections and coagulopathies and had to have no history of autoimmune, cardiovascular, or respiratory disease. Because the teratogenic risks of Flt3L are unknown, patients were excluded if they were pregnant or planning a pregnancy.
Patient Treatment and Monitoring
Immunex Corporation (Seattle, WA, U.S.A.) provided the Flt3L. A total of 14 patients received Flt3L alone at 25 µg/kg/dose daily (seven patients with renal cell cancer and seven patients with melanoma, referred to as patients A1–A7 and B1–B7 in figures and tables). Another seven patients with HLA-A2 positive melanoma received both Flt3L at 25 µg/kg/dose daily and in addition, a vaccine consisting of four tumor antigen peptides (see below), referred to as patients C1–C7 in figures and tables. Each peptide was given separately as a 1 mg dose emulsified in Incomplete Freund’s Adjuvant subcutaneously at a different site on day 12 of Flt3L administration. Ten patients with renal cell cancer received 100 µg/kg/dose daily of Flt3L, referred to as patients D1–D10 in figures and tables.
There were two cycles per course of Flt3L, each cycle consisting of subcutaneous injections for 14 consecutive days. Each cycle was 2 weeks apart, and the two courses were 3 weeks apart. Most of the patients received multiple cycles of Flt3L injection. All patients underwent leukapheresis before starting therapy and on day 15 at the completion of a 14-day cycle of therapy. Peripheral blood mononuclear cells were collected from the leukaphereses by Ficoll-Hypaque density gradient centrifugation and cryopreserved by standard procedures until use. Blood was drawn at several times before, during, and after the cycles for serum chemistry, WBC count with differential, and for phenotypic characterization. Average values were computed ± the standard error of the mean. In addition, all patients underwent computed tomography scans of the chest, abdomen, and pelvis before and after therapy to monitor disease progression. A clinical response was defined as >50% reduction in all tumors, as measured by the sum of the perpendicular dimensions of each tumor.
Thus, of 31 patients who were administered Flt3L, there were 14 cases of metastatic melanoma and 17 cases of metastatic renal cell cancer. The age range was 21–78 years, with a median age of 55 years. All patients had a good performance status, and all had received some form of therapy before. As reported in other studies (17,18), Flt3L was well tolerated. Twenty-six of 31 (84%) patients experienced no toxicity during the course of therapy. One patient developed a grade 2 toxicity (bone pain), two patients developed grade 3 toxicity (infection), and one patient developed a grade 4 toxicity (thrombosis) during the course of therapy.
Phenotypic Analysis
FACS analysis for some patients was performed on days 0 and 15. Pheresis samples and intervening research blood was drawn to establish phenotypic characteristics and changes in the PBMC. Flow cytometry consisted of two color analyses, utilizing antibodies specific for CD1a, CD3, CD11c, CD14, CD16, CD19, CD33, CD80, CD83, CD86 (BD Pharmingen, San Diego, CA, U.S.A.), and HLA-DR (Biosource International, Camarillo, CA, U.S.A.), labeled with either fluorescein or phycoerythrin. A ‘cocktail‘ of non-DC antibodies against differentiated hematopoietic lineage cells (CD3, CD14, CD16, CD19, and CD56) was created with fluorescein-labeled antibodies and used to distinguish non-DC populations (24). This non-DC lineage cocktail is referred to as the “Lin.” Forward angle and side scatter gates were set to exclude subcellular debris and cell clumps. Dead cells were excluded from the analysis by propidium iodide staining. Each cell profile was based on an analysis of 10,000 cells using a Becton Dickinson FACScan instrument. For phenotypic analysis of cultured DC, forward angle and side scatter gates were set to exclude small lymphoid cells to focus on DC. Isotype controls were used to detect nonspecific antibody binding.
Cell Culture
T2 cells (American Type Culture Collection, Manassas, VA, U.S.A.), a TAP-deficient line with empty surface HLA-A2 (25), were grown in RPMI 1640 supplemented with 10% FCS, 1 mmol/L glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin (all from Biofluids, Rockville, MD, U.S.A.).
Dendritic Cell Culture
Based on the original protocol of Sallusto et al. (3), DC were derived from patient PBMC. Dendritic cells were generated by thawing cryopreserved PBMC and culturing the PBMC overnight at 37°C, in complete medium composed of Iscove’s Modified Dulbecco’s Medium (IMDM; Biofluids) with 25 mmol/L HEPES buffer, 10% human AB serum (heat inactivated; Gemini Bio-Products, Calabasas, CA, U.S.A.), 2 mmol/L glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin (all from Biofluids). Nonadherent cells were removed on day 1. Adherent cells were cultured for 7 days in complete medium supplemented with 100 ng/mL GMCSF (1,000 U/mL) and 500 ng/mL IL-4 (1,000 U/mL) (both from Peprotech, Rocky Hill, NJ, U.S.A.). Granulocyte-macrophage colony-stimulating factors and IL-4 were added again on days 3 and 5. Dendritic cells generated were stained with mouse antihuman CD11c and CD14 labeled antibodies and analyzed by flow cytometry to determine their phenotype.
Mixed Leukocyte Reaction Analysis of Fresh Peripheral Blood Mononuclear Cells
The mixed leukocyte reaction, as described by Steinman and Inaba (26), was used to measure functional differences between PBMC collected before and after patient treatment with Flt3L. Briefly, pre-Flt3L (day 0) and post-Flt3L PBMC (day 15) were thawed, counted, and irradiated with 2000 cGy, and their ability to stimulate allogeneic T-cell proliferation was subsequently determined. Naive, allogeneic responder T cells, from a single donor not known to have been preimmunized against alloantigens, were purified on a human T-cell enrichment column (R&D Systems, Minneapolis, MN, U.S.A.), aliquoted, cryopreserved, and thawed on the day of use. These responder T cells were then plated in groups of triplicate wells, at 1.0 × 105 cells per well in a 96-well flat bottom plate in complete media and cocultured with 1.0 × 105 allogeneic stimulator cells (pre- and post-Flt3L patient PBMC) in 0.2 mL volumes at serial dilutions from 4:1 to 1:256. The cultures were pulsed with 1 µCi of 3H-thymidine (Nycomed Amersham, Buckinghamshire, U.K.) on day 5 and harvested after a 16-hour incubation using a Tomtec Cell Harvester 96 (Tomtec, Hamden, CT, U.S.A.).
Thymidine incorporation was subsequently measured as an indicator of cellular proliferation on the LKB 1205 Betaplate Liquid Scintillation Counter (Wallac, Turku, Finland). Stimulator cells, responder T cells, and complete media were also plated independently in each assay, and their background subtracted from the final counts of T-cell proliferation.
Mixed Leukocyte Reaction Analysis of Cultured Dendritic Cells
Dendritic cells were generated as described above from pre- (day 0) and post- (day 15) patient PBMC. On day 7 of culture, a mixed leukocyte reaction (MLR) was performed (similar to the method described above) by plating the DC generated in groups of triplicate wells at 1.0 × 105 cells per well in a 96-well flat bottom plate in complete media. These were cocultured with 1.0 × 105 purified T cells from the same healthy donor used in all MLR in this study in 0.2 mL volumes at serial dilutions from 4:1 to 1:256. The cultures were pulsed with 1 µCi of 3H-thymidine (Nycomed Amersham) on day 5 and harvested after a 16-hour incubation. Proliferation was measured by tritiated thymidine incorporation (counts per minute). Before the MLR, the DC generated were stained with mouse antihuman CD11c and CD14 labeled antibodies and analyzed by flow cytometry to determine their phenotype.
Peptides
Four different peptides, MART-1:27–35 (MART-1, AAGIGILTV), the modified gp100:209–217 (210M) (g209–2M, IMDQVPFSV), gp100:280–288(288V) (g280–9V, YLEPGPVTV), and tyrosinase:368–376(370D) (tyrosinase, YMDGTMSQV), were used for vaccination and in vitro testing as later specified (Multiple Peptide Systems, San, Diego, CA, U.S.A. for all peptides except MART-1, which was produced by Peninsula Laboratories, San Carlos, CA, U.S.A.). The Flu-M1:58–66 peptide (Flu, GILGFVFTL) was used as control in sensitization assays. The Cancer Therapy Evaluation Program (National Cancer Institute) provided all peptides, with the exception of Flu. The peptides were produced to good manufacturing procedure grade by solid phase synthesis techniques and solubilized in either sterile water or dimethyl sulfoxide (Sigma, St. Louis, MO, U.S.A.) according to their biochemical characteristics. The identity of each of the peptides was confirmed by mass spectral analysis. The peptides were > 98% pure as assessed by high-pressure liquid chromatography.
In Vitro Assessment of Immunologic Reactivity to the Four Peptides
Cryopreserved PBMC were thawed and simultaneously tested for peptide reactivity as described previously (21). Briefly, 3 × 106 PBMC were suspended in 2 mL of complete media, and 1 µmol/L of the same peptide as used for patient immunization was added. Two days later, recombinant 300 IU/mL IL-2 (Chiron Corp., Emeryville, CA, U.S.A.) was added to the cultures. Every 3–4 days thereafter, half of the medium was replaced with fresh medium containing IL-2 (300 IU/mL). Cultures were harvested on day 12, and lymphocyte reactivity assayed. Cells were washed once in Hank’s balanced salt solution, and 105 cells/0.1 mL were added to wells of flat-bottom 96-well plate. Stimulator cells were added consisting of 105 T2 cells/0.1 mL pulsed with 1 µmol/L each of the four peptides in the native form naturally expressed on tumors, and flu peptide as a control. Cultures were incubated 18–24 hours, and the supernatant tested for interferon-γ using enzyme-linked immunosorbent assay kits (R&D Systems). Peripheral blood mononuclear cells were considered reactive to peptide if cytokine values were greater than 100 pg/mL and twice those of peptide controls.
RESULTS
Hematologic Analyses After Flt3 Ligand Administration
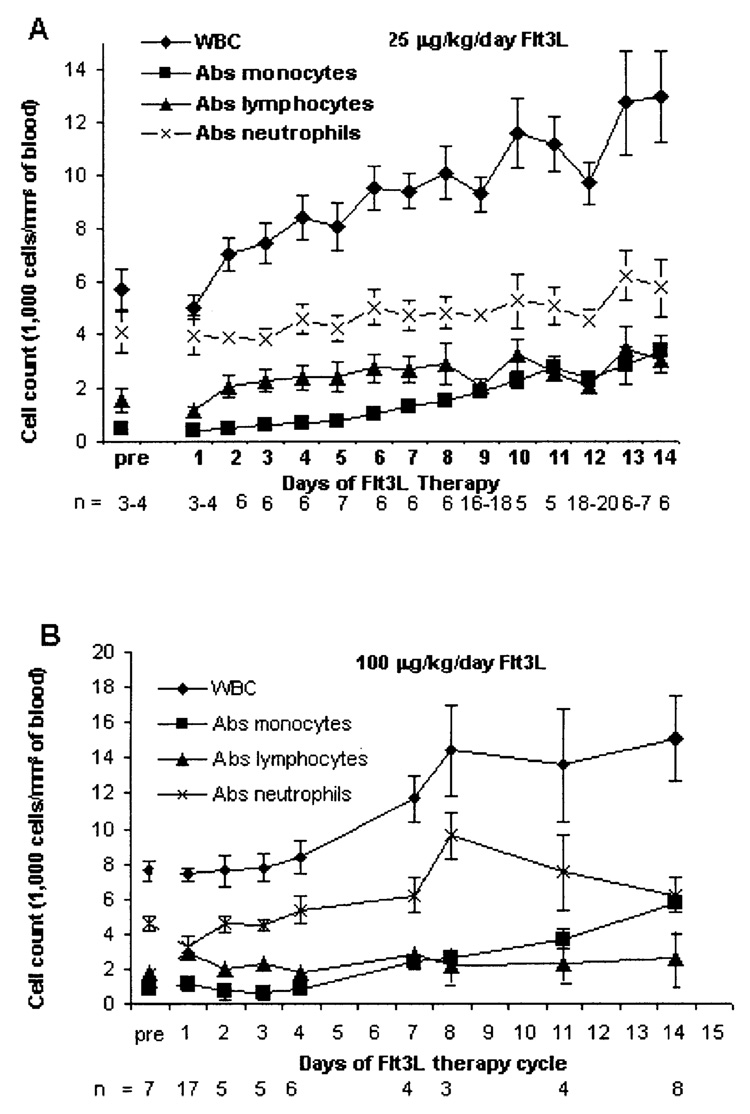
As expected, patients demonstrated an increase in WBC and monocyte counts (Fig. 1A and B). Patients receiving daily Flt3L injections at 25 µg/kg/d are shown in Figure 1, panel A for course 1/cycle 1. The mean WBC count of all patients increased from 5,700 cells/mm3 of blood to 13,000 cells/mm3 of blood. The greatest increase was observed in the mean absolute monocyte count, which increased from 470 cells/mm3 to 3,400 cells/mm3 (7.2-fold increase). However, neutrophil and lymphocyte numbers changed only modestly after Flt3L administration (from 4,100 to 6,200 cells/mm3 and 1,600 to 3,400 cells/mm3, respectively).
FIG. 1.
White blood cells counts with differentials for patients treated with 25 µg/kg/d Flt3 ligand (Flt3L) (A) and with 100 µg/kg/d Flt3L (B). Counts were determined during the first cycle of each treatment (average values ± the standard error of the mean are presented).
Ten patients received a higher dose of Flt3L at 100 µg/kg/d. As shown in Figure 1B, a 2-fold increase in the WBC count again occurred, and fold increases of the differential counts were similar in magnitude to those of patients on the lower Flt3L dose.
Consistent with the increase in WBC counts, there were transient increases in spleen sizes. Volumetric analysis of spleen size by computed tomography scan of the first seven patients revealed a 13%–84% increase in splenic volume of the post-Flt3L spleen size compared with the pre-Flt3L spleen size.
Phenotypic Analyses
Having observed increases in the absolute monocyte count that paralleled the rising WBC count, a phenotypic analysis was performed to characterize the cell types that were being induced by Flt3L. Patients were leukapheresed, and their PBMC were isolated by Ficoll/Hypaque density gradient centrifugation, which physically removed granulocytes, thus enriching for mononuclear cells. Dendritic cells were defined by the absence of markers for other cell types. Cells were stained with a non-DC lineage cocktail (CD3, CD14, CD16, CD19, and CD56: “Lin”). Thus, T cells, B cells, natural killer cells, and monocytes could be eliminated, because they are Lin+ (1,27,28). In peripheral blood, myeloid DC have been described, which are CD11c+/CD33+, and take up antigen (24,29,30). Dendritic cells were analyzed for expression of HLA-DR, T-cell costimulatory molecules CD80 and CD86, the DC maturation marker CD83, and CD1a, which is expressed by epidermal DC (31).
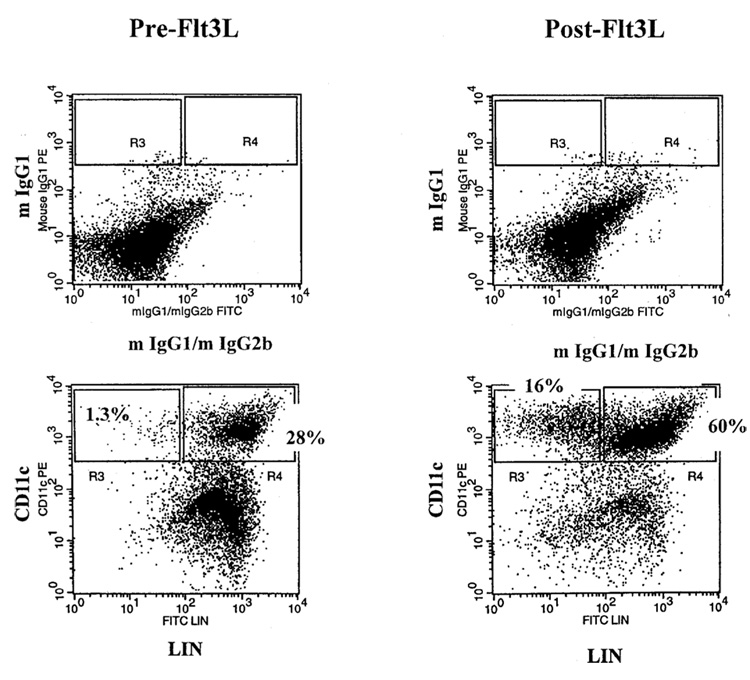
The main differences observed when pretreatment PBMC were compared with posttreatment PBMC can be seen in Figure 2 and Table 1A for patients who received 25 µg/kg/d of Flt3L. The CD11c+/Lin− DC subset increased after treatment on average 19-fold after 14 days of Flt3L compared with levels before treatment. A parallel increase was seen in the CD33+/Lin− population, indicating an increase in the myeloid DC population. Increased levels were also seen in the expression of HLA-DR and the costimulatory molecule CD86. There was no appreciable increase in another costimulatory molecule, CD80. The vast majority of DC were negative for the maturation marker CD83. Comparable increases in DC were seen in patients receiving 100 µg/kg/d Flt3L (Table 1B). Monocyte counts increased 4 fold in patients who received 25 µg/kg/d and 6–7 fold in patients receiving 100 µg/kg/d of Flt3L (Table 1B).
FIG. 2.
Flow cytometry analysis of peripheral blood mononuclear cells (PBMC) from a representative patient before and after treatment with Flt3 ligand. The PBMC were stained with an antibody binding to CD11c and a cocktail of antibodies binding to non-dendritic cell lineage (Lin) markers (CD3−CD14−CD16−CD19−CD56−).
TABLE 1A.
PBMC subsets collected from patients before and after Flt3L (25 µg/kg/day Flt3L therapy)
| Mean absolute cell count* (cells/mm3)‡ |
|||
|---|---|---|---|
| Principal cell types and phenotypic markers | Day 0 | Day 15 | Fold increase† |
| DC subsets | |||
| CD11c+/Lin−‡ | 36 | 671 | 19 |
| CD11c+/CD14− | 82 | 788 | 10 |
| CD33+/Lin− | 25 | 620 | 25 |
| CD1a+/Lin− | 0 | 1 | — |
| CD83+/Lin− | 0.6 | 14 | 23 |
| CD80+(B7.1)/Lin− | 0 | 0 | 0 |
| CD86+(B7.2)/Lin− | 47 | 404 | 9 |
| HLA-DR+/Lin− | 39 | 362 | 9 |
| Monocyte subsets | |||
| CD11c+/CD14+ | 460 | 1,934 | 4 |
| CD11c+/Lin+ | 665 | 2,322 | 3.5 |
| Other cell types | |||
| CD34+/Lin− (stem cells) | 2 | 22 | 11 |
| CD19+ (B cells) | 249 | 185 | 0.7 |
| CD3+ (T cells) | 927 | 924 | 0.9 |
|
TABLE 1B. PBMC subsets collected from patients before and after Flt3L (100 µg/kg/day Flt3L therapy) | |||
|---|---|---|---|
| Mean absolute cell count* (cells/mm3)‡ |
|||
| Principal cell types and phenotypic markers | Day 0 | Day 15 | Fold increase† |
| DC subsets | |||
| CD11c+/Lin−‡ | 30 | 519 | 18 |
| CD11c+/CD14− | 134 | 745 | 6 |
| CD33+/Lin− | 49 | 616 | 12 |
| CD83+/Lin− | 0 | 0 | — |
| CD80+(B7.1)/Lin− | 0 | 0 | — |
| Monocyte subsets | |||
| CD11c+/CD14+ | 1015 | 6973 | 7 |
| CD11c+/Lin+ | 886 | 6724 | 8 |
| Other cell types | |||
| CD19+ (B cells) | 250 | 247 | 1 |
| CD3+ (T cells) | 1105 | 879 | 0.8 |
n = 9–10
Fold increase = mean absolute cell count day 15/mean absolute cell count day 0
Lin−= CD3−CD14−CD16−CD19−CD56− (excludes T, B, NK cells, and monocytes)
DC, dendritic cells; Flt3L, Flt3 ligand; NK, natural killer; PBMC, peripheral blood mononuclear cells.
n = 5
Fold increase = mean absolute cell count day 15/mean absolute cell count day 0
Lin−− = CD3−CD14−CD16−CD19−CD56− (excludes T, B, NK cells, and monocytes)
DC, dendritic cells; Flt3L, Flt3 ligand; NK, natural killer; PBMC, peripheral blood mononuclear cells.
Functional Analysis of Patient Peripheral Blood Mononuclear Cells
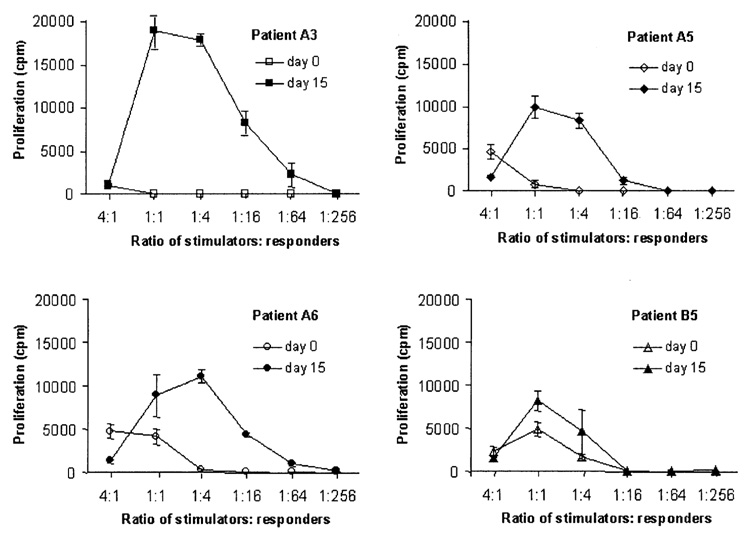
Once it was established that Flt3L induced changes in the WBC profile after 14 days of therapy, it became important to determine whether any functional differences could be detected as a result of the increased DC and monocyte counts. The MLR detects proliferation of naive allogeneic T cells in response to stimulator cells, particularly DC (26). In this case, the MLR was used to assess T-cell proliferation in response to pretreatment PBMC compared with posttreatment PBMC from ten patients receiving 25 µg/kg/d Flt3L (Fig. 3). Peripheral blood mononuclear cells from nine of ten patients reproducibly stimulated better T-cell proliferation after Flt3L administration.
FIG. 3.
T-cell proliferation stimulated by noncultured peripheral blood mononuclear cells (PBMC) from patients before and after treatment with Flt3 ligand (Flt3L). The mixed leukocyte reactions (MLR) were performed using cryopreserved, noncultured, patient PBMC as stimulator cells. Peripheral blood mononuclear cells from pre- and post-Flt3L patient samples were cultured with purified T cells prepared from the same healthy donor used in all MLR. Proliferation was measured by tritiated thymidine incorporation (counts per minute). Patients A3, A5, and A6 were patients with renal cell cancer, and patient B5 was a patient with melanoma, given 25 µg/kg/d Flt3L.
Flt3 Ligand Administration With Peptide Vaccination
To determine if the increase in circulating DC induced by Flt3L can lead to a better antigen response in vivo, a cohort of patients with melanoma was immunized with Flt3L and a vaccine consisting of four tumor antigen peptides. Previous clinical trials with a variety of immunization protocols in which patients with melanoma were immunized with the melanoma peptide antigen g209–2M have demonstrated immunization in as high as 91% of patients by in vitro monitoring (21,23). In a recent trial, patients immunized with g209–2M and the same three peptides as used in the current study, demonstrated g209–2M immunization in 18 of 29 (62%) patients by in vitro monitoring (32). That is, the patients produced specific T-cell precursors reactive to the peptide in vitro. In general, we previously found that most patients can be immunized against g209–2M, but with less success against the other peptides MART-1, g280–9V, or tyrosinase. Analysis of peptide reactivity after immunization with Flt3L plus four peptides for six patients in our study (Table 2) showed no difference in what was seen historically in the absence of Flt3L. Of the five patients who did not show any peptide reactivity in the pre-Flt3L samples, four reproducibly generated cells reactive to gp100:209–217 after two–four vaccinations. Only one of five tested patients reproducibly generated cells reactive to MART-1, and no patients reproducibly generated cells reactive to gp100:280–288 or tyrosinase. Flt3 ligand appeared to neither enhance immunization nor decrease reactivity to peptide compared with that seen historically (21,23).
TABLE 2.
Analysis of peptide reactivity after treatment with Flt3L with peptide immunization*
| Patient† | Number of vaccinations | gp100:280–288‡ | gp100:209–217 | Tyrosinase (368–376; 370D) | MART-1 (27–35) | Flu (M1; 58–66) |
|---|---|---|---|---|---|---|
| C2 | Pre | 0/4§ | 0/5 | 0/4 | 0/4 | 4/4 |
| Post 2 | 0/4 | 3/5 | 0/4 | 0/4 | 1/4 | |
| Post 4 | 0/2 | 2/2 | 0/2 | 0/2 | 0/2 | |
| C3 | Pre | 0/4 | 0/5 | 0/4 | 0/4 | 2/4 |
| Post 2 | 1/4 | 3/4 | 0/4 | 1/4 | 2/4 | |
| Post 4 | 0/3 | 3/3 | 1/3 | 1/3 | 2/3 | |
| C4 | Pre | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 |
| Post 2 | 0/2 | 1/2 | 0/2 | 0/2 | 2/2 | |
| C5 | Pre | 0/4 | 0/4 | 0/4 | 0/4 | 3/4 |
| Post 2 | 1/4 | 0/5 | 0/4 | 0/4 | 3/4 | |
| Post 4 | 0/2 | 2/2 | 1/2 | 2/2 | 2/2 | |
| C6 | Pre | 3/3 | 3/3 | 0/3 | 2/3 | 3/3 |
| Post 2 | 3/3 | 2/3 | 0/3 | 0/3 | 3/3 | |
| C7 | Pre | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 |
| Post 2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
Cryopreserved PBMC were simultaneously thawed and tested for peptide reactivity by incubation for 12 days with 1 µmol/L of the same peptides used for immunization (see “Patients and Methods” section). Cells were washed and 105 cells were added to 105 T2 cells pulsed with 1 µmol/L each of the four peptides in the native form naturally expressed on tumors, and flu peptide as a control, then incubated for 18–24 hours, and the supernatant tested for IFN-γ
C2–C7 were patients with melanoma given 25 µg/kg/day Flt3L.
Peptide used during the 18–24 hour in vitro incubation.
Number of positive assays/number assays. PBMC were considered reactive to peptide if cytokine values were greater than 100 pg/mL and twice those of peptide control (T2 cells pulsed with 1 µmol/L Flu).
Flt3L, Flt3 ligand; Flu, influenza; IFN, interferon; PBMC, peripheral blood mononuclear cells.
Peripheral Blood Mononuclear Cells Yields From Leukaphereses
The most common method to produce DC in vitro is to obtain PBMC from a patient by leukapheresis, and then incubate the adherent cells with cytokines, such as IL-4 and GM-CSF, which induce monocytes to differentiate into DC. Production of cultured DC in vitro from PBMC is therefore, limited by the number of monocytes that can be harvested from each patient’s blood. Therefore, we analyzed leukapheresis yields after Flt3L administration to determine if the monocyte yield was enhanced as would be expected from data in Figure 1. For patients receiving 25 µg/kg/d of Flt3L, there was an average 7-fold increase in monocyte yield after the first cycle of Flt3L (Table 3A). For subsequent cycles, there was a 12-fold, 9-fold, and 11-fold increase, respectively. For patients receiving 100 µg/kg/d of Flt3L, there was an 8-fold increase in monocyte yield after the first cycle of Flt3L (Table 3B).
TABLE 3A.
Fold increase in monocyte yield per liter after treatment with Flt3L (25 µg/kg/day Flt3L therapy)
| Fold increase in monocyte yield* Course/Cycle† |
||||
|---|---|---|---|---|
| Patient‡ | C1C1 | C1C2 | C2C1 | C2C2 |
| A1 | 10.0 | 9.2 | ||
| A2 | 15.5 | 66.7 | ||
| A3 | 7.8 | 12.7 | 17.1 | 21.8 |
| A4 | 30.8 | 18.0 | 21.5 | 23.5 |
| A5 | 0.6 | 2.0 | 2.8 | 3.1 |
| A6 | 1.5 | 8.4 | ||
| A7 | 6.4 | 11.0 | 21.8 | 28.1 |
| B1 | 6.5 | 14.1 | 8.6 | 3.4 |
| B2 | 1.3 | 1.1 | ||
| B3 | 1.2 | 1.2 | ||
| B4 | 11.0 | 34.5 | ||
| B5 | 1.1 | 1.7 | 1.4 | 2.3 |
| B6 | 8.4 | 7.2 | 7.6 | 8.8 |
| C1 | 5.8 | |||
| C2 | 4.3 | 1.5 | 3.3 | 3.5 |
| C3 | 2.5 | 4.8 | 3.9 | 4.1 |
| C4 | 0.3 | 3.7 | ||
| C5 | 6.5 | 9.5 | 5.9 | 8.3 |
| C6 | 7.9 | 8.3 | ||
| C7 | 9.5 | 0.3 | ||
| n | 20 | 19 | 10 | 10 |
| Mean fold increase‡ | 6.9 | 11.8 | 9.4 | 10.7 |
| Std error of the mean | 1.6 | 3.7 | 2.5 | 3.1 |
|
TABLE 3B. Fold increase in monocyte yield per liter after treatment with Flt3L (100 µg/kg/day Flt3L therapy) | |
|---|---|
| Patient* | Fold increase in monocyte yield† |
| D1 | 12.8 |
| D2 | 4.4 |
| D3 | 8.3 |
| D4 | 5.5 |
| D5 | 14.2 |
| D6 | 5.7 |
| D7 | 8.5 |
| D8 | 10.6 |
| D9 | 4.5 |
| n | 9 |
| Mean fold increase | 8.3 |
| Std error of the mean | 1.2 |
Fold increase = monocyte yield post-Flt3L/monocyte yield pre-Flt3L
C1C1 = course 1/cycle 1, C1C2 = course 1/cycle 2, C2C1 = course 2/cycle 1, C2C2 = course 2/cycle 2.
A1–A7, patients with renal cell cancer given 25 µg/kg/day Flt3L; B1–B6, patients with melanoma given 25 µg/kg/day Flt3L; C1–C7, patients with melanoma given 25 µg/kg/day Flt3L + 4 peptides.
Flt3L, Flt3 ligand.
D1–D9, patients with renal cell cancer given 100 µg/kg/day Flt3L.
Fold increase = monocyte yield post-Flt3L/monocyte yield pre-Flt3L
Flt3L, Flt3 ligand.
Generation of Dendritic Cells From Cultured Peripheral Blood Mononuclear Cells Pre- and Post-Flt3 Ligand
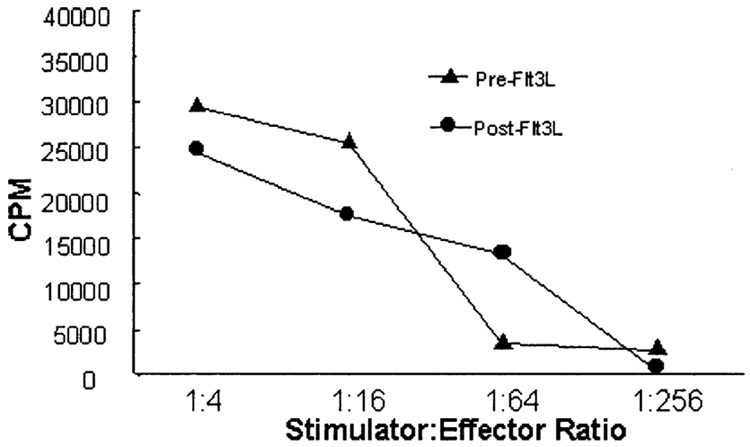
Because the yield of monocytes from patient leukaphereses was greatly increased after Flt3L administration, it was important to determine if the function of the DC generated from the PBMC from these post-leukaphereses was comparable with those generated from pre-Flt3L PBMC. Dendritic cells were generated from PBMC in vitro as described by culturing adherent cells in GM-CSF and IL-4. The percentage yield of DC (number of DC obtained/number of PBMC plated) was found to be 2.5% ± 1.77 (standard error of the mean) pre-Flt3L and 2.5% ± 0.62 post-Flt3L. Phenotypes were similar for cells cultured from pre-Flt3L PBMC compared with post-Flt3L PBMC (Table 4). Cultured DC were CD11c+/CD14− and highly positive for CD86. The DC generated produced similar T-cell proliferation in a MLR (Fig. 4). That is, the DC derived from cultured PBMC of patients after Flt3L administration were functionally similar to those derived from the PBMC taken before Flt3L administration. Because there is an average 7-fold increase in monocytes collected from leukaphereses of patients after Flt3L administration (Table 3A), one would expect a corresponding greater total yield of DC that could be generated from PBMC from patients after Flt3L administration.
TABLE 4.
Phenotype of DC generated from PBMC before and after Flt3L
| Initial PBMC used to generate DC | Percentage of DC population generated* | ||
|---|---|---|---|
| CD11C+/CD14− | CD80+/CD86+ | CD80−/CD86+ | |
| Pre-Flt3L (day 0) | 99.7% | 45.3% | 51.2% |
| Post-Flt3L (day 15) | 99.3% | 53.4% | 34.9% |
DC were generated by thawing cryopreserved PBMC from pre-and post-Flt3L patient samples, and culturing adherent cells with GMCSF and IL-4 (see “Patients and Methods”). This table is representative of culture results performed on five patients.
DC, dendritic cells; Flt3L, Flt3 ligand; GM-CSF, granulocytemacrophage colony-stimulating factor; IL, interleukin; PBMC, peripheral blood mononuclear cells.
FIG. 4.
T-cell proliferation stimulated by cultured dendritic cells (DC). Dendritic cells were generated by simultaneously thawing cryo-preserved peripheral blood mononuclear cells from pre- and post-Flt3 ligand patient samples, and culturing the adherent monocytes in granulocyte-macrophage colony-stimulating factor and interleukin-4 (see “Patients and Methods”). On day 7, a mixed leukocyte reaction (MLR) was performed with purified T cells from the same healthy donor. Proliferation was measured by tritiated thymidine incorporation (counts per minute). This graph is representative of MLR performed using DC prepared from ten patients.
DISCUSSION
The goal of this study was to determine if Flt3L could be used by either enhancing the immunization of patients to peptides in vivo, or whether the mobilization of DC or precursor DC by Flt3L could be exploited to increase yields of these cells that could then be manipulated in vitro and used for clinical therapy of patients with metastatic melanoma and renal cell cancer.
Consistent with earlier reports, Flt3L was capable of enhancing numbers of circulating DC and monocytes. However, despite Flt3L administration aimed at treating melanoma and renal tumors, which are cytokine responsive and immunogenic, none of the 31 patients in this trial clinically responded or showed enhanced immunization with melanoma peptides. This may possibly be a result of the lack of activation of DC. Although numbers of circulating DC were increased, the DC did not express significant levels of CD80 or CD83, both important activation markers on DC. Dhodapkar et al. (33) found that injection of immature DC (in which only 3%–7% of cells were expressing CD83 compared with mature DC in which 92%–93% were expressing CD83) in humans led to specific inhibition of CD8+ T-cell effector function, and that immature DC could dampen pre-existing antigen-specific T-cell function.
If the intermediate-stage DC could receive the necessary maturational signals, they may develop the ability to present specific antigens and provide an optimal costimulatory signal by expression of CD80 and CD86. Cella et al. (34) reported enhanced stimulatory function of DC with addition of CD40L to cultured DC. These findings were confirmed by studies in our laboratory (35). Our own experience suggests that PBMC harvested after 14 days of Flt3L therapy and cultured in the presence of GM-CSF, IL-4, and CD40L produce mature DC that activate naïve T cells to become functionally reactive after a single stimulation. These mature DC express greater concentrations of HLA-DR, CD80, CD86, and CD83, as well as increased IL-12 secretion, after culturing for 6 days. The importance of delivering fully mature DC was demonstrated by Labeur et al. (36) who found that induction of antitumor responses in mice by bone marrow-derived DC correlated with their degree of maturation, and by Dhodapkar et al. (33) who found that injection of immature DC in humans led to specific inhibition of CD8+ T-cell effector function.
Dendritic cell ex vivo therapy, such as peptide- or tumor lysate-pulsed DC (7,37), or vaccination with tumor cell-DC hybrids (6) demonstrated objective clinical responses in patients. Nestle et al. (7) reported clinical responses in five of sixteen patients with advanced melanoma (two complete responses) when patients received DC pulsed with a cocktail of peptides or with tumor lysate. Kugler et al. (6) administered hybrids of autologous tumor and allogeneic DC to patients with renal cancer and reported that seven of seventeen patients showed clinical responses (four complete responses). Dendritic cell therapy is thus, a promising area of research. The techniques in these studies required the generation of mature DC ex vivo. Flt3 ligand may be a means by which larger numbers of DC or monocytes could be generated in vivo, harvested by leukapheresis, manipulated ex vivo to generate mature DC, and then administered to the patient.
The use of Flt3L to mobilize precursor DC in the peripheral blood of patients is analogous to the present use of granulocyte colony-stimulating factor to mobilize circulating hematopoietic stem cells (38). Granulocyte colony-stimulating factor mobilizes large numbers of stem cells in the peripheral blood, which are then harvested from each patient or healthy donor before bone marrow transplantation and then transferred to the patient after high-dose chemotherapy. Similarly, IL-2 has been used (39) to mobilize greater numbers of lymphocytes in peripheral blood, which were then harvested, cultured in vitro to higher numbers, and reinfused into the patient.
The subcutaneous administration of Flt3L resulted in increased numbers of circulating PBMC, which we found could be harvested from patients, cultured in vitro to generate DC, and which were capable of stimulating similar T-cell proliferation as those generated from PBMC before Flt3L administration. The ability of Flt3L to mobilize cells with DC and monocyte phenotypes provides a source of antigen-presenting cells for in vitro manipulation and clinical trials. Currently, others and we are studying cultured DC that are being activated in vitro by CD40L in a clinical trial.
Acknowledgments
The authors thank Paul Spiess and My Do for their technical assistance. Dania Caron is employed by Immunex Corporation, whose product is studied in the present work.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.McKenna HJ. Generating a T cell tumor-specific immune response in vivo: can flt3-ligand-generated dendritic cells tip the balance? Cancer Immunol Immunother. 1999;48:281–286. doi: 10.1007/s002620050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porgador A, Snyder D, Gilboa E. Induction of antitumor immunity using bone marrow-generated dendritic cells. J Immunol. 1996;156:2918–2926. [PubMed] [Google Scholar]
- 5.Celluzzi CM, Mayordomo JI, Storkus WJ, et al. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kugler A, Stuhler G, Walden P, et al. Regression of human meta-static renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 7.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 8.Dhodapkar MV, Steinman RM, Sapp M, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyman SD. Biology of flt3 ligand and receptor. Int J Hematol. 1995;62:63–73. doi: 10.1016/0925-5710(95)00389-a. [DOI] [PubMed] [Google Scholar]
- 10.Matthews W, Jordan CT, Gavin M, et al. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci U S A. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews W, Jordan CT, Wiegand GW, et al. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65:1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 12.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994;91:459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maraskovsky E, Pulendran B, Brasel K, et al. Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligandtreated mice. Adv Exp Med Biol. 1997;417:33–40. doi: 10.1007/978-1-4757-9966-8_6. [DOI] [PubMed] [Google Scholar]
- 14.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch DH, Andreasen A, Maraskovsky E, et al. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 16.Esche C, Subbotin VM, Maliszewski C, et al. FLT3 ligand administration inhibits tumor growth in murine melanoma and lymphoma. Cancer Res. 1998;58:380–383. [PubMed] [Google Scholar]
- 17.Maraskovsky E, Daro E, Roux E, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 18.Morse MA, Nair S, Fernandez-Casal M, et al. Preoperative mobilization of circulating dendritic cells by flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol. 2000;18:3883–3893. doi: 10.1200/JCO.2000.18.23.3883. [DOI] [PubMed] [Google Scholar]
- 19.Hanada K, Perry-Lalley DM, Ohnmacht GA, et al. Identification of fibroblast growth factor-5 as an overexpressed antigen in multiple human adenocarcinomas. Cancer Res. 2001;61:5511–5516. [PubMed] [Google Scholar]
- 20.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–4757. [PubMed] [Google Scholar]
- 23.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 24.O’Doherty U, Peng M, Gezelter S, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 25.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM, Inaba K. Stimulation of the primary mixed leukocyte reaction. Crit Rev Immunol. 1985;5:331–348. [PubMed] [Google Scholar]
- 27.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 28.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 29.Robinson SP, Patterson S, English N, et al. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Thomas R, Lipsky PE. Human peripheral blood dendritic cell sub-sets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016–4028. [PubMed] [Google Scholar]
- 31.Randolph GJ, Beaulieu S, Lebecque S, et al. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Yang J, Schwartzentruber D, et al. Immunologic and clinical studies of patients with metastatic melanoma administered immunodominant peptides from melanoma-melanocyte differentiation antigens. Manuscript submitted. [Google Scholar]
- 33.Dhodapkar MV, Steinman RM, Krasovsky J, et al. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapointe R, Toso JF, Butts C, et al. Human dendritic cells require multiple activation signals for the efficient generation of tumor antigen-specific T lymphocytes. Eur J Immunol. 2000;30:3291–3298. doi: 10.1002/1521-4141(200011)30:11<3291::AID-IMMU3291>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Labeur MS, Roters B, Pers B, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 37.Dhodapkar MV, Krasovsky J, Steinman RM, et al. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molineux G, Pojda Z, Hampson IN, et al. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153–2158. [PubMed] [Google Scholar]
- 39.Rosenberg SA, Lotze MT, Muul LM, et al. A new approach to the therapy of cancer based on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2. Surgery. 1986;100:262–272. [PubMed] [Google Scholar]