Abstract
Uncoupling proteins (UCPs), which dissipate the mitochondrial proton gradient, have the ability to decouple mitochodrial respiration from ATP production. Since mitochondrial electron transport is a major source of free radical production, it is possible that UCP activity might impact free radical production. Free radicals can react with and damage cellular proteins, DNA and lipids. Accumulated damage from oxidative stress is believed to be a major contributor to cellular decline during aging. If UCP function were to impact mitochondrial free radical production, then one would expect to find a link between UCP activity and aging. This theory has recently been tested in a handful of organisms whose genomes contain UCP1 homologs. Interestingly, these experiments indicate that UCP homologs can affect lifespan, although they do not support a simple relationship between UCP activity and aging. Instead, UCP-like proteins appear to have a variety of effects on lifespan, and on pathways implicated in lifespan regulation. One possible explanation for this complex picture is that UCP homologs may have tissue-specific effects that complicate their effects on aging. Furthermore, the functional analysis of UCP1 homologs is incomplete. Thus, these proteins may perform functions in addition to, or instead of, mitochondrial uncoupling. Although these studies have not revealed a clear picture of UCP effects on aging, they have contributed to the growing knowledge base for these interesting proteins. Future biochemical and genetic investigation of UCP-like proteins will do much to clarify their functions and to identify the regulatory networks in which they are involved.
Keywords: UCP, Mitochondria, Lifespan, Oxidative stress, Insulin, Caloric restriction
1. Uncoupling proteins can “short-circuit” oxidative phosphorylation in mitochondria
1.1. Electron transport in coupled mitochondria drives ATP synthesis
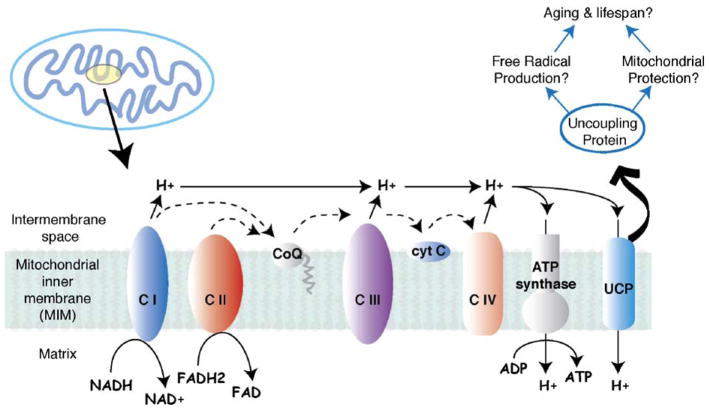
Uptake of extracellular sugar, such as circulating glucose, provides the reducing equivalents necessary to initiate electron transport through the mitochondrial electron transport chain (ETC), a series of protein complexes that reside in the mitochondrial inner membrane (MIM). Either NADH or FADH2 can initiate electron transfer through the ETC (Fig. 1). NADH is produced as a byproduct of malate oxidation and is, in turn, oxidized by complex I (CI), the NADH-CoQ reductase, which passes electrons to the membrane-bound electron carrier, coenzyme Q (CoQ). FADH2 is an alternative substrate for initiating mitochondrial respiration and is oxidized by complex II (CII), succinate dehydrogenase, which then passes electrons onto CoQ. Reduced CoQ from either CI or CII then passes electrons to complex III, CoQ-cytochrome c reductase, which then transfers them to oxidized cytochrome c. Reduced cytochrome c passes electrons to complex IV, cytochrome c oxidase, which reduces molecular oxygen to water in the final step. Electron transfer by complexes I, III and IV is coupled to proton transport across the MIM to the intermembrane space. Thus, electron transport through the ETC is coupled to the export of 2 (via CII) or 3 (via CI) protons into the mitochondrial intermembrane space.
Fig. 1.
Schematic of mitochondrial electron transport chain (ETC). Dashed lines indicate the flow of electrons donated from either NADH or FADH2 to oxidative phosphorylation complexes I–IV (CI–CIV). As a result of electron transport, protons (H+) are translocated into the intermembrane space of the mitochondria creating a proton gradient across the inner mitochondrial membrane. The proton gradient is necessary to drive ATP production via ATP synthase, but under certain conditions, uncoupling proteins (UCP) are used to dissipate the proton gradient. Uncoupling proteins may be associated with cell protection, the prevention of free radical production, and lifespan.
What is the purpose of the proton gradient formed by mitochondrial respiration? The major function of this proton gradient is to power mitochondrial complex V, ATP synthase, which couples ATP synthesis to proton translocation back into the mitochondrial matrix. Since ATP synthase couples ATP production to proton translocation, this enzyme is completely dependent on the production of the proton gradient across the MIM by the ETC. However, under some circumstances, ATP production is undesirable, particularly during ADP depletion. What, then, would be the fate of the proton gradient in the absence of ATP synthesis? Under this scenario, ATP synthase would be unable to diffuse the mitochondrial proton gradient, hypothetically leading to unrestricted proton accumulation in the intermembrane space. An excessively high proton gradient could have adverse effects, such as promoting reverse reactions of respiratory chain complexes or side reactions between the reactive species trapped in the ETC. Indeed, the formation of the reactive superoxide molecule is enhanced in the presence of high proton gradient (Korshunov et al., 1997). For this reason, it could be advantageous for mitochondria to possess pathways that can diffuse the proton gradient across the MIM. This activity is carried out by uncoupling proteins (UCPs).
1.2. Identification and cellular functions of the mammalian UCP gene family
The first UCP identified was UCP1, which is highly induced in brown adipose tissue (BAT) for non-shivering thermogenesis in the cold (Bouillaud et al., 1985). Proton gradient diffusion by UCP1 in BAT stimulates mitochondrial respiratory chain (MRC) electron transport activity, generating heat. Several UCP1 homologs have also been identified that do not have apparent roles in thermogenesis, although only a few have been shown to function as bona fide uncoupling proteins (Klingenberg and Echtay, 2001; Krauss et al., 2005). UCP2 is widely expressed and plays roles in the regulation of insulin release (see below), immunity and neuroprotection (Arsenijevic et al., 2000; Zhang et al., 2001; Diano et al., 2003; Krauss et al., 2003; Mattiasson et al., 2003). UCP3 is primarily expressed in muscle and UCP3-knockout mice exhibit symptoms of increased oxidative stress, consistent with a role for UCP3 in antioxidant defense (Vidal-Puig et al., 1997, 2000; Cline et al., 2001). UCP4 and UCP5/BCMP are preferentially expressed in the nervous system, where they may also contribute to antioxidant defenses (Mao et al., 1999; Yu et al., 2000; Kim-Han et al., 2001; Liu et al., in press). Additional UCP1 homologs have also been identified in several invertebrate species, including the nematode, Caenorhabditis elegans, and fruitfly, Drosophila melanogaster (Hanak and Jezek, 2001; Sokolova and Sokolov, 2005). In vitro uncoupling activity has been demonstrated for UCP2, UCP3 and DmUCP5 (Vidal-Puig et al., 2000; Krauss et al., 2002; Fridell et al., 2004). However, the biochemical function of most UCP1 homologs remains poorly characterized.
UCP-mediated proton pumping activity can be positively or negatively regulated by several factors. Fatty acids stimulate uncoupling by the UCPs (Klingenberg and Echtay, 2001; Fridell et al., 2004; Krauss et al., 2005). This could mean that UCPs can function to transport fatty acids across the MIM, or may be a sign that UCPs are coordinately regulated in a shift to a fat-based metabolism (Sullivan et al., 2004; Krauss et al., 2005). Another factor that stimulates UCP activity is the reactive oxygen species (ROS) superoxide, which is generated as a side-reaction between coenzyme Q and molecular oxygen (Echtay et al., 2002; Brand et al., 2004). Purine nucleotides are inhibitory to UCP activity in vitro, although the physiological basis for this feature of UCPs is not known (Klingenberg and Echtay, 2001; Krauss et al., 2005). These regulatory inputs may be important factors for setting the level of proton leak under a variety of in vivo conditions.
1.3. Why investigate the effect of UCP function on aging and lifespan?
In all organisms, tissue deterioration is associated with increased age. One major cause for this deterioration is believed to be cumulative damage to cellular components from reactions with free radical species, which are primarily generated by mitochondria. This theory is usually referred to as the free radical theory of aging and was first formally proposed by Harman in the 1950s (Harman, 1956). This hypothesis has been extensively investigated and debated, but has not yet been clearly validated or disproven (Finkel and Holbrook, 2000; Golden et al., 2002). Most tests of the free-radical theory of aging have taken the approach of altering cellular antioxidant capacity and examining the effects on aging-related phenotypes. This approach has produced positive results, suggesting that damage from free radicals play some role in aging and lifespan determination (Sun et al., 2002; Sampayo et al., 2003; Walker and Lithgow, 2003; Sun et al., 2004; Landis and Tower, 2005). In addition, a genetic mutation in the nematode, Caenorhabditis elegans, that disrupts mitochondrial electron transport, causing superoxide overproduction and increased oxidative stress, has been associated with reduced lifespan (Ishii et al., 1998; Senoo-Matsuda et al., 2001). However, lifespan was not shortened in mutant mice with deficiencies in one antioxidant defense, despite significantly increased oxidative stress, possibly contradicting the importance of free radicals in aging (Van Remmen et al., 2003). In light of such discrepancies, investigators have endeavored to reduce mitochondrial free radical production as an alternative means of testing the free-radical theory of aging. In C. elegans, reductions of mitochondrial electron transport have consistently led to increased lifespan, although it has been difficult to separate the effects of reduced oxidative stress from reductions in metabolic rate itself, as the causal factor in lifespan extension (Feng et al., 2001; Dillin et al., 2002; Lee et al., 2003). However, there is evidence linking reduced metabolic rate with long lifespan in C. elegans (Van Voorhies, 2004).
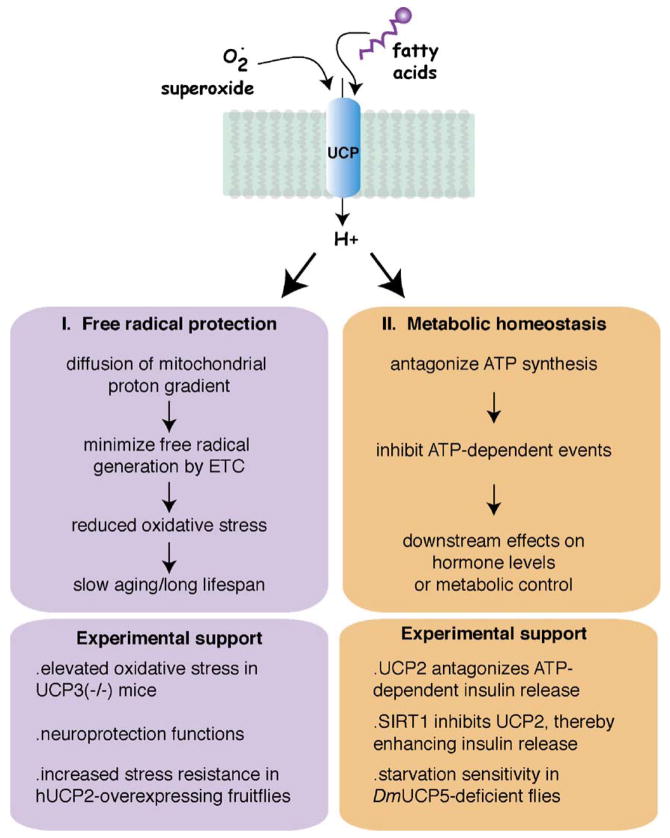
An alternative approach to altering free radical production may be by manipulating the proton gradient across the mitochondrial inner membrane. It has been shown that the magnitude of the proton gradient is directly correlated with superoxide production by the ETC (Korshunov et al., 1997). The basis for this feature of mitochondria is that the rate of electron passage through the ETC is impeded by high magnitude proton gradients. This scenario would then favor side reactions between molecular oxygen and the reduced electron carrier, coenzyme Q, producing superoxide. Thus, if free radical production indeed promotes aging and shortens lifespan, and if UCPs can alleviate free radical production, then manipulations of UCP levels should affect aging (Fig. 2). Specifically, animals with high uncoupling should have lower oxidative stress and age slowly and, conversely, animals with low uncoupling should have relatively greater levels of oxidative stress and age more quickly (Van Voorhies, 2004).
Fig. 2.
Modalities by which UCP homologs could affect aging and lifespan. Evidence from both biochemical and genetic experimentation suggests two possible modalities by which UCP function could affect aging: free radical protection (purple) or by modulating metabolic homeostasis (orange). Upper boxes outline the basic theory behind each potential modality. Lower boxes list some of the evidence supporting each theory. The upper part of the figure illustrates the UCP-stimulatory factors, superoxide and fatty acids. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2. Testing the ‘uncoupling-to-survive’ hypothesis
One approach to assess the importance of mitochondrial UCP function in aging and lifespan is to examine the correlation between the level of mitochondrial uncoupling and lifespan in individual animals. This has recently been done using population cohorts of non-sibling mice of an outbred strain (Speakman et al., 2004). A positive correlation was observed between lifespan of individual animals and whole-animal energy consumption. This correlation was most evident among individuals within the highest and lowest energy consumption quartiles. A positive correlation was also found for mitochondrial proton leak and whole-body energy consumption, for the highest and lowest quartiles of energy consumption. Together, these findings suggest that animals with the highest overall energy consumption also possessed the most uncoupled mitochondria in the population, and these characteristics appeared to be correlated with increased longevity. The lesson from this set of experiments seems to be that more uncoupled mitochondria may be favorable for long lifespan. These observations may provide indirect support for the hypothesis that oxidative stress can be reduced by higher proton flux through uncoupling proteins (Van Voorhies, 2004). Subsequent studies will be necessary to determine whether there is also a direct correlation between mitochondrial uncoupling and symptoms of oxidative stress in vivo.
3. The effect of genetic manipulation of UCP levels on aging and lifespan
A handful of studies have directly examined the effects of UCP deletion or overexpression on aging and lifespan. To date, the physiological functions of UCP family members have been best studied in mammals. However, it is costly and slow to measure aging and lifespan phenotypes in mammals. An alternative approach to directly examining the effects of UCPs on lifespan is to use shorter-lived invertebrate species, such as the fruitfly, Drosophila melanogaster, and nematode, Caenorhabditis elegans. The genomes of both species contain genes encoding UCP-like proteins, suggesting that functions of UCP-like proteins may have been conserved in these species.
3.1. Characterization of a UCP5/BMCP-like protein in Drosophila
According to phylogenic analysis, the genome of the fruitfly, Drosophila melanogaster, contains three genes encoding UCP4-like proteins, and one gene encoding a protein most closely resembling UCP5/BMCP, named DmUCP5 (Hanak and Jezek, 2001). To date, only DmUCP5 has been characterized. Evidence in support of DmUCP5’s role as a bona fide uncoupling protein comes from heterologous expression in yeast. DmUCP5 expression was associated with increased oxygen consumption in the presence of NADH, a phenotype consistent with increased uncoupling (Fridell et al., 2004). In addition, this rise in oxygen consumption was further stimulated by addition of fatty acids, such as laurine, and reduced in the presence of GDP. Together, these findings provide evidence that DmUCP5 can diffuse the mitochondrial proton gradient. However, there are caveats to this interpretation, due to inherent problems with such heterologous overexpression systems (Krauss et al., 2005). Furthermore, genetic disruption of the DmUCP5 locus was not correlated with increased ATP levels, one phenotype that might be expected in animals with more coupled mitochondria (Sanchez-Blanco et al., 2006). This negative result is also difficult to interpret since other UCP family members could functionally substitute for DmUCP5 in the mutant animals. In any case, more remains to be discovered about the biochemical function of DmUCP5.
Regardless of whether DmUCP5 turns out to be a bona fide uncoupling protein, its phylogenetic relationship to the vertebrate UCPs makes its functional characterization of interest in an evolutionary sense. DmUCP5 was preferentially expressed in the fruitfly head, suggesting neuronal function for certain UCP family members may be evolutionary conserved (Fridell et al., 2004). To study the functional role of DmUCP5, a genetic mutation in DmUCP5 was identified in a strain carrying a P-element transposon insertion within the DmUCP5 coding region. The P-element insertion abrogated expression of the DmUCPS gene, as determined by the absence of detectable DmUCPS transcript by RT-PCR (Sanchez-Blanco et al., 2006). The lifespan of DmUCP5-deficient animals was not different from wildtype controls under normal growth conditions. However, DmUCP5-deficient flies lived longer during calorie-restriction, but had reduced survival during starvation, as compared to wildtype flies. During starvation, triglyceride stores were consumed more rapidly in the DmUCP5-deficient flies compared with wildtype, suggesting that DmUCP5 may be necessary for the proper regulation of metabolic homeostasis during periods of reduced food availability. This function may be specific to conditions where it is favorable for the animals to shift to a fat-based metabolism, although there could be a more general role for this protein as well. There are clearly more exciting insights to be learned about the function of this invertebrate UCP homolog.
3.2. Extension of Drosophila lifespan by overexpression of human UCP2
In an experimental tour de force, the group studying DmUCP5 also determined that fruitfly lifespan could be lengthened approximately 10–30% by overexpression of human UCP2 in the nervous system (Fridell et al., 2005). Ubiquitous hUCP2 overexpression was lethal. Closer inspection indicated that the production of ROS was reduced in hUCP2-expressing flies and the transgenic flies were more resistant to exogenously applied oxidative stress than non-transgenic controls. This evidence supports the hypothesis that high UCP activity may augment antioxidant defenses and increase lifespan.
However, there have been consistent problems with interpretation of data obtained from heterologous overexpression of UCP proteins, particularly when UCP homologs were overexpressed in yeast cells (Heidkaemper et al., 2000). To demonstrate that hUCP2 was functional when expressed in Drosophila neurons, respiration in the absence of ATP synthesis was measured in mitochondria isolated from the heads of transgenic hUCP2-expressing flies. As expected, transgenic hUCP2-expression was correlated with increased oxygen consumption under conditions of low ATP synthesis, consistent with increased proton leak in the transgenic flies.
3.3. Deletion of a C. elegans UCP-4-like protein did not affect lifespan
The genome of the nematode, C. elegans, encodes one protein with significant similarity to vertebrate UCPs, which is referred to as CeUCP-4 (Hanak and Jezek, 2001). Thus, in this organism, redundancy between UCP-family members does not complicate experimental analysis, as it does with flies and vertebrates. CeUCP-4 is highly expressed in the muscles of the pharynx, which is the C. elegans feeding organ, and in the body muscles, which promote locomotion (Iser et al., 2005). In order to examine the requirement for CeUCP-4 for normal lifespan, a deletion mutation was obtained using standard chemical mutagenesis and the phenotypes of the mutant animals were characterized (Iser et al., 2005). ATP levels were determined to be elevated in the UCP-4(ok195) knock-out animals, compared with isogenic wildtype animals. This would be consistent with an antagonistic effect of CeUCP-4 on mitochondrial respiration, as expected if CeUCP-4 increased the mitochondrial proton leak. Nevertheless, CeUCP-4 knockout animals displayed no differences in lifespan or stress resistance in comparison to wildtype animals. However, further characterization of the mitochondria of CeUCP-4(ok195) animals is necessary to more clearly determine whether CeUCP-4 is a true uncoupler.
4. Mammalian UCP1 homologs provide a link between mitochondrial respiration and metabolic control
As mentioned earlier, direct examination of the effects of vertebrate UCPs on aging has not be performed, primarily because of the cost and long duration of lifespan studies in these species. However, functional studies of mammalian UCPs, primarily in genetically altered mice, have revealed several important functions of UCPs that could impact aging and lifespan (Fig. 2).
4.1. UCP2 regulates metabolism via pancreatic insulin release
As noted above, UCP-mediated proton leak is favored under conditions of reduced ATP synthesis, such as during periods of low ADP. Thus, deficiency of UCP activity could lead to increased ATP production. This connection to ATP/ADP ratios may link UCPs to mitochondrial energy balance and cellular functions that are sensitive to ATP levels. One such ATP-requiring function is the release of insulin from intracellular vesicles by beta cells in the pancreas. Theoretically, UCP-mediated uncoupling should antagonize ATP-promoted insulin release by attenuating ATP production. Indeed, several studies have demonstrated a role for UCP2, which is expressed in pancreatic beta cells, as an antagonist of insulin release (Zhang et al., 2001; Krauss et al., 2003). The theory behind UCP2’s role in insulin signaling is as follows (Krauss et al., 2005). Glucose uptake by pancreatic beta cells contributes electrons to mitochondrial respiration for the production of ATP from ADP. The increase in ATP concentrations stimulates fusion of insulin-containing vesicles with the cell surface, allowing insulin to be released into the bloodstream. When the ATP/ADP ratios reach a high threshold, UCP2 may be activated, diffusing the proton gradient to antagonize ATP synthesis, thus attenuating ATP production. The consequential drop in ATP levels in the beta cells would reduce the rate of insulin vesicle fusion, decreasing insulin release, and attenuating glucose uptake. The sensor of ATP/ADP ratio may be the production of superoxide by the complexes of the ETC, which can stimulate UCP2-mediated proton translocation.
There are two ways that UCP2 regulation of insulin release could affect lifespan. First, dysfunction of the UCP2-mediated switch controlling insulin release from pancreatic beta cells could result in hyper- or hypoinsulinemia, depending on the type of dysfunction. Overactivation of UCP2 via enhanced superoxide production during respiration prematurely attenuated insulin release from pancreatic beta cells, resulting in impaired glucose uptake and hyperglycemia, one of the hallmarks of diabetes (Krauss et al., 2003). Diabetes is a serious and potentially costly disease with aging-related onset. Therefore, the relationship between UCP function and diabetes may provide new therapeutic approaches for treating this disease.
There is also a second possible mechanism for UCP regulation of insulin secretion to affect lifespan. This possible mechanism draws on findings that reductions in insulin-like signaling have dramatic effects on lifespan in C. elegans and D. melanogaster. In both species, mutations that decrease insulin-like signaling lead to significantly increased lifespan and stress resistance (Tatar et al., 2003; Gami and Wolkow, 2006). These effects are mediated by FOXO-transcription factors encoded by the C. elegans gene daf-16 and by the Drosophila dFOXO gene (Ogg et al., 1997; Kramer et al., 2003). Signaling downstream of the insulin receptor antagonizes FOXO function via phosphorylation by AKT/PKB (Paradis and Ruvkun, 1998). Vertebrates contain at least three DAF-16 orthologs, named FOXO1, 3 and 4, and at least one has been shown to enhance stress resistance in response to IGF-I signaling (Brunet et al., 1999, 2001). It is possible that lifespan regulation is a conserved output of insulin-like signaling pathways across metazoan evolution, with tantilizing hints that this is the case from the finding that mice heterozygous for an IGF receptor knockout allele reportedly lived longer than controls (Holzenberger et al., 2003; Ahamed et al., 2005). If the parallels hold true, and the vertebrate FOXOs are regulated by insulin, as well as by IGF-I, then it is possible that attenuation of insulin release by UCP2 could provide a protective effect against the stresses that contribute to aging.
4.2. Sirtuins regulate UCP2 expression in the pancreas
Restriction of caloric intake can extend lifespan in a large variety of species, including rodents, fruitflies, nematodes and, even, yeast cells. While the health benefits of CR are relatively well-known, the mechanisms behind these effects have only begun to be uncovered. Sirtuins are critical mediators of CR in every organism examined. Sirtuins are a class of NAD-dependent histone deacetylases that were first identified by the SIR2 gene in Saccharomyces cerevisiae. In yeast, SIR2 represses expression of the HML and HMR mating type loci and SIR2 mutations relieve transcriptional repression at these loci (Rine and Herskowitz, 1987). More recently, increased SIR2 activity was also found to prolong replicative lifespan in S. cerevisiae, which is measured as the number of daughter cells a single mother cell is able to produce (Kennedy et al., 1997; Guarente, 1999). Although this effect of SIR2 was due to repression of recombination at the genomic rDNA loci, which produced toxic extrachromosomal circles (ERCs), SIR2’s dependence on the metabolic intermediate, NAD, also suggested an additional role for this gene in reponses to metabolic conditions (Vaziri et al., 2001). Since CR was hypothesized to cause dramatic change in metabolic balance, a role for SIR2 in this process was investigated and successfully identified (Lin et al., 2000). Since these landmark studies in S. cerevisiae, further work has elegantly demonstrated that some physiological effects of CR in other species, including mice, are also dependent on the presence of the sirtuin gene family (Wood et al., 2004; Chen et al., 2005; Guarente, 2005).
Recently, a direct link was identified between a vertebrate sirtuin, SIRT1, and the metabolic control pathways involving insulin and UCP2. Deficiencies in SIRT1 activity had been correlated with hypoinsulinaemia, as well as increased glucose uptake upon glucose challenge (Bordone et al., 2005). Unexpectedly, ATP/ADP ratios were reduced in the SIRT1 -deficient background, even in the presence of added glucose. These findings suggest that rapid glucose uptake did not immediately translate to greater ATP production, as would have normally been expected. Thus, it was possible that the energy derived from glucose uptake was diverted away from ATP production, possibly by increased uncoupling. Indeed, further investigation revealed that SIRT1-deficient cells had greatly increased levels of UCP2 mRNA and protein, indicating that SIRT1 actually functions to repress UCP2 expression. In addition, reductions in UCP2 levels could relieve the metabolic changes associated with SIRT1-deficiencies and restore normal insulin production in response to glucose administration.
5. Conclusions and caveats about possible roles for UCPs in regulating lifespan
In this review, we have attempted to highlight the findings from several elegant investigations into the connections between UCP-mediated uncoupling and lifespan. However, the conclusions from this work do not provide a clear picture of the effect of UCP-mediated uncoupling on lifespan. For instance, while hUCP2 overexpression was associated with increased lifespan in Drosophila, the deletion of one of the endogenous Drosophila UCP homologs, DmUCP5, could also extend lifespan (Fridell et al., 2005; Sanchez-Blanco et al., 2006). Furthermore, SIRT1 has been found to negatively regulate UCP2 in pancreatic beta cells, thus promoting insulin release, despite the fact that the overwhelming evidence supports the model that SIRT1 activity is limiting for long lifespan. In both these cases, the problem likely stems from our incomplete understanding of the endogenous functions of UCP homologs, both in invertebrate and vertebrate species. Further investigation of these interesting proteins is sure to reveal new ways that mitochondria can impact physiology of the entire organism.
Acknowledgments
We thank members of the Wolkow laboratory, Phil Morgan and the NIA/IRP for helpful discussions. This work was funded by the Intramural Research Program of the NIH, National Institute on Aging and by the Ellison Medical Foundation.
References
- Ahamed K, Epaud R, Holzenberger M, Bonora M, Flejou JF, Puard J, Clement A, Henrion-Caude A. Deficiency in type 1 insulin-like growth factor receptor in mice protects against oxygen-induced lung injury. Respir Res. 2005;6:31. doi: 10.1186/1465-9921-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2005;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillaud F, Ricquier D, Thibault J, Weissenbach J. Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc Natl Acad Sci USA. 1985;82:445–448. doi: 10.1073/pnas.82.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Rad Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem. 2001;276:20240–20244. doi: 10.1074/jbc.M102540200. [DOI] [PubMed] [Google Scholar]
- Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology. 2003;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Devel Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook N. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Functional characterization of a Drosophila mitochondrial uncoupling protein. J Bioenerg Biomembr. 2004;36:219–228. doi: 10.1023/b:jobb.0000031973.20153.c6. [DOI] [PubMed] [Google Scholar]
- Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Gami MS, Wolkow CA. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell. 2006;5:31–37. doi: 10.1111/j.1474-9726.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–123. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Guarente L. Diverse and dynamic functions of the Sir silencing complex. Nat Genet. 1999;23:281–285. doi: 10.1038/15458. [DOI] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and SIR2 genes-towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hanak P, Jezek P. Mitochondrial uncoupling proteins and phylogenesis—UCP4 as the ancestral uncoupling protein. FEBS Lett. 2001;495:137–141. doi: 10.1016/s0014-5793(01)02338-9. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Heidkaemper D, Winkler E, Muller V, Frischmuth K, Liu Q, Caskey T, Klingenberg M. The bulk of UCP3 expressed in yeast cells is incompetent for a nucleotide regulated H+ transport. FEBS Lett. 2000;480:265–270. doi: 10.1016/s0014-5793(00)01949-9. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Iser WB, Kim D, Bachman E, Wolkow C. Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans. Mech Age Dev. 2005;126:1090–1096. doi: 10.1016/j.mad.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, Pak SM, Laroche T, Gasser SM, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Kim-Han JS, Reichert SA, Quick KL, Dugan LL. BMCP1: a mitochondrial uncoupling protein in neurons which regulates mitochondrial function and oxidant productions. J Neurochem. 2001;79:658–668. doi: 10.1046/j.1471-4159.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M, Echtay KS. Uncoupling proteins: the issues from a biochemist point of view. Biochim Biophys Acta. 2001;1504:128–143. doi: 10.1016/s0005-2728(00)00242-5. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Davidge JT, Lockyer JM, Staveley BE. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3:5. doi: 10.1186/1471-213X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Nat1 Acad Sci USA. 2002;99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126:365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie estriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan S, Slevin JJ, Wersto R, Zhan M, de Souza-Pinto N, Mustafa K, de Cabo R, Mattson M. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol Med. doi: 10.1385/NMM:8:3:389. in press. [DOI] [PubMed] [Google Scholar]
- Mao W, Yu XX, Zhong A, Li W, Brush J, Sherwood SW, Adams SH, Pan G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999;443:326–330. doi: 10.1016/s0014-5793(98)01713-x. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo JN, Gill MS, Lithgow GJ. Oxidative stress and aging—the use of superoxide dismutase/catalase mimetics to extend lifespan. Biochem Soc Trans. 2003;31:1305–1307. doi: 10.1042/bst0311305. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blanco A, Fridell YW, Helfand SL. Involvement of Drosophila uncoupling protein 5 in metabolism and aging. Genetics. 2006;172:1699–1710. doi: 10.1534/genetics.105.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J Biol Chem. 2001;276:4155341558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- Sokolova IM, Sokolov EP. Evolution of mitochondrial uncoupling proteins: novel invertebrate UCP homologues suggest early evolutionary divergence of the UCP family. FEBS Lett. 2005;579:313–317. doi: 10.1016/j.febslet.2004.11.103. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Sun J, Molitor J, Tower J. Effects of simultaneous over-expression of Cu/ZnSOD and MnSOD on Drosophila melanogaster life span. Mech Age Dev. 2004;125:341–349. doi: 10.1016/j.mad.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe S, Alderson N, Baynes J, Epstein C, Huang T, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genom. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Van Voorhies WA. Live fast - live long? A commentary on a recent paper by Speakman et al. Aging Cell. 2004;3:327–330. doi: 10.1111/j.1474-9728.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun. 1997;235:79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yu XX, Mao W, Zhong A, Schow P, Brush J, Sherwood SW, Adams SH, Pan G. Characterization of novel UCP5/BMCP1 isoforms and differential regulation of UCP4 and UCP5 expression through dietary or temperature manipulation. FASEB J. 2000;14:1611–1618. doi: 10.1096/fj.14.11.1611. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]