Summary
A major obstacle limiting the efficacy of adoptive T-cell transfer (adoptive immunotherapy) to treat patients with cancer is the short survival of the transferred cells. These in vitro activated T cells depend on the growth factor, interleukin (IL)-2, and may undergo apoptosis in vivo when they are transferred. The authors previously reported that the need for an exogenous source of IL-2 could be abrogated in vitro by retrovirally transducing antitumor T lymphocytes with an exogenous IL-2 gene. Here they report that this growth of IL-2 transductants depended on restimulation of the T-cell receptor complex and appeared to be regulated at the transcriptional level of the transduced IL-2 gene. The transduced IL-2 transcript was barely detectable in IL-2–transductants just before they died without restimulation, and they expressed a low level of the CD25 molecule, the alpha chain of the IL-2 trimeric receptor complex. Melanoma-specific tumor-infiltrating lymphocytes (either bulk or CD8+ cells alone), when transduced with an IL-2 retroviral vector, could produce IL-2 upon tumor stimulation and proliferated after the destruction of autologous tumor cells in the absence of added IL-2. Control vector–transduced tumor-infiltrating lymphocytes failed to do so under the same conditions. These findings provide a foundation for the development of clinical efforts to adoptively transfer melanoma-specific tumor-infiltrating lymphocytes transduced with an IL-2 retroviral vector for the treatment of patients with metastatic melanoma to evaluate the fate and therapeutic effect of these IL-2 gene-modified antitumor T lymphocytes in vivo.
Keywords: Cytokines, Cytotoxic T Lymphocytes, Gene Therapy, Immunotherapy, Retroviral Transduction
Interleukin (IL)-2 is an important cytokine that regulates T-cell growth and can promote T-cell survival by inducing the expression of multiple proteins, including bcl-2 (1-5). Interleukin-2–dependent activated cells undergo apoptotic death when IL-2 is withdrawn either in vitro or after in vivo cell transfer (6-8). Thus, a source of IL-2 has been shown to be an essential component of the eradication of established widespread tumors in murine models and in humans after the adoptive transfer of antitumor T cells (9,10). The prolonged administration of IL-2 can be associated with substantial toxicity, limiting the amount of this cytokine that can be given safely to humans (11), and thus limiting the survival of CD8+ T cells after their adoptive transfer (7). This may severely limit the effectiveness of adoptive immunotherapy.
To overcome this problem and to improve the efficacy of adoptive immunotherapy, we introduced an exogenous IL-2 gene into melanoma-reactive T lymphocytes by retroviral transduction. We found that IL-2 gene-transduced T cells could secrete IL-2 and proliferate in an IL-2 autocrine manner for more than 8 weeks. Importantly, such gene modification of these cells did not affect their antitumor activity (12).
In the current study, we explored the mechanisms involved in the regulation of the growth of these IL-2 gene-transduced antitumor T lymphocytes and tested the ability of IL-2 gene-modified tumor-infiltrating lymphocytes (TILs) to proliferate with tumor stimulation.
MATERIALS AND METHODS
Interleukin-2YFP Peripheral Blood Mononuclear Cell Transductants
Preparation of IL-2 yellow fluorescent protein (YFP) transductants has been described previously (12). Briefly, cryopreserved peripheral blood mononuclear cells (PBMCs) obtained from patients with melanoma after immunization with the gp100:209–217 (210 M) peptide (209–2M; IMDQVPFSV) in incomplete Freund's adjuvant (13) were restimulated in vitro with the cognate peptide, transduced with a retrovirus (IL-2YFP) encoding human IL-2 gene under the control of the retroviral 5′ LTR promoter, and selected by fluorescence-activated cell sorting (FACS).
Rapid-Expansion Protocol
Cells were expanded as described previously (14) with minor modifications. T cells (2 × 105) as indicated were added to 25 mL complete medium (CM) without IL-2 in a 25-cm2 tissue culture flask containing 2.5 × 107 irradiated (35 Gy) allogeneic PBMCs and 30 ng/mL OKT3. The CM consisted of RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10 mM HEPES buffer, 100 U/mL penicillin, and 100 μg/mL streptomycin (Biofluids, Rockville, MD, U.S.A.), 20 μM β-mercaptoethanol, and 10% heat-inactivated freshly pooled normal human male serum (Biochemed Pharmacologicals, Winchester, VA, U.S.A.). Interleukin-2 was added to some flasks on the next day to produce a final concentration of 300 IU/mL. Twenty milliliters of media were removed on day 7 and replaced with fresh CM with or without IL-2 (300 IU/mL). Viable cells of each condition were counted by trypan blue exclusion on the days indicated. For rapid expansion protocols (REPs) performed in the 175-cm2 flasks (Costar Corp., Corning, NY, U.S.A.), 1 × 106 effector T cells were incubated with 2 × 108 irradiated (35 Gy) allogeneic PBMCs in 175 mL media containing 30 ng/mL OKT3.
Reverse Transcription–Polymerase Chain Reaction and Genomic DNA Polymerase Chain Reaction for Interleukin-2YFP Peripheral Blood Mononuclear Cell Transductants
At the indicated days of REP4, total RNA was extracted from 1 × 106 cells using STRATAGENE Absolutely RNA Reverse Transcription–Polymerase Chain Reaction (RT-PCR) Miniprep Kit (Stratagene, La Jolla, CA, U.S.A.). Two micrograms of total RNA was subjected to first-strand cDNA synthesis with random priming using an Amersham Pharmacia Biotech Kit (Pisscat-away, NJ, U.S.A.). One eighth of the first-strand cDNA reaction was subjected to PCR reaction containing primer 16 (5′GTCAGCGGGGGTCTTTCATT3′) and primer 2 (5′GGGTCGACGGATCCTCAAGTTAGTGTTGAGATGA3′). The PCR products were fractionated on a 1% agarose gel. The vector-derived IL-2 transcript directed the synthesis of an 885-bp fragment. In the control reactions containing sense primer (5′ACACTGTGCCCATCTACGAGG′) and antisense primer (5′AGGGGCCGGACTCGTCATACT3′), the beta-actin gene transcript directed the synthesis of a 621-bp fragment (15).
Genomic DNA was prepared as follows. A cell pellet of 1 × 105 was resuspended in 1,000 μL QuickExtract DNA extraction solution 1.0 (Epicenter Technologies, Madison, WI, U.S.A.). Ten microliters of this solution containing 1,000-cell-equivalent DNA was subjected to PCRs containing primers 16 and 2, as described before. The integrated retroviral genome directed the synthesis of an 1,837-bp fragment.
Flow Cytometric Studies
Cells at the time indicated were stained with antibodies as indicated according to the instruction manual. Stained cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, U.S.A.).
Construction of the SBIL-2 Retroviral Vector
The SBIL-2 retroviral vector was derived from a retroviral plasmid IL-2-IRES-eGFP that contained exactly the same genetic information as IL-2YFP (12) except eGFP replaced YFP. The retroviral backbone (SB) was prepared from IL-2-IRES-eGFP by digestion with Bam H1, followed by blunt-ending with Klenow fragment and digestion with Not I. The IL-2 cDNA insert was also prepared from IL-2-IRES-eGFP by digestion with Sal I, followed by blunt-ending with Klenow fragment and digestion with Not I. The resultant insert was directionally cloned into SB. The resultant SBIL-2 vector (Surgery Branch IL-2) did not contain any other genes, as confirmed by nucleotide sequencing analysis. The construct was pseudotyped in the packaging cell line PG13, which provided Gibbon Ape Leukemia Virus (GaLV) envelope protein. A stable PG13SBIL-2 producer clone #3 was established, and genomic DNA analysis revealed that this producer clone contained three copies of the integrated retroviral IL-2 DNA. The vector supernatant produced by this producer clone was shown to be biologically active by transducing a human non–IL-2-producing melanoma cell line to produce IL-2 as detected by enzyme-linked immunosorbent assay (ELISA). The control-vector YFP was prepared in parallel from PG13 packaging cell lines.
Transduction of Tumor-Infiltrating Lymphocytes
TIL1913 and TIL1941 were derived from subcutaneous lesions from patients with metastatic melanoma. Transductions were performed in wells of six-well plates (Becton Dickinson) coated with 10 μg/cm2 Retronectin (Takara, Shuzo Co., Otsu, Shiga, Japan) according to the manufacturer's instructions. Two million TIL cell pellets from day 7 REP cultures were resuspended with 8 mL freshly prepared YFP or SBIL-2 retroviral supernatants, respectively. Interleukin-2 was added to YFP supernatants to a final concentration of 6,000 IU/mL. No exogenous IL-2 was added to SBIL-2 supernatants, which already contained comparable levels of IL-2. This cell-viral supernatant mixture was applied to a Retronectin-coated well and incubated at 37°C 5% carbon dioxide for 6 hours. Four retroviral transductions in the same Retronectin-coated wells were performed in two consecutive days. At the end of second day, the transduced cells were washed twice with CM and resuspended in 50–50 medium consisting of a 1:1 volume ratio of CM and AIM V medium (Life Technologies, Grand Island, NY, U.S.A.) supplemented with 6,000 IU/mL IL-2. The cultures were maintained at the cell density of 0.7 to 1 × 106/mL at 37°C, 5% carbon dioxide. On days 4 or 5 after transduction, transduction efficiency was determined as the percentage of YFP+ cells for YFP-transduced cells by a FACScan (FITC channel). Transduction efficiency for SBIL-2 was determined by genomic DNA PCR (see below).
Genomic DNA Polymerase Chain Reaction of the IL-2 Gene To Determine the Transduction Efficiency of Tumor-Infiltrating Lymphocytes by SBIL-2
Genomic DNA samples were prepared from the cells as described. Equivalent amounts of DNA representing 1,250 cells per sample were subjected to PCR cycles of 22, 25, 27, 29, and 35, respectively. The upstream primer (5′ATGTACAGGATGCAACTCCT 3′) was derived from exon 1, and downstream primer (5′ CTTCTTGGGCATGTAAAACT 3′) was derived from exon 2 of the IL-2 gene. Endogenous genomic IL-2 gene directed the synthesis of 311-bp fragment containing the first intron sequences of IL-2 gene. The transduced vector-derived IL-2 gene (IL-2 cDNA) directed the synthesis of a 221-bp fragment without intron sequences. The PCR products were fractionated on a 2% tris/acetate agarose gel.
The products from 29-cycle reactions were subjected to Southern blot and hybridization using an oligo probe containing sequences from exon 1 of the IL-2 gene.
The ratio (percentage) of vector-derived IL-2 DNA relative to endogenous genomic IL-2 DNA was derived from dividing the phosphoimager signal of the vector DNA band by that of the genomic DNA band. Genomic DNA from the PG13SBIL-2 packaging clone #3 (containing three copies of the retroviral IL-2 gene) was used as the control for the vector-derived IL-2 gene.
Coculture of Tumor-Infiltrating Lymphocytes Transduced With Either YFP or SBIL-2 With Autologous Tumor Cells
TIL1913 cells (1 × 105) in replicates as indicated were obtained from a REP culture on day 13 (5 days after transduction), washed extensively, resuspended in 100 μL CM without or with 300 IU/mL or 6,000 IU/mL IL-2, and plated onto the 96 flat-bottom wells. Autologous tumor cells (1 × 105; the same tumor specimen used to derive TIL1913) in 100 μL CM were irradiated at 100 Gy and added to each well containing TIL.
Six days later, cells were transferred to 48-well plates and 200 μL fresh media was added (± IL-2).
On day 8, cultures were photographed and 400 μL fresh media was added (± IL-2).
On day 9, cells were transferred to 24-well plates. One milliliter fresh media (± IL-2) was added (1.8 mL total/well).
On day 14, viable cells were counted by trypan blue exclusion. No viable cells from wells containing tumor cells alone were seen on day 14. On day 15, cells from the SBIL-2-transduced TIL cocultured well were analyzed for CD8 surface marker and intracellular IL-2 staining with phycoerythrin (PE)-conjugated anti–IL-2 antibody (Pharmingen, San Diego, CA, U.S.A.) according to the instruction manual. On day 17, 60 μL media was taken from each well of the no–IL-2 condition and assayed for IL-2 by ELISA.
Depletion of CD4+ Cells
Seven days after transduction, SBIL-2–transduced TIL1913 cells were expanded in a REP protocol without IL-2 and YFP-transduced cells were expanded in the presence of 300 IU/mL IL-2. Fourteen days from the onset of this expansion, CD4+ cells were depleted using Dynal beads (Dynal ASA, Oslo, Norway). The efficiency of the depletion was more than 99.5%, as determined by FACS analysis.
Immunofluorescent Staining for Intracellular Interleukin-2 by Flow Cytometry in the CD4+-Depleted Cells
The CD4+-depleted cells (1 × 106) were washed with medium without IL-2 three times before replating in 1 mL medium onto the wells of a 24-well plate precoated with OKT3 (1 μg/mL) without IL-2. After 16 hours incubation in the presence of Golgi stop (PharMingen) at 37°C and 5% carbon dioxide, the cells were harvested and subjected to intracellular FACS with PE-conjugated anti–IL-2 or an isotype-control antibody (PharMingen) according to the instruction manual.
RESULTS
Interleukin-2–Independent Growth of IL-2YFP PBMC Transductants was Regulated at the Transcriptional Level of the Transduced IL-2 Gene
Previously we found that PBMCs, when retrovirally transduced with an exogenous IL-2 gene (IL-2YFP), could secrete IL-2 after stimulation with OKT3 and continue to grow in the absence of exogenous IL-2 for more than 8 weeks in two REPs (REP1 and REP2) (12). The same results could be reproduced in a REP3 experiment (data not shown). Rapid expansion protocol cultures consistently ceased to proliferate approximately 2 or 3 weeks after stimulation with OKT3 and allogenic feeders, began to lose viability at approximately 4 to 6 weeks in the absence of added IL-2, and eventually died without further stimulation.
To explore the mechanisms regulating their growth, we restimulated IL-2YFP PBMC transductants in a REP4 experiment without IL-2 and studied the expression of the transduced IL-2 gene. Total RNA was extracted from an equal number of IL-2YFP PBMC transductants on day 8 when the cells were actively proliferating, on day 26 when the cell growth reached a plateau, and on day 34 when the cells began to lose viability. cDNA templates were prepared by reverse transcription with random priming, followed by amplification in PCR reactions containing primers encompassing 5′ retroviral and 5′ IL-2 gene sequences. Under these conditions, the transduced vector-specific IL-2 transcript directed a synthesis of an 881-bp DNA fragment.
The transduced IL-2 gene was actively expressed on day 8 of REP4 during the logarithmic growth period (Fig. 1A, lane 1). The amount of IL-2 as measured by ELISA was 240 pg/mL ± 10 (mean ± SEM) in a volume of 200 μL by 2 × 105 cells. This expression was only slightly decreased when the cell growth peaked on day 26 (Fig. 1A, lane 2). However, the level of transduced IL-2 gene transcript was barely detectable on day 34 of the REP4, when the IL-2YFP PBMC transductants began to die (Fig. 1A, lane 3). As a control, there was no difference of beta-actin gene expression in these cells during the REP4 (Fig. 1B). The decrease in the transduced IL-2 gene expression was not a result of the loss of the integrated retroviral genome, because there was no significant difference in the integrated proviral DNA in these cells, as shown by genomic DNA PCR (Fig. 1C).
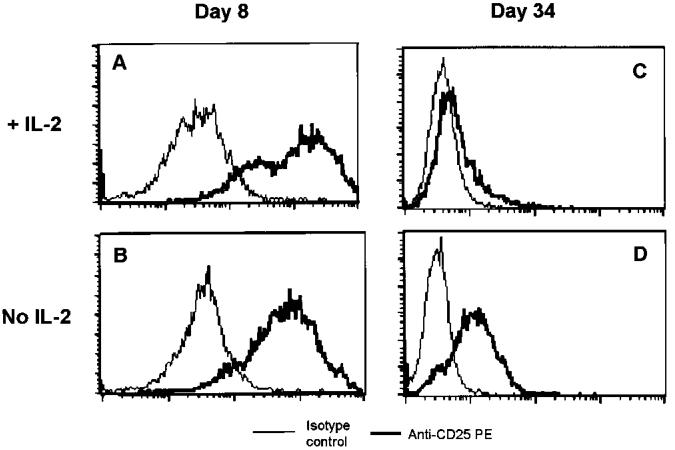
FIG. 1.
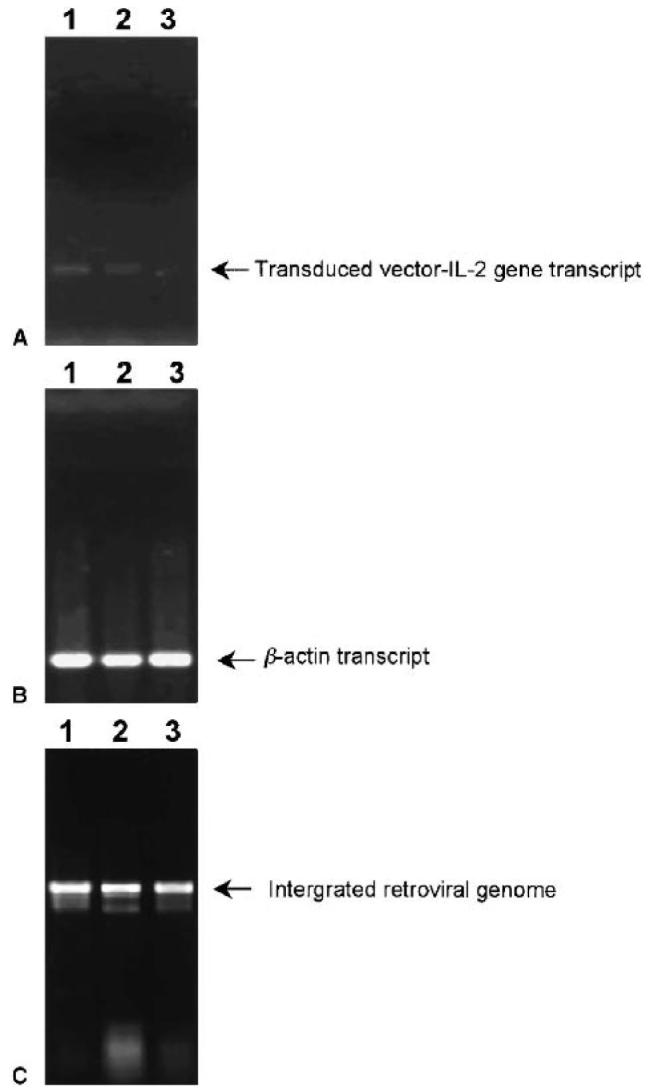
IL-2YFP PBMC transductants self-regulated the transduced IL-2 gene in the absence of added IL-2 without further stimulation. Viable cells were counted by trypan blue exclusion and collected on days 8 (lane 1), 26 (lane 2), and 34 (lane 3) of the REP4 experiment without added IL-2, as described in the text. The RT-PCR was performed using primers for vector-derived (A) IL-2 gene and (B) beta-actin gene. (C) Genomic DNA PCR was performed using primers for retroviral genome. Different cycles of PCR were performed with the same pattern of results seen (data not shown). Shown here are photographs of 1% agarose gel electrophoresis of the PCR products after 29 cycles of amplification.
The expression of CD25 (Tac), the alpha chain of the IL-2 receptor complex, is upregulated by antigenic stimulation and by IL-2 itself. Downregulation of CD25 is hypothesized to be one of the mechanisms by which activated T cells stop responding to IL-2 in vitro (16). Therefore, we examined the CD25 expression of IL-2YFP cells grown in the absence or presence of exogenous IL-2 (300 IU/mL) by flow cytometry during the REP4.
On day 8 of REP4, 100% of IL-2YFP transductants expressed CD25 on their cell surface either with or without exogenous IL-2 (Figs. 2A and B). However, on day 34, cells grown in the presence of IL-2 expressed very little CD25 when their growth reached a plateau (Fig. 2C). In contrast, under the no-IL-2 condition on day 34 when cells were dying, nearly all of IL-2YFP transductants still expressed CD25 (Fig. 2D). However, the median fluorescence index was much lower when compared with the median fluorescence index on day 8.
FIG. 2.
IL-2YFP PBMC transductants still expressed CD25 when they started to lose viability in the absence of exogenous IL-2. Viable cells were counted by trypan blue exclusion and taken on day 8 (A and B) and day 34 (C and D) of a REP4 experiment (B and D) without or (A and C) with 300 IU/mL added IL-2. Cells (5 × 105) were subjected to FACS analysis after staining with PE-conjugated antibodies. Shown here are histograms of anti-CD25–stained cells (thick line) superimposed over those of isotype control– stained cells (thin line).
These data suggested that the growth of IL-2YFP PBMC transductants under the no-added IL-2 condition was limited by downregulation of the expression of the transduced IL-2 gene in the absence of further stimulation and by the downregulation of CD25.
Construction of a Retroviral IL-2 Vector (SBIL-2) and Transduction of Bulk Tumor-Infiltrating Lymphocytes
The characteristics of IL-2 independence but OKT3 stimulation dependence exhibited by these IL-2YFP PBMC transductants prompted us to hypothesize that the growth of IL-2 gene-transduced antitumor T cells might also become independent of exogenous IL-2 when stimulated with tumor.
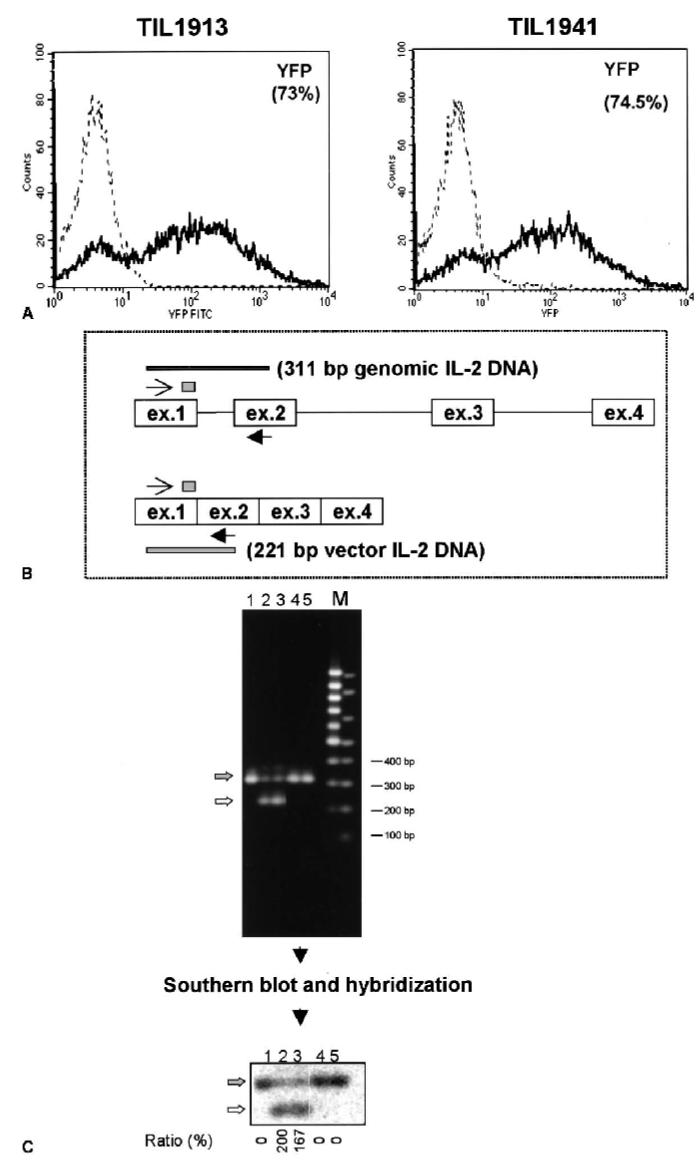
To test this possibility ex vivo, we constructed a new IL-2 retroviral vector (SBIL-2) without any marker gene and used this retrovirus to transduce bulk TILs. Five days after transduction, gene-transfer efficiency was evaluated. Because the SBIL-2 had no marker gene, we compared its transduction efficiency with that of a control vector (YFP) that contained the same retroviral sequence and envelope protein in parallel experiments. We reproducibly achieved at least 73% gene transfer efficiency into human TILs (Fig. 3A).
FIG. 3.
The SBIL-2 vector-transduced human melanoma-specific TILs at frequencies greater than 73%. Bulk TIL1913 and TIL1941 were obtained and transduced with SBIL-2 and YFP retroviral vector, respectively, as described in Materials and Methods. Five days after transduction, the cells were subjected to FACS analysis and TIL 1913 cells were subjected to genomic DNA PCR. (A) Histograms of the percentage of YFP+ cells for YFP vector-transduced TILs (thick line) superimposed on the untransduced cells (thin line). (B) A schema for the genomic DNA PCR of the transduced TIL1913 cells. The forward primer indicated by a thin arrow and the reverse primer indicated by a thick arrow contained sequences from the exon 1 and exon 2 of the IL-2 gene, respectively. This pair of primers amplified a 311-bp fragment from the endogenous IL-2 gene containing the intron sequences, whereas the transduced retroviral IL-2 gene directed a synthesis of 221-bp fragment without intron sequences. (C, upper) A 2% agarose electrophoretic gel for the amplified PCR products using the schema illustrated in panel B. An equivalent amount of DNA representing 1,250 cells per sample was subjected to 29 cycles. No PCR cycle difference was seen in the pattern of amplified fragments (data not shown). Lane 1, untransduced cells; lanes 2 and 3, duplicate samples of the SBIL-2–transduced TIL1913 cells; lanes 4 and 5, duplicate samples of the control-vector YFP-transduced cells; M, DNA markers. The gel was subjected to Southern blotting and hybridization using a 32P-labeled oliogonucleotide containing the sequences of the exon 1 of the IL-2 gene (indicated by a small stippled box of panel B). (C, lower) A phosphoimage of the blot. The endogenous IL-2 gene band was indicated by a filled arrow, and the vector-derived IL-2 gene band was indicated by a solid arrow. After quantification of the signals by the phosphoimager, the ratio (percentage) shown at the bottom of the image was calculated by dividing the signal of the vector IL-2 gene band by that of the genomic DNA band. The fact that this ratio is greater than 100% in the SBIL-2–transduced cells implies that nearly all cells harbored one copy of the transduced IL-2 gene (a transduction efficiency of 100%), with some cells containing more than one copy of the retroviral sequences.
We also used genomic DNA PCR of the IL-2 gene to confirm the transduction efficiency of SBIL-2. This test takes advantage of the intron difference between the endogenous genomic IL-2 gene and the vector-derived IL-2 gene. Using primers flanking the intron 1 of the IL-2 gene, the genomic IL-2 gene directed the synthesis of a 311-bp fragment containing the intron, whereas amplification of the vector-derived IL-2 gene (cDNA derivative) would give rise to a 211-bp product without the intron (Fig. 3B). The intensity of these bands was quantified by phosphoimaging after hybridization with a 32P-labeled oligonucleotide probe common to both products. The relative ratio of a 211-bp fragment compared with a 311-bp PCR product was then calculated. The vector-derived IL-2 gene band was stronger than the endogenous IL-2 gene band (ratio >1), which implied that most of the cells were transduced by SBIL-2 (Fig. 3C, lanes 2 and 3). The SBIL-2–transduced bulk TIL1913 and TIL1941 produced IL-2 and proliferated in the absence of exogenous IL-2 after stimulation with OKT3, whereas YFP-transduced cells failed to do so (data not shown), consonant with the data presented previously on the IL-2YFP PBMC transductants.
The SBIL-2–Transduced TILs Could Proliferate in the Absence of Exogenous IL-2 After the Destruction of Autologous Tumor Cells
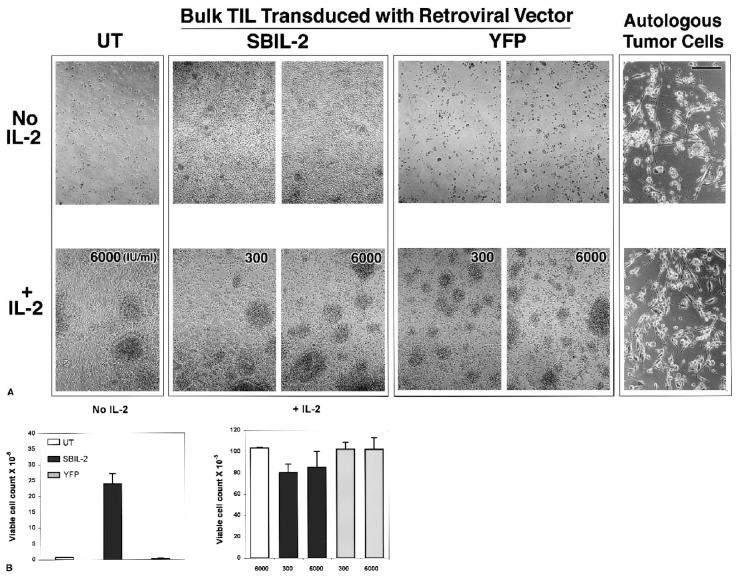
We tested the growth of these SBIL-2–transduced TIL1913 cells in coculture assays with irradiated autologous tumor cells. Eight days after the initial onset of cocultures, the cells in each well were photographed. In the absence of added IL-2, in the wells containing TILs transduced with SBIL-2, the growth of T cells was apparent with T-cell clusters (Fig. 4A, upper middle panels). In contrast, in the absence of exogenous IL-2, few or no T cells were seen in the wells containing untransduced or YFP control-vector-transduced TIL cells (Fig. 4A, upper left and right panels). Instead, scattered tumor cells and cell debris were seen. In the presence of added IL-2 (either 300 or 6,000 IU/mL), no difference was seen in all cocultured wells (Fig. 4A, lower panels): All of them destroyed tumor cells and subsequently proliferated. On day 14 after the onset of the coculture, viable cells were counted from each cocultured well. In the absence of exogenous IL-2, SBIL-2–transduced TILs expanded 25 to 30 times after tumor stimulation (Fig. 4B, left panel) but only three times without stimulation in the absence of IL-2 (data not shown). These cells were 100% CD8+ and positive for intracellular IL-2 by FACS analyses (data not shown). In contrast, there were no viable cells either in untransduced or in YFP-transduced cocultured wells (Fig. 4B, left panel). No growth difference was seen when exogenous IL-2 was added at either 300 or 6,000 IU/mL to the coculture media (Fig. 4B, right panel).
FIG. 4.
The SBIL-2–transduced TIL1913 cells proliferated after the destruction of the autologous tumor cells in the absence of added IL-2. Untransduced and transduced cells (1 × 105) were plated in duplicate 5 days after transduction in 100 μL medium in the wells of a 96-well flat-bottom plate, followed immediately by the addition of 1 × 105 autologous melanoma tumor cells previously irradiated at 100 Gy in 100 μL medium. Interleukin-2 was added to some wells at the indicated concentrations. Six days from the onset of the coculture at 37°C, 5% carbon dioxide, the cells in each well were transferred individually to wells of 48-well plates. (A) On day 8, each well was photographed (scale bar, 100 μm). Fourteen days after the onset of the experiment, viable cells were counted by trypan blue exclusion. Tumor-alone wells contained no viable cells. (B) The mean cell count ± SEM. The SBIL-2–transduced TIL1913 cells proliferated after the destruction of the autologous tumor cells in the absence of any exogenous IL-2. Untransduced or the control vector YFP-transduced cells failed to proliferate under the same condition.
We followed the growth of tumor-stimulated, SBIL-2–transduced cells without the addition of IL-2. Their proliferation peaked approximately 2 weeks after tumor stimulation, and these cells eventually died without further stimulation in the absence of added IL-2. With re-stimulation with fresh autologous tumor cells, SBIL-2–transduced TIL cells proliferated again in the absence of added IL-2. These results were the same as our previous results with IL-2YFP PBMC transductants.
We also tested the presence of IL-2 by ELISA in the coculture media harvested from the no–IL-2 condition on day 17 after the onset of the coculture. Interleukin-2 (150 ± 9 pg/mL [mean ± standard error of the mean] by 2.2 × 106 cells in 1.4 mL medium) could be detected from the wells containing SBIL-2–transduced TIL cells cocultured with the tumor cells. This level reflected a balance between the production and consumption by these SBIL-2–transduced cells. No IL-2 could be detected from the wells containing either YFP-transduced or untransduced TIL cells cocultured with the tumor cells.
When considered together, these data show that SBIL-2–transduced TILs could secret IL-2 when stimulated with tumor antigen and proliferate in the absence of added IL-2 after the destruction of autologous tumor cells, whereas untransduced or control-vector YFP-transduced cells failed to do so under the same conditions.
SBIL-2–Transduced CD8+ TIL Cells Alone Could Proliferate After the Destruction of Tumors Cells Without Help From CD4+ Cells in the Absence of Exogenous IL-2
Having shown that bulk TIL1913 cells (containing 15% CD4+ and 85% CD8+ cells) could proliferate after the destruction of autologous tumor cells when transduced with SBIL-2, we wanted to determine whether SBIL-2–transduced CD8+ cells alone could do the same.
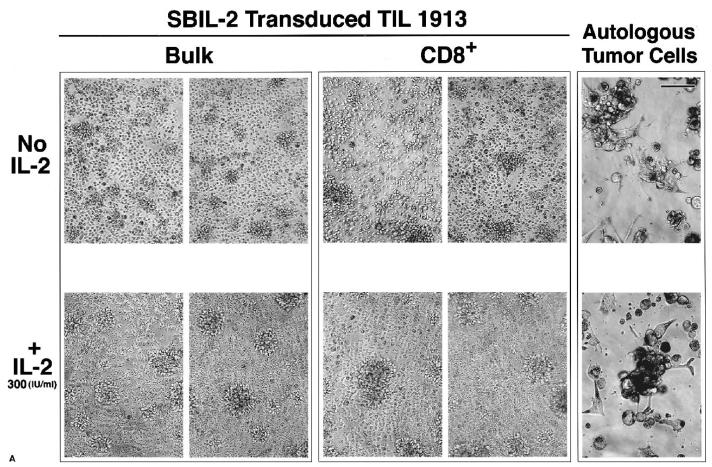
Five days after the initial transduction, SBIL-2–transduced bulk TIL1913 cells were expanded in the absence of IL-2 in a REP experiment and YFP-transduced cells were grown in the presence of 300 IU/mL IL-2. On day 14 of this REP, CD4+ cells were depleted using Dynal beads, with 99.5% cells being CD8+ cells after the depletion. These CD8+ cells were assayed for intracellular IL-2 by FACS and subjected to coculture assay with autologous tumor cells, as described previously without or with IL-2 (300 IU/mL). On day 12 from the initial onset of this coculture experiment, photographs of each well were taken.
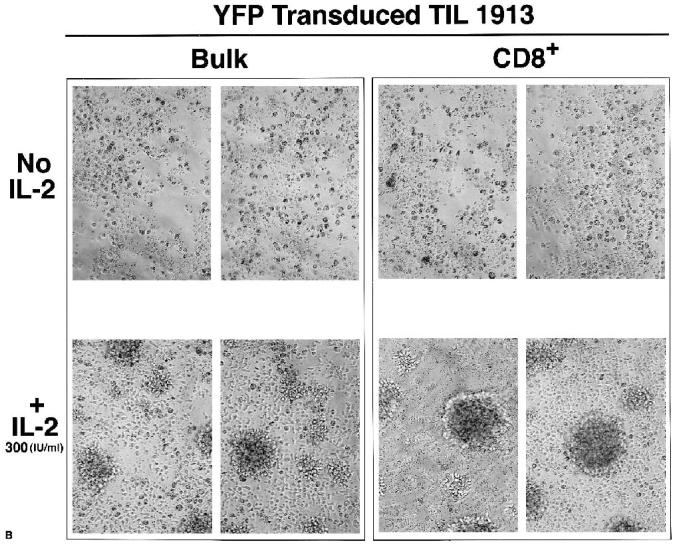
SBIL-2–transduced TILs produced intracellular IL-2, but YFP-transduced cells did not (Fig. 5, compare panels A and B). After an additional round of expansion in the absence of IL-2, bulk SBIL-2–transduced TILs still retained the property of destroying tumors in the absence of IL-2 and continued to proliferate (Fig. 6A, upper left panel), confirming the data presented in Figure 4. The same pattern was seen for CD4-depleted cells: These SBIL-2–transduced CD8+ cells proliferated after the destruction of tumor cells (Fig. 6A, upper middle panel). In contrast, neither bulk nor CD8+ cells of YFP-transduced cultures could do so under the same conditions (Fig. 6B, upper panels). In the presence of IL-2, no difference was seen between transduced or untransduced cultures: All cells could kill the tumor and subsequently proliferated (Fig. 6A and B, lower panels).
FIG. 5.
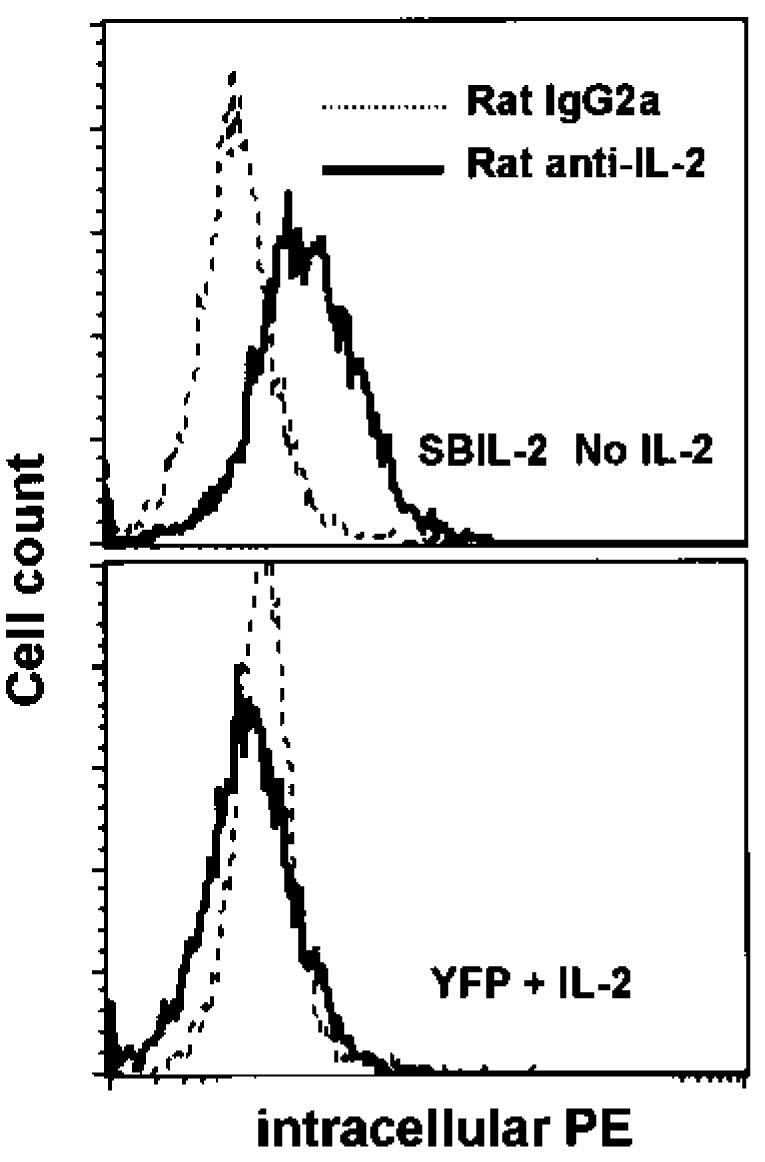
SBIL-2–transduced TIL1913 cells produced intracellular IL-2 after additional expansion. Seven days after transduction, SBIL-2–transduced TIL cells were expanded in a REP protocol without IL-2 and YFP-transduced cells were expanded in the presence of 300 IU/mL IL-2. Fourteen days from the onset of this expansion, CD4+ cells were depleted using Dynal beads. The remaining CD8+ cells were washed with medium without IL-2 three times before replating onto the wells of a 24-well plate precoated with OKT3 without IL-2. After overnight stimulation in the presence of Golgi stop, cells were harvested and subjected to intracellular FACS with PE-conjugated antibodies. Shown here are histograms of cells stained for IL-2 superimposed over those of cells stained with an isotype-control antibody. Only SBIL-2–transduced TIL cells were positive for intracellular IL-2.
FIG. 6.
SBIL-2–transduced CD8+ TIL cells alone could destroy tumor cells and proliferate in the absence of added IL-2. Seven days after transduction, SBIL-2–transduced TIL1913 cells were expanded in a REP protocol without IL-2, and YFP-transduced cells were expanded in the presence of 300 IU/mL IL-2. Fourteen days from the onset of this expansion, CD4+ cells were depleted using Dynal beads. The remaining CD8+ cells and undepleted (bulk) cells were washed with medium without IL-2 three times before replating onto the wells of a 96-well plate containing preirradiated autologous tumor cells (100 Gy) without or with 300 IU/mL IL-2, as described in Figure 4. Six days from the onset of the coculture with tumor cells, each well was split one to two and 100 μL fresh medium with or without IL-2 was added. Twelve days after the onset of the coculture, photographs were taken of each well. (A) SBIL-2–transduced cells (scale bar, 100 μm). (B) YFP-transduced cells. Upper panels, no IL-2. Lower panels, +IL-2.
These data showed that when CD8+ TILs transduced with an exogenous IL-2 gene alone, they were able to produce IL-2 and proliferate after the destruction of autologous tumor cells without help from CD4+ cells.
DISCUSSION
In this study, we found that TILs transduced with an exogenous IL-2 gene could proliferate after the stimulation by and the destruction of autologous tumor cells in the absence of added IL-2. This IL-2–independent growth depended on tumor stimulation. Without further stimulation, these cells stopped proliferating and eventually died.
Our goal for the genetic modification of antitumor T lymphocytes with the IL-2 gene was to use them for adoptive immunotherapy in patients with cancer. Our finding that tumor stimulation could support the growth of antitumor IL-2–transduced T cells in the absence of added IL-2 may have profound implications for adoptive immunotherapy. In the presence of tumor, these cells can be stimulated to grow and eradicate tumor cells. When there no tumor antigens are present, these cells will no longer grow and will eventually be eliminated. Therefore, this restimulation-dependent characteristic of IL-2–transduced T cells could serve as a safeguarding mechanism in vivo when they are adoptively transferred, making them ideal candidates for the adoptive immuno-therapy of patients with cancer.
Because of the establishment of an IL-2 autocrine loop of these IL-2 gene transductants, there is a theoretical concern that this could have led to their transformation to cancer. Our data do not support this because neither IL-2YFP–transduced PBMCs nor SBIL-2–transduced TILs continued to grow without further stimulation in the absence of added IL-2. As a further test, we analyzed the growth of 16 individual T-cell cultures retrovirally transduced with an IL-2 gene in the absence of exogenous IL-2. These cultures included TILs, CD8+ T-cell clones, and T cells from PBL. All cultures stopped growing and eventually died without further restimulation.
One mechanism that regulates the stimulation-dependent growth exhibited by IL-2–transduced T cells appears to be at the level of the expression of the transduced IL-2 gene. At the end of a given growth cycle before restimulation, the level of the expression of the transduced IL-2 gene was significantly decreased (Fig. 1A). With restimulation, the transgene was activated and an autocrine loop was reestablished (12). These results also correspond with other reports that expression of transgenes in primary T cells is positively correlated with the activation status of the transductants (17,18). The mechanisms involved in the decreased expression of the transgene without restimulation are not known. Among other possibilities, cytokine-induced suppressors may play a role in the negative regulation of the activated T lymphocytes. It has been shown that these suppressors bind and inactivate key regulators such as STAT-5, or the kinase domain of the Janus family of protein tyrosine kinases (19), thus shutting off positive growth signal transduction and converting the cells into a quiescent state. Similarly unclear is how stimulation of T cells upregulates transgene expression. Preliminary data suggested that transcription factors involved in the activation of the retroviral promoter might mediate the increase in transgene expression in T cells activated by anti-CD3 and anti-CD28 (20). Thus, one strategy to further increase the transgene expression in retrovirally modified T cells might be to replace the retroviral promoter with a strong element. One example is the CMMP retroviral construct in which the murine leukemia virus LTRs are replaced with the corresponding myeloproliferative sarcoma virus LTRs and the normal murine leukemia virus tRNA primer binding site is replaced by a glutamine tRNA primer binding site (21).
The expression of CD25 (Tac), the alpha chain of the IL-2 receptor complex, is upregulated by antigenic stimulation and by IL-2 itself. Downregulation of CD25 is hypothesized to be one of the mechanisms by which the activated T cells stop responding to IL-2 in vitro (16). The level of CD25 expression on the cell surface of IL-2YFP transductants on day 34 of REP4 before they died without restimulation in the absence of exogenous IL-2 was dramatically decreased when compared with the amount of CD25 during the peak of their growth (compare Figures 2B and D). Consequently, it would be interesting to determine whether the growth of IL-2 transductants can be enhanced by the introduction of an exogenous constitutive CD25 gene. To test this possibility, we are actively trying to retrovirally transduce anti-melanoma T lymphocytes with IL-2 and CD25 genes.
The role of CD4+ cells in mediating tumor regression after adoptive transfer remains to be defined. Our previous data, in limited numbers of patients, suggested that adoptive transfer of heterogeneous TIL populations containing both CD8+ and CD4+ cells were more effective than the transfer of highly avid melanoma-reactive CD8+ clones in mediating tumor regression in patients with metastatic melanoma (7,14,22). Conceivably, these CD4+ cells could have provided necessary cytokines such as IL-2 to sustain the survival of CD8+ cells in vivo. The finding of this study that CD8+ cells alone when transduced with an exogenous IL-2 gene could destroy tumor cells and proliferate (Fig. 6A, middle panel) supports the notion that these IL-2–CD8+ transductants may not need help from CD4+ cells to proliferate and destroy tumor cells in vitro. Whether they can mediate tumor regression in vivo without CD4+ help must be proved.
Based on the findings in this study, we developed a clinical protocol to retrovirally transduce human melanoma-reactive TIL cells with an IL-2 gene (SBIL-2) to investigate the fate and therapeutic effect of these IL-2 gene–modified cells when transferred to patients with metastatic melanoma. Accrual of patients to this clinical protocol is expected to begin soon.
Acknowledgment
The authors thank Dr. Mark Dudley for helpful discussions; Dr. John Wunderlich and the TIL laboratory of the Surgery Branch for providing T cells and tumor cells; Arnold Mixon and Shawn Farid for their technical support for FACS analyses and sorting; and Rick Dreyfuss for photographic assistance.
REFERENCES
- 1.Sprent J. T-cell survival and the role of cytokines. Immunol Cell Biol. 2001;79:199–206. doi: 10.1046/j.1440-1711.2001.00999.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki TZ, Liu J, Kawahara A, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 4.Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J Immunol. 1998;161:4627–4633. [PubMed] [Google Scholar]
- 5.Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 6.Akbar AN, Borthwick NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not proapoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Yang JC, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with meta-static melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke RC, Cohen JJ. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 9.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley M, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L, Guan Y, Li L, et al. Expression in normal human tissues of five nucleotide excision repair genes measured simultaneously by multiplex reverse transcription-polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 1999;8:801–807. [PubMed] [Google Scholar]
- 16.Waldmann TA. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 17.Pollok KE, Hanenberg H, Noblitt TW, et al. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol. 1998;72:4882–4892. doi: 10.1128/jvi.72.6.4882-4892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plavce I, Agarwal M, Ho KE, et al. High transdominant RevM10 protein levels are required to inhibit HIV-1 replication in cell lines and primary T cells: implication for gene therapy of AIDS. Gene Ther. 1997;4:128–139. doi: 10.1038/sj.gt.3300369. [DOI] [PubMed] [Google Scholar]
- 19.Yasukawa H, Sasaki S, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Pollok KE, van der Loo JC, Cooper RJ, et al. Costimulation of transduced T lymphocytes via T cell receptor-CD3 complex and CD28 leads to increased transcription of integrated retrovirus. Hum Gene Ther. 1999;10:2221–2236. doi: 10.1089/10430349950017202. [DOI] [PubMed] [Google Scholar]
- 21.Klein C, Bueler H, Mulligan RC. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med. 2000;191:1699–1708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with anti-tumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]