Abstract
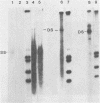
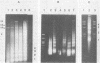
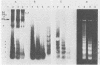
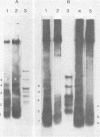
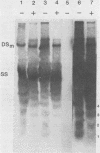
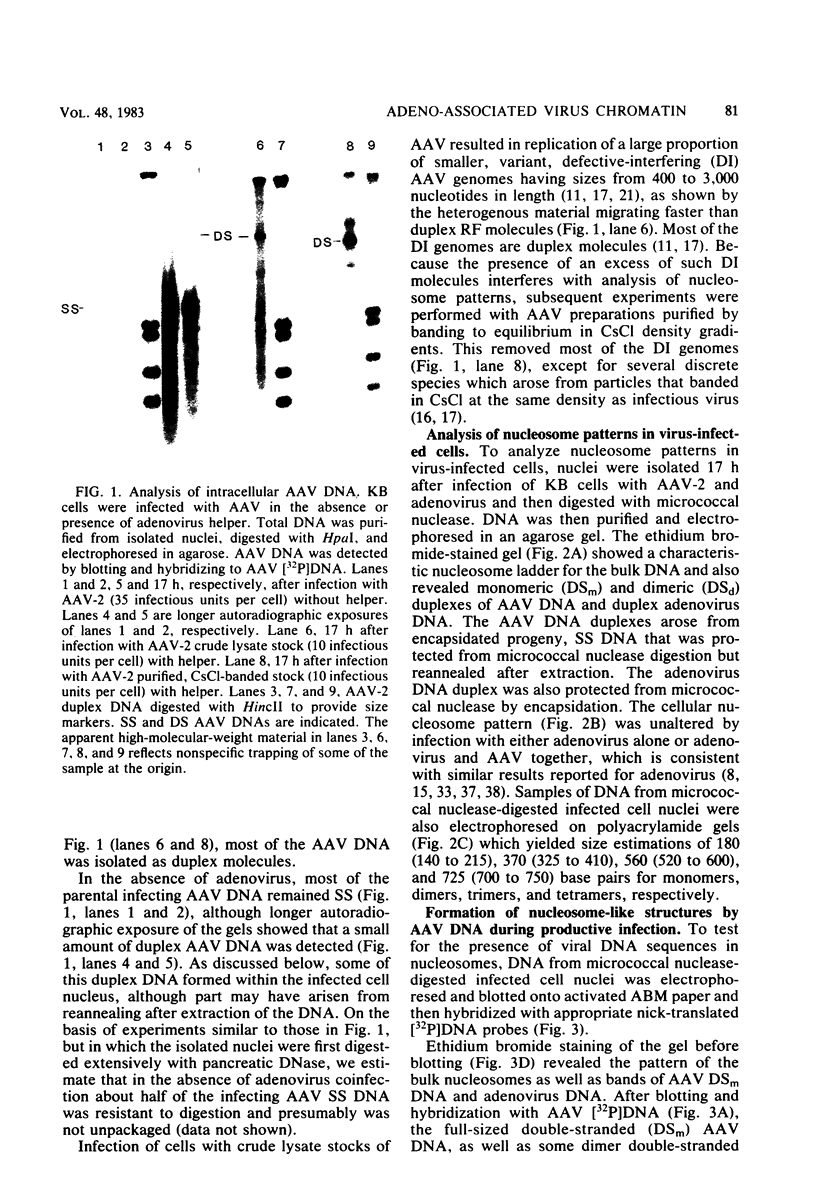
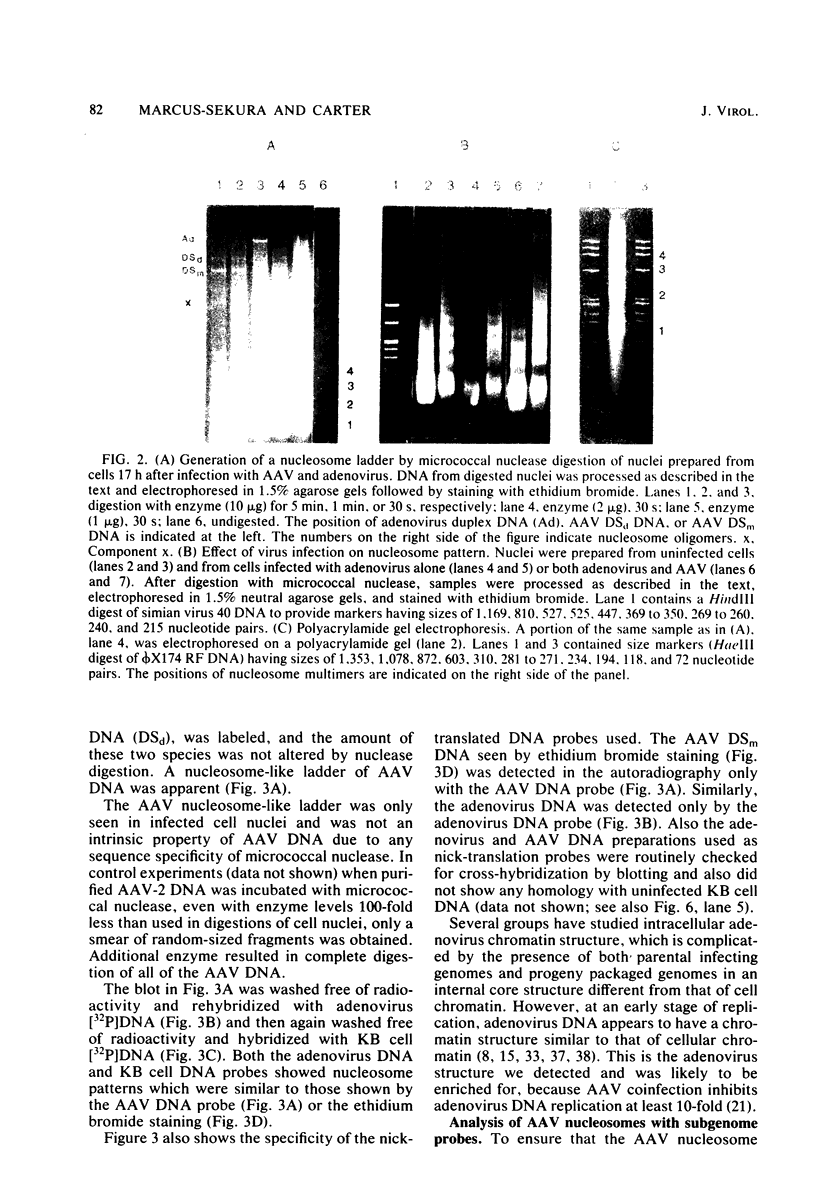
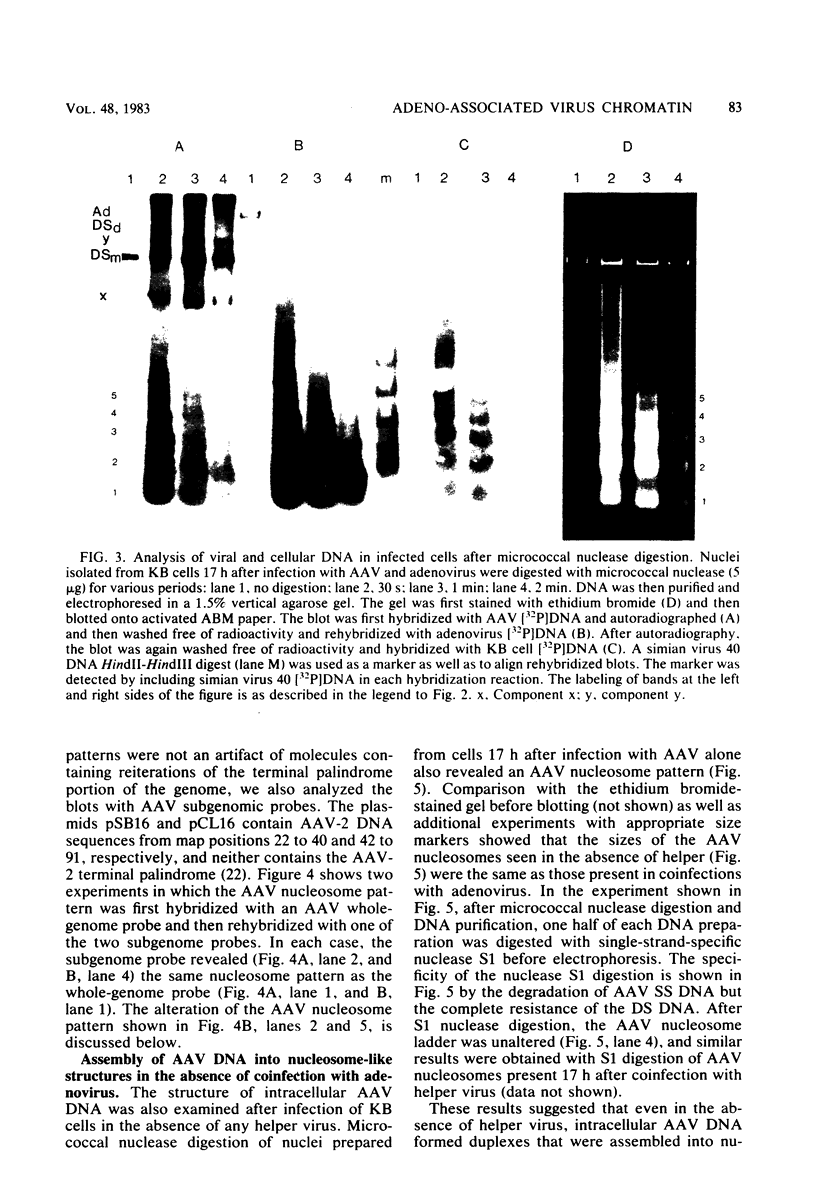
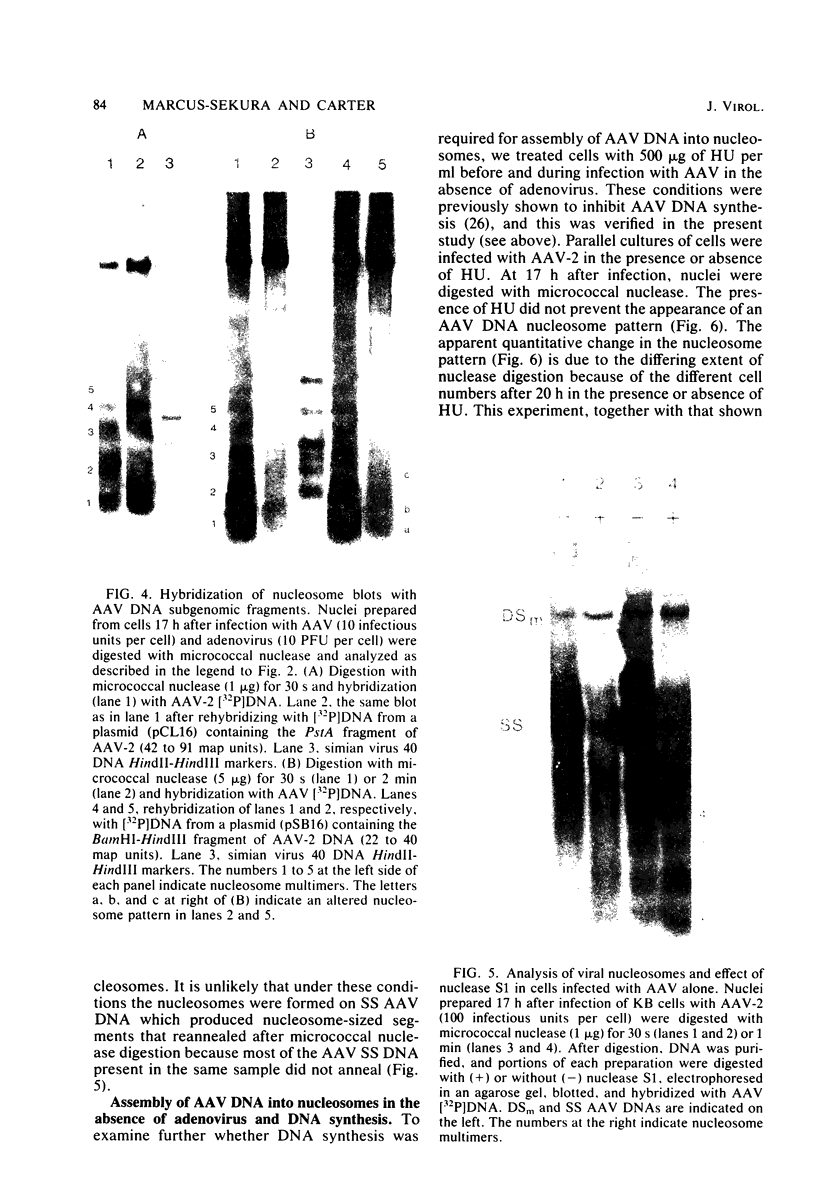
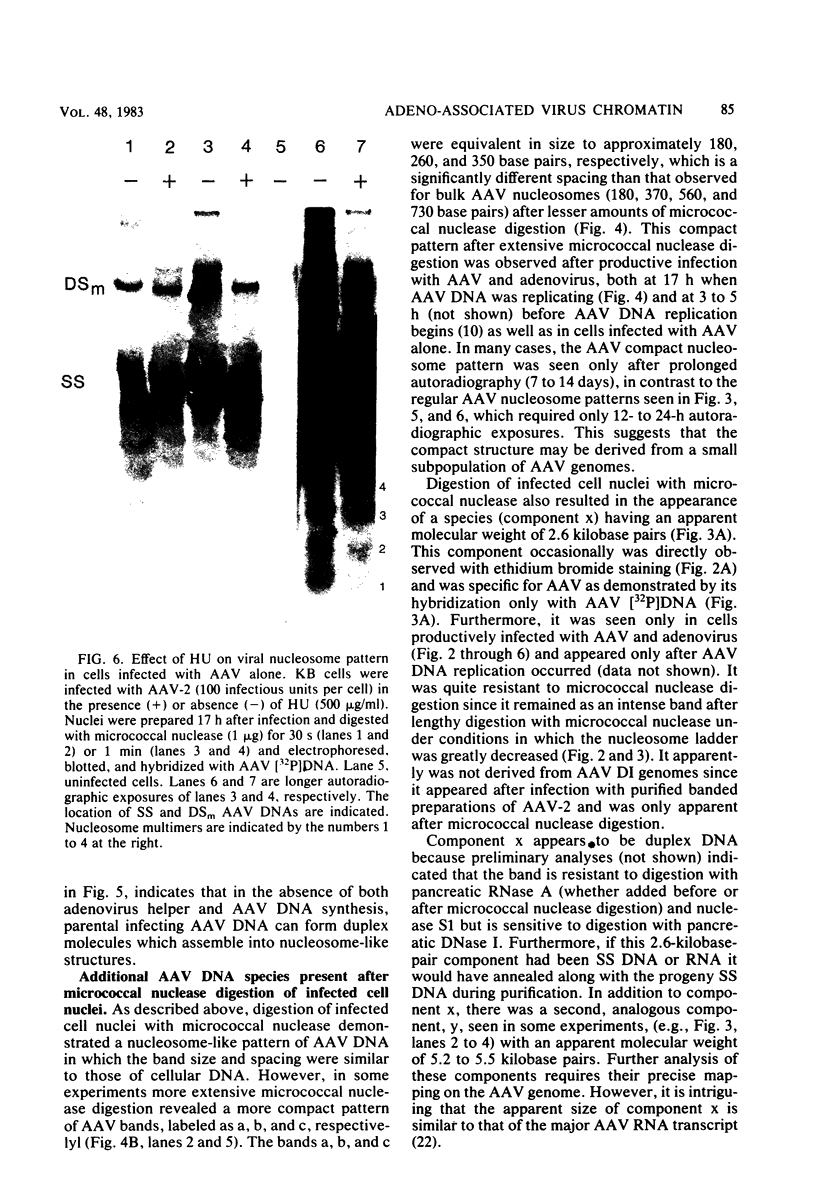
Adeno-associated virus (AAV) is a single-stranded DNA virus which requires adenovirus as a helper for productive infection. We studied whether intracellular AAV DNA in KB cells was present in a chromatin-like structure by digesting infected cell nuclei with micrococcal nuclease. Virus DNA was detected by agarose gel electrophoresis followed by blotting and hybridization to nick-translated [32P]DNA probes. After coinfection with adenovirus, AAV DNA was present in nucleosome-like structures which were similar to cell nucleosomes and were double stranded as judged by insensitivity to S1 nuclease digestion. In the absence of adenovirus, intracellular AAV DNA also formed similar nucleosome-like structures which were also insensitive to S1 digestion and were formed in both the presence and absence of hydroxyurea. These latter structures probably formed on AAV duplexes created either by reassociation of infecting parental single-stranded DNA or by covalent integration into the cell genome rather than by de novo AAV DNA synthesis. These results have implications for the mechanism of AAV genome replication, transcription, and integration into the cell genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Beard P. Mobility of histones on the chromosome of simian virus 40. Cell. 1978 Nov;15(3):955–967. doi: 10.1016/0092-8674(78)90279-9. [DOI] [PubMed] [Google Scholar]
- Ben-Asher E., Bratosin S., Aloni Y. Intracellular DNA of the parvovirus minute virus of mice is organized in a minichromosome structure. J Virol. 1982 Mar;41(3):1044–1054. doi: 10.1128/jvi.41.3.1044-1054.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W., Fife K. H., Lusby E. Adeno-associated virus DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):781–787. doi: 10.1101/sqb.1979.043.01.085. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Pinkerton T. C., Thomas G. F., Hoggan M. D. Detection of adeno-associated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology. 1975 Dec;68(2):556–560. doi: 10.1016/0042-6822(75)90298-6. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Immunofluorescent studies of the potentiation of an adenovirus-associated virus by adenovirus 7. J Exp Med. 1967 May 1;125(5):755–765. doi: 10.1084/jem.125.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Cambon P. A simple and efficient method to remove ribonuclease contamination from pancreatic deoxyribonuclease preparations. Anal Biochem. 1976 Oct;75(2):402–409. doi: 10.1016/0003-2697(76)90094-4. [DOI] [PubMed] [Google Scholar]
- Brown M., Weber J. Adenoassociated virus has a unique chromatin structure. Can J Biochem. 1982;60(10):1001–1005. doi: 10.1139/o82-128. [DOI] [PubMed] [Google Scholar]
- Brown M., Weber J. Virion core-like organization of intranuclear adenovirus chromatin late in infection. Virology. 1980 Nov;107(1):306–310. doi: 10.1016/0042-6822(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Laughlin C. A., de la Maza L. M., Myers M. Adeno-associated virus autointerference. Virology. 1979 Jan 30;92(2):449–462. doi: 10.1016/0042-6822(79)90149-1. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Marcus-Sekura C. J., Laughlin C. A., Ketner G. Properties of an adenovirus type 2 mutant, Ad2dl807, having a deletion near the right-hand genome terminus: failure to help AAV replication. Virology. 1983 Apr 30;126(2):505–516. doi: 10.1016/s0042-6822(83)80008-7. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Transcription in vivo of a defective parvovirus: sedimentation and electrophoretic analysis of RNA synthesized by adenovirus-associated virus and its helper adenovirus. Virology. 1974 Sep;61(1):182–199. doi: 10.1016/0042-6822(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Hoggan M. D., Hauswirth W. W., Berns K. I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980 Feb;33(2):739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Groff D. E., Fedor M. J. Adenovirus chromatin structure at different stages of infection. Mol Cell Biol. 1981 Dec;1(12):1094–1105. doi: 10.1128/mcb.1.12.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Shiroki K., Shimojo H. Establishment and characterization of KB cell lines latently infected with adeno-associated virus type 1. Virology. 1977 Oct 1;82(1):84–92. doi: 10.1016/0042-6822(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Laughlin C. A., Myers M. W., Risin D. L., Carter B. J. Defective-interfering particles of the human parvovirus adeno-associated virus. Virology. 1979 Apr 15;94(1):162–174. doi: 10.1016/0042-6822(79)90446-x. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Laughlin C. A., Carter B. J. Adeno-associated virus RNA transcription in vivo. Eur J Biochem. 1981 Dec;121(1):147–154. doi: 10.1111/j.1432-1033.1981.tb06443.x. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Carter B. J. Adeno-associated virus replication. The effect of L-canavanine or a helper virus mutation on accumulation of viral capsids and progeny single-stranded DNA. J Biol Chem. 1981 Jan 25;256(2):567–570. [PubMed] [Google Scholar]
- Myers M. W., Carter B. J. Assembly of adeno-associated virus. Virology. 1980 Apr 15;102(1):71–82. doi: 10.1016/0042-6822(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Duckworth D. H., Berns K. I. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology. 1981 Sep;113(2):521–533. doi: 10.1016/0042-6822(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Foe V. E., Alberts B. M. Evidence for the formation of nucleosome-like histone complexes on single-stranded DNA. Cell. 1979 Oct;18(2):451–467. doi: 10.1016/0092-8674(79)90064-3. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981 Nov;27(1 Pt 2):133–141. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Van Holde K. E. Compact oligomers and nucleosome phasing. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3583–3587. doi: 10.1073/pnas.75.8.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayda M. E., Rogers A. E., Flint S. J. The structure of nucleoprotein cores released from adenovirions. Nucleic Acids Res. 1983 Jan 25;11(2):441–460. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischet W. O., Van Holde K. E. Nuclease digestion promotes structural rearrangements in H1-depleted chromatin. Nucleic Acids Res. 1980 Sep 11;8(17):3743–3755. doi: 10.1093/nar/8.17.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Heavy and light particles of adeno-associated virus. J Virol. 1980 Mar;33(3):1129–1137. doi: 10.1128/jvi.33.3.1129-1137.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Molecular structure of adeno-associated virus variant DNA. J Biol Chem. 1980 Apr 10;255(7):3194–3203. [PubMed] [Google Scholar]