Abstract
The functional status of circulating vaccine-induced, tumor-specific T cells has been questioned to explain their paradoxical inability to inhibit tumor growth. We enumerated with HLA-A*0201/peptide tetramers (tHLA) vaccine-elicited CD8+ T cell precursor frequency among PBMC in 13 patients with melanoma undergoing vaccination with the HLA-A*0201-associated gp100: 209–217(210 M) epitope. T cell precursor frequency increased from undetectable to 12,400 ± 3,600 × 106 CD8+ T cells after vaccination and appeared heterogeneous according to previously described functional subtypes: CD45RA+CD27+ (14 ± 2.6% of tHLA-staining T cells), naive; CD45RA−CD27+ (14 ± 3.2%), memory; CD45RA+CD27− (43 ± 6%), effector; and CD45RA−CD27− (30 ± 4.1%), memory/effector. The majority of tHLA+CD8+ T cells displayed an effector, CD27− phenotype (73%). However, few expressed perforin (17%). Epitope-specific in vitro stimulation (IVS) followed by 10-day expansion in IL-2 reversed this phenotype by increasing the number of perforin+ (84 ± 3.6%; by paired t test, p < 0.001) and CD27+ (from 28 to 67%; by paired t test, p = 0.01) tHLA+ T cells. This conversion probably represented a change in the functional status of tHLA+ T cells rather than a preferential expansion of a CD27+ (naive and/or memory) PBMC, because it was reproduced after IVS of a T cell clone bearing a classic effector phenotype (CD45RA+CD27−). These findings suggest that circulating vaccine-elicited T cells are not as functionally active as inferred by characterization of IVS-induced CTL. In addition, CD45RA/CD27 expression may be more informative about the status of activation of circulating T cells than their status of differentiation.
Active-specific vaccination against melanoma using peptide epitopes derived from melanoma Ags (MAGE-1, MAGE-3, NY-ESO-1, MART-1, and gp100) demonstrated effectiveness in inducing vaccine-specific T cells that can recognize HLA-matched melanoma cells (1, 2). However, these immune responses are only sporadically associated with clinical regression (3). In addition, no correlation between clinical response and T cell precursor frequency (Tc-pf)3 could be identified by direct ex vivo HLA/epitope tetrameric complexes (tHLA) enumeration of vaccine-induced lymphocytes (2, 4).
Because tHLA-based phenotyping of T cells does not yield information about their effector function, it has been postulated that the lack of function of tumor Ag-specific T cells could be due to a suboptimal status of activation (5). Pittet et al. (6) noted that tumor Ag-specific T cells in patients with melanoma are, contrary to those analyzed in healthy individuals, CD45RAlow and can secrete IFN-γ upon cognate stimulation compatible with a functional differentiation into competent effector/memory T cells in vivo. We have previously argued that vaccine-induced T cells should be at least partially functional based on the fact that they can express IFN-γ mRNA and protein ex vivo upon cognate stimulation (7). Stimulation with vaccine-related epitopes induced IFN-γ expression detectable by intracellular cytokine analysis and quantitative real-time PCR, suggesting that vaccine-elicited CTL retain Ag responsiveness (4). However, recent studies suggest that IFN-γ expression by T cells may not be the functional parameter that might best describe their potential as effector cells. Appay et al. (8) observed in patients with chronic HIV infection that CMV-specific differ from HIV-specific circulating CD8+ T cells because they express significantly lower levels of perforin despite retaining the ability to secrete IFN-γ in response to epitope stimulation. In addition, these lymphocytes retained the expression of CD27, which was interpreted by the authors as a sign of impaired maturation toward fully developed effector cells. It is therefore possible that Ag-experienced vaccine-induced T cells may be in an incomplete stage of differentiation, which explains their limited effect on tumor growth. Functionally distinct phenotypes of CD8+ T cells spanning from a naive to an effector and/or memory stage of differentiation have been described by several groups (9, 10). According to Hamman et al. (9), memory-type CD8+ T cells are CD45RA−CD27+, can express a broad range of cytokines (IL-2, IFN-γ, TNF-α, and IL-4) in response to cognate stimulation, and do not display cytolytic activity without prior in vitro stimulation (IVS). In contrast, effector T cells have a CD45RA+C27− phenotype, release a more stringent array of cytokines upon stimulation (IFN-γ and TNF-α), and display strong cytolytic activity with high expression of perforin and granzyme without the need for in vitro prestimulation. Thus, combining phenotypic with functional proprieties, the authors suggested four distinct populations of CD8+ T cells depending on their CD45RA and CD27 expression levels: naive T cells (CD45RA+CD27+), effector T cells (CD45RA+CD27−), memory T cells (CD45RA−CD27+), and effector/memory T cells (CD45RA−CD27−). This classification has been extensively used subsequently in the context of various infectious diseases and other immune pathologies (11–13).
Independent of their intrinsic status of activation and/or differentiation, circulating lymphocytes need to be able to localize in target organs to exert their effector function. It has been suggested that two subsets of memory T lymphocytes can be identified with distinct homing potentials and effector functions based on their level of expression of the chemokine receptor CCR7 (14). CCR7− memory cells express receptors for migration to target tissues and are ready to exert their effector function. Conversely, CCR7+ memory cells express lymph node-homing receptors and lack immediate effector function. Champagne et al. (15) suggested that CD8+ T lymphocytes follow a consistent differentiation pattern that flows from CD45RA+CCR7+→CD45RA−CCR7+→ CD45RA−CCR7−3CD45RA+CCR7−. In HIV patients, HIV-specific CD8+ T cell populations appear to be composed predominantly of the preterminally differentiated CD45RA−CCR7− subset, while, at the same time, CMV-specific CD8+ T cells display in large majority a terminally differentiated phenotype (CD45RA+ CCR7−). Thus, it appears from the literature that T cells differentiate upon Ag exposure through a continuum display of evolving functions that might, in turn, affect the overall effectiveness of a given immune response.
Epitope-specific vaccination offers the unique opportunity of evaluating the immune response in relation to a well-defined time of Ag exposure. In particular, immunization with the HLA-A* 0201-associated gp100:209–217(210M) epitope (g209-2M) dramatically converts a largely undetectable T cell response in vaccine-naive patients to the frequently observable induction of vaccine-specific CD8+ T cells documentable by tHLA phenotyping (2). Thus, subsets of Ag-experienced/memory T cells can be characterized in their phenotypic and functional properties. Therefore, in this study we determined the expression of CD45RA and CD27 in epitope-specific CD8+ T cells. Expression was determined from the PBMC of patients undergoing vaccination with g209-2M modified from the wild-type epitope of the melanoma Ag gp100/Pmel17 by substitution of a methionine in position 2 (16). This peptide has been previously shown to efficiently induce epitope-specific CD8+ T cell responses in most patients when administered emulsified in IFA (17). These responses could be easily identified using tHLA complexes reconstituted with either the g209-2M or the wild-type gp100:209–217 (g209) peptide (2). Thus, the goal of this study was to characterize the CD45RA CD27 profile of tHLA+ vaccine-induced CD8+ T cells with the assumption that these cells are Ag-experienced, because none was detectable before vaccination. CCR7 expression was also evaluated in a subpopulation of patients because of its predictive significance concerning the ability of T cells to localize and exert effector function in the target organ. In addition, perforin expression was included in the phenotypic characterization as a marker of “ready for action” effector activity. More specifically, in this study we questioned whether these molecules could be regarded as informative markers of the level of differentiation and/or function of immunization-induced T cells.
Materials and Methods
Patients’ selection
In this study we randomly selected 13 patients with metastatic melanoma among 20 who demonstrated a specific immune response to vaccination with a gp100 peptide. These 20 immunization-responsive patients were identified among 35 HLA-A*0201 patients with melanoma. These 35 patients had received repeated s.c. injections of the g209-2M peptide in IFA in a protocol approved by the institutional review board of the National Cancer Institute. No concomitant treatment, including IL-2, was administered. The HLA class I phenotype of patients was determined in PBMC using sequence-specific primer-PCR (18). All patients had been surgically determined to be without evidence of disease before enrollment in the vaccination protocol that was administered with adjuvant purposes. Thus, no correlation with clinical outcome could be evaluated in this study.
Peptide
The g209-2M (IMDQVPFSV) peptide used for vaccination was prepared according to good manufacturing practice by Multiple Peptide Systems (San Diego, CA). The g209-2M peptide used for tHLA synthesis and IVS studies was commercially synthesized by Princeton Biomolecules (Columbus, OH). The peptides were purified by gel filtration to >95% purity, and their identities were confirmed by mass spectral analysis.
Cells and culture conditions
PBMC were obtained by leukapheresis both before the patients received their first vaccine and 3 wk after vaccination. As discussed in Results, samples were obtained from patients who had received different numbers of vaccinations administered according to various time schedules. PBMC were isolated by Ficoll gradient separation and frozen until analysis. Analysis of vaccine-specific T cells was performed after overnight resting of thawed PBMC in complete medium consisting of Iscove’s medium (Biofluids, Rockville, MD) supplemented with 10 mM HEPES buffer, 100 U/ml penicillin-streptomycin (Biofluids), 10 µg/ml ciprofloxacin (Bayer, West Haven, CT), 0.03% L-glutamine (Biofluids), 0.5 mg/ml amphotericin B (Biofluids), and 10% heat-inactivated human AB serum (Gemini Bioproducts, Calabasas, CA). This step allowed depletion of adherent monocytes.
The PBMC were also analyzed 10 days following IVS with 1 µM g209-2M peptide, followed by culture in IL-2 (300 IU/ml)-containing medium that was added on the day following stimulation and every 2–3 days thereafter. Bulk CTL cultures were cloned by limiting dilution according to a modification of Riddell’s technique (19, 20). Briefly, bulk CTL cultures were plated in 96-well plates at 0.6 cell/well with OKT3 (30 ng/ml), 50,000/well irradiated allogeneic PBMC (3,000 rad), and IL-2 (300 IU/ml). The clone used for this study (P1G9) was selected according to its reactivity against HLA-matched, gp100-expressing tumor targets as previously described (21). For stimulation of T cells, two melanoma cell lines were used characterized by expression (624.38 MEL) or lack of expression (624.28 MEL) of HLA-A*0201.
Kinetics of surface marker expression in response to Ag-specific stimulation
The g209- and g209-2 M-specific CTL clone P1G9 was cocultured in the presence of the HLA-A*0201/gp100-expressing melanoma cell line 624.38 MEL or a sister clone (624.28 MEL) that lacked expression of HLAA* 0201 but retained expression of gp100 (22, 23). Coculture was performed at a 1:4 cancer cell:CTL ratio in a six-well Costar plate (Corning Glass, Corning, NY) in complete medium at a concentration of 106 CTL/ml. Twenty-four hours after starting the coculture IL-2 (300 IU/ml) was added and was replenished every 2–3 days. Cocultured cells were harvested at various time intervals (as described for individual experiments) and analyzed by FACS analysis. To simulate the conditions of high epitope density used for IVS of PBMC, P1G9 clone was also stimulated in vitro with 1 µM g209-2M peptide. However, this stimulation could not be performed by directly adding soluble peptide to the medium as was done for the PBMC because this caused extensive apoptotic death of the T cell clone. To avoid this, g209-2M peptide was pulsed on 624.38 MEL and 624.28 MEL for 1 h and then washed away. The melanoma cell lines were then used for epitope presentation in conditions identical with those described previously.
T cell staining
Tetrameric peptide-HLA-A*0201 complexes were produced as described previously (2, 24). Recombinant HLA-A*0201 H chain containing a biotinylation site and recombinant β2-microglobulin were synthesized and used for refolding of soluble HLA molecules in the presence of g209-2M. Monomeric HLA/peptide complexes were biotinylated with BirA (Avidity, Denver, CO) and tetramerized by adding avidin-PE (Pierce, Rockford, IL).
Purified anti-CD27 mAb was obtained from BD PharMingen (San Diego, CA) and was used with the secondary PE-conjugated rat anti-mouse IgG1 from BD Biosciences (San Jose, CA). PE-conjugated anti-CD27 mAb was obtained from BD PharMingen. FITC-conjugated mAb against CD45RA was obtained from Caltag Laboratories (Burlingame, CA). Purified CCR7-specific mAb was obtained from BD PharMingen and was used with the secondary CY5.5-conjugated rat anti-mouse IgM from BD Biosciences. Perforin staining was performed using the BD Biosciences kit. CD8 mAb was obtained from BD Biosciences.
After overnight depletion of monocytes (or 10–11 days following IVS), nonadherent PBMC were resuspended at 106 cells/50 µl FACS buffer (phosphate buffer plus 5% FCS; Biofluids). Cells were incubated at 4°C with 1 µl tHLA for 15 min, and then incubation was continued for 30 min with the specific mAb. A similar staining procedure was applied for staining CTL cultures and the CTL clone P1G9. After tHLA staining, cells were directly stained with Abs for surface markers (CD27, CD8, CD45, CCR-7) and kept for 45 min at 4°C or fixed in 4% paraformaldehyde and then permeabilized with acetone as previously described (25) for perforin staining. The totally CD8+ P1G9 clone was stained with CD27-PE, CD45RA-FITC, tHLA-PE, CD8-PerCP, and Vβ17-FITC (Endogen, Woburn, MA). After staining, cells were washed in 2 ml FACS buffer and analyzed by FACS (BD Biosciences). The acquisition data were analyzed after pregating for lymphocyte size and tetramer positivity. The CTL clone P1G9 was discriminated from cocultured cancer cells based on forward scatter as described in Results. Twenty thousand events were acquired for analysis of the T cell clone, and 200,000 were used for PBMC or CTL after IVS.
Statistical analysis
Vaccine-specific Tc-pf was calculated as the number of g209 tHLA-staining CD8+ T cells per 106 CD8+ cells adopting the following formula: f = URQ/(URQ + LRQ) × 106 CD8+ cells, with URQ (upper right quadrant) containing the tHLA+CD8+ cells and LRQ (lower right quadrant) containing all other CD8+ cells. From these frequencies the background with CD8+ staining only was subtracted for each sample to obtain the corrected frequency. The corrected Tc-pf is presented as the number of vaccine-specific T cells per 106 CD8+ T cells.
Comparison of values between pre- and postimmunization specimens or comparing identical specimens before and after IVS was performed using a paired sample t test. The p values are reported as the level of significance for a two-tailed analysis.
Results
Priming of circulating CD8+ T cells by epitope-based immunization
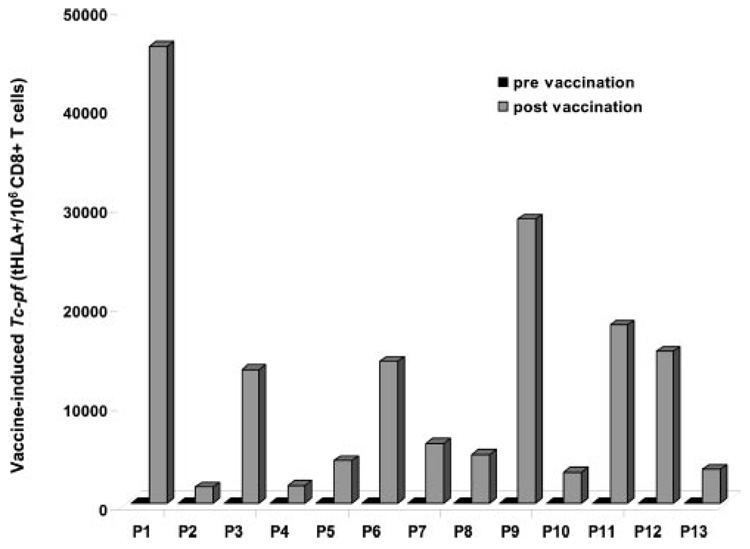
PBMC from 13 HLA-A*0201-expressing patients with metastatic melanoma undergoing repeated immunization with s.c. injection of g209-2M peptide emulsified in IFA were phenotypically characterized by g209-2M tHLA staining of CD8+ T cells. For this study PBMC were selected from patients who had developed detectable vaccine-specific Tc-pf after immunization (Fig. 1). Tc-pf was considered detectable when >1,000 g209-2M tHLA-staining T cells were counted per 106 CD8+ T cells. This value, according to our previous experience, corresponded to evidence of immunization and correlated with results obtained by IVS (2) or functional ex vivo assays (7). In none of these patients could >1,000 g209-2M tHLA staining T cells/106 CD8+ T cells be detected in vaccine naive settings, a finding consistent with previous reports (2, 4, 7). After immunization, Tc-pf ranged between 1,600 and 46,100 × 106 CD8+ T cells. This broad range in Tc-pf is not uncharacteristic of vaccine-induced immune responses. In this particular case the different schedule of administration and the different number of vaccinations received by individual patients (Table I) might have affected Tc-pf (4). In fact, the patients included in this study were among those participating in a randomization protocol in which different schedules of administration of the g209-2M in IFA were compared. According to the arm assigned, the patients received the peptide injection weekly or at 3-wk intervals or for 4 consecutive days at 3-wk intervals. In each arm of the study the patients received four cycles of the assigned schedule and were then immunologically reassessed 3 wk after administration of the last peptide injection by obtaining a leukapheresis to compare vaccine-induced Tc-pf with pretreatment samples. This study was designed to compare the effectiveness of different administration schedules, and it is still under investigation. Therefore, the results are beyond the purpose of this report. However, PBMC were randomly sampled from individuals who were treated in different arms of this protocol and who had received a different number of vaccinations to address whether any of these factors may bear any effect on the phenotypic characteristics of vaccine-induced T cells and deserve further investigation. Thus, the only constant parameter studied here is the phenotypic and functional portrait of vaccine-induced T cells obtained after different vaccination schedules 3 wk after the last administration of vaccine. Results obtained from the 13 patients studied suggested, as shown in the next section, that this factor significantly affected the CD45RA CD27 CCR7 phenotypic profile of vaccine-induced T cells and, therefore, the diversity of treatment schedule and the number of vaccinations were not investigated further. Thus, in all patients selected for this study Ag-experienced circulating CD8+ T cells were detected 3 wk after the last immunization that could be further characterized (9).
FIGURE 1.
Induction of vaccine-specific T cells by repeated immunization with g209-2M peptide. Vaccine-specific Tc-pf are shown in PBMC obtained before (■) and after ( ) vaccination in 13 patients undergoing immunization with g209-2M peptide administered s.c. emulsified in IFA. Tc-pf is shown as the number of tHLA-staining T cells per 106 CD8+ T cells.
) vaccination in 13 patients undergoing immunization with g209-2M peptide administered s.c. emulsified in IFA. Tc-pf is shown as the number of tHLA-staining T cells per 106 CD8+ T cells.
Table I.
Schedule and number of administrations and corresponding vaccine-induced T cell precursor frequencies in PBMC of patients with melanoma receiving immunization with g209-2M epitope emulsified in IFAa
| Patients | Schedule | No. of Immunizations Receivedb | Tc-pfc |
|---|---|---|---|
| 1 | Every 3 wk | 12 | 46,100 |
| 2 | Weekly | 4 | 1,600 |
| 3 | 4 consecutive days every 3 wk | 4 | 13,500 |
| 4 | 4 consecutive days every 3 wk | 4 | 1,800 |
| 5 | Every 3 wk | 4 | 4,300 |
| 6 | Weekly | 16 | 14,300 |
| 7 | Weekly | 8 | 6,000 |
| 8 | Every 3 wk | 8 | 4,900 |
| 9 | Every 3 wk | 8 | 28,700 |
| 10 | 4 consecutive days every 3 wk | 4 | 3,100 |
| 11 | 4 consecutive days every 3 wk | 8 | 18,000 |
| 12 | Weekly | 8 | 15,300 |
| 13 | Every 3 wk | 8 | 3,400 |
PBMC were randomly obtained before and after various numbers of immunizations from patients who belonged to various arms of a randomized clinical study where the length and frequency of immunization schedule is under investigation. The three randomization arms include one immunization every 3 wk, one immunization every week, and immunization for four consecutive days followed by 3 wk of rest.
The number of injections of vaccine received by the patients at the time when the specimen presented in this study was collected.
Tc-pf was calculated as number of g209 tHLA-staining CD8+ T cells per 106CD8+ cells adopting the following formula: f = URQ/(URQ + LRQ) × 106 CD8+cells, with URQ (upper center quadrant) containing the tHLA+CD8+ cells and LRQ (lower center quadrant) containing all other CD8+ cells. From these frequencies, the background with CD8+ staining only was subtracted for each sample to obtain the corrected frequency. The corrected Tc-pf includes this correction and is presented as the number of vaccine-specific T cells × 106 CD8+ T cells.
Phenotypic characterization of vaccine-induced circulating CD8+ T cell subsets based on CD45RA and CD27 expression
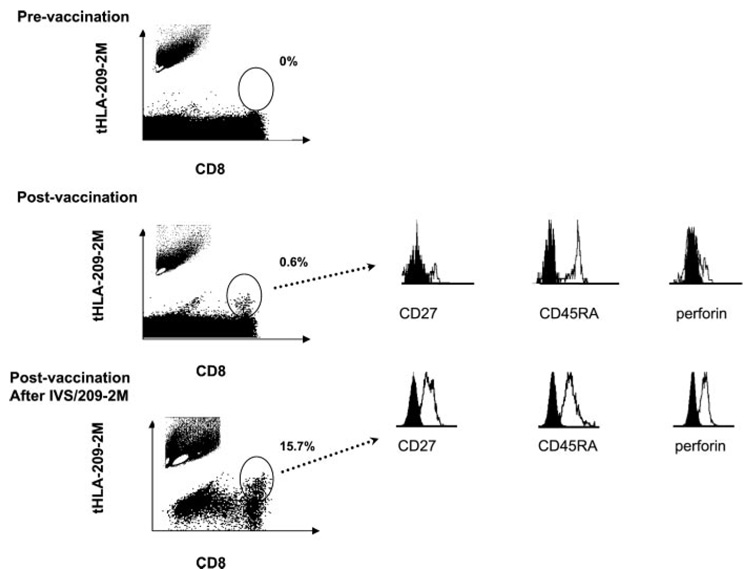
Vaccine-induced tHLA+CD8+ T cells were further characterized according to the classification suggested by Hamann et al. (9) based on CD45RA and CD27 expression (Fig. 2). The following subtypes were considered: CD45RA+CD27+ (naive), CD45RA−CD27+ (memory), CD45RA+CD27− (effector), and CD45RA− CD27− (effector/memory) (Table II). Circulating vaccine-induced T cells analyzed ex vivo were characterized by a small size, as demonstrated by side and forward scatter, suggesting a small size characteristic of resting T cells. In this respect they did not display any morphological feature significantly different from the remaining circulating CD8+ T cells. In the majority of cases vaccine-induced T cells belonged to effector (43 ± 6% of tHLA-staining CD8+ T cells) and effector/memory (30 ± 4.1%) subsets, while only a small proportion displayed naive or memory markers. Because Hamann et al. (9) had previously reported that memory type CD8+ T cells are characterized by a high expression of perforin, we tested the expression of this cytotoxin in tHLA+CD8+ T cells. Surprisingly, only a small proportion of these cells expressed detectable amounts of perforin (17 ± 4.5%). In addition, to further characterize vaccine-induced T cells, we tested PBMC from six patients for CCR7 expression that differentiates between central memory and effector memory T cells characterized, respectively, by the presence or the absence of expression of this marker (14). Sixty-three percent of vaccine-induced T cells did not express CCR7. Of them the majority (73%; 46% of all vaccine-induced T cells) were CD27−, while a second smaller subpopulation was CCR7+CD27+ (Table III). This observation suggested a spectrum of vaccine-induced T cells of which the preponderance appeared to bear two phenotypic characteristics associated with effector (CD27−CCR7−) function. Thus, vaccine-induced T cells appear to belong in large majority to an effector phenotype. However, contrary to other reports these effector cells appear deprived of perforin.
FIGURE 2.
Phenotyping of vaccine-induced CD8+ T cells according to CD45RA, CD27, and perforin expression. PBMC obtained before and after vaccination were gated according to g209-2M tHLA staining and phenotyped according to their level of expression of CD27, CD45RA, and perforin. In addition, CTL cultures from postvaccination PBMC were expanded in 300 IU/ml IL-2 after IVS with 1 µM g209-2M peptide. A representative patient sample (patient 7 in Table I) is presented. Percentages refer to the frequency of tHLA staining T cells over CD8+ T cells. In the inset of each scatter plot the side and forward scatter of the monocyte population analyzed is shown with a white gate representing the area of higher density of CD8+ T cells. The size of CD8+ T cells increases after IVS compared with circulating T cells.
Table II.
Percentage of CD45RA CD27 coexpression in tHLA-staining CD8+ T cells from fresh PBMC or 10 days following IVSa
| Patients | CD45RA+CD27+ (naive) | CD45RA−CD27+ (memory) | CD45RA+CD27− (effector) | CD45RA−CD27− (effector/memory) | Perforin+ |
|---|---|---|---|---|---|
| PBMC | |||||
| 1 | 16 | 9 | 54 | 21 | 1 |
| 2 | 6 | 3 | 77 | 14 | 2 |
| 3 | 6 | 9 | 67 | 20 | 2 |
| 4 | 11 | 13 | 32 | 44 | 19 |
| 5 | 5 | 4 | 53 | 38 | 3 |
| 6 | 3 | 2 | 68 | 27 | 61 |
| 7 | 8 | 12 | 51 | 29 | 19 |
| 8 | 23 | 40 | 17 | 21 | 14 |
| 9 | 17 | 2 | 55 | 26 | 31 |
| 10 | 27 | 29 | 27 | 16 | 17 |
| 11 | 10 | 15 | 17 | 58 | 15 |
| 12 | 16 | 14 | 17 | 54 | 29 |
| 13 | 34 | 28 | 20 | 18 | 14 |
| Average | 14 | 14 | 43 | 30 | 17 |
| SEM | 2.6 | 3.2 | 6.0 | 4.1 | 4.5 |
| After IVS | |||||
| 3 | 11 | 74 | 1 | 14 | 94 |
| 4 | 28 | 69 | 0 | 3 | 66 |
| 5 | 34 | 63 | 0 | 3 | 97 |
| 6 | 73 | 21 | 1 | 5 | 95 |
| 7 | 58 | 32 | 3 | 7 | 77 |
| 8 | 8 | 57 | 2 | 33 | 83 |
| 9 | 15 | 0 | 84 | 1 | 68 |
| 10 | 6 | 72 | 1 | 21 | 96 |
| 11 | 4 | 32 | 5 | 59 | 93 |
| 12 | 4 | 20 | 1 | 75 | 72 |
| 13 | 13 | 49 | 5 | 33 | 81 |
| Average | 23 | 44 | 9 | 23 | 84 |
| SEM | 7.0 | 7.5 | 7.5 | 7.5 | 3.6 |
Value of p < 0.001 (paired t test) for increase in perforin-expressing vaccine-specific T cells after IVS compared with PBMC (patients 3–13). Value of p = 0.01 (paired t test) for increase in CD27-expressing vaccine-specific T cells after IVS compared with PBMC (patients 3–13).
Table III.
Percentage of CCR7 and CD27 coexpression in tHLA-staining CD8+ T cells from fresh PBMC or 10 days following IVS
| Patients | CCR7+CD27+ | CCR7+CD27− | CCR7−CD27+ | CCR7−CD27− |
|---|---|---|---|---|
| PBMC | ||||
| 8 | 37 | 9 | 26 | 27 |
| 9 | 13 | 4 | 14 | 69 |
| 10 | 32 | 5 | 28 | 34 |
| 11 | 15 | 3 | 12 | 70 |
| 12 | 28 | 12 | 8 | 52 |
| 13 | 52 | 12 | 10 | 26 |
| Average | 30 | 7 | 17 | 46 |
| SEM | 5.9 | 1.6 | 3.5 | 8.2 |
| After 10 days of IVS (209-2M) | ||||
| 8 | 7 | 2 | 70 | 21 |
| 9 | 1 | 5 | 12 | 82 |
| 10 | 6 | 1 | 83 | 10 |
| 11 | 4 | 3 | 43 | 51 |
| 12 | 2 | 1 | 14 | 83 |
| 13 | 7 | 2 | 60 | 30 |
| Average | 5 | 2 | 55 | 38 |
| SEM | 1.0 | 0.6 | 12.0 | 12.7 |
Phenotypic characterization of vaccine-induced CD8+ T cell subsets based on CD45RA and CD27 expression after cognate IVS, followed by expansion in IL-2
CTL cultures from postvaccination PBMCs were expanded in 300 IU/ml IL-2 for 10 days after IVS with 1 µM g209-2M. Forward and side scatter analysis demonstrated that CD8+ T cells (whether vaccine-specific or not) were condensed in a population of T cells of a larger size than that observed in the generating PBMC (inset in scatter plot, Fig. 2). This increase in size was seen in all cultures tested (P3–P13) and was consistent with the typical morphology of IL-2 and/or stimulation of activated T cells after in vitro culture. Phenotyping of these cultures demonstrated a significant increase in CD27+ cells independent of the expression of CD45RA (from 28 to 67%; by paired t test, p = 0.01; Table II). In particular, 23% of IVS-derived CTL were CD45RA+CD27+, corresponding to a naive phenotype. However, their naive status was considered unlikely, first because no evidence of tHLA staining T cells could be identified before vaccination, and second because, as we have repeatedly shown, IVS does not induce g209-2M CTL in vaccination-naive circulating cells (2, 4). This suggests that it is unlikely that the CD8+ tHLA-staining T cells expressing both CD45RA and CD27 resulted from the in vitro expansion of a subliminal number of Ag-inexperienced T cells undetectable by tHLA, because this does not occur in PBMC obtained before vaccination. In addition, CD45 RA positivity significantly decreased in response to IVS from 54 to 32% of vaccine-specific T cells (P3–P13; by paired t test p = 0.02). Interestingly, together with changes in CD27 status, a dramatic increase in perforin expression was noted among the tHLA-staining postvaccination CTL cultures (from 17 ± 4.5% to 84 ± 3.6%; by paired t test for P3–P13, p < 0.001). Similarly, an almost complete loss of expression of CCR7 was noted, with 93% of vaccine-induced T cells being CCR7 negative (Table III). Taken together, these data suggest that upon IVS the vaccine-elicited T cells undergo a further step in the process of activation/differentiation that appears to increase their effector potential, which includes larger cellular size, decreased expression of CCR7, and increased expression of perforin. In contrast, small cellular size and lack of perforin expression in circulating PBMC in the absence of IVS and expansion suggest a resting status of vaccine-induced CD8+ T cells. Puzzling, however, remained the interpretation of the increased frequency of CD27+ vaccine-specific T cells after IVS. It is not clear whether the phenotypic changes observed after IVS were due to the epitope-specific stimulation rather than to the presence of IL-2 (300 IU/ml) in the culture medium. Analysis of tHLA-negative cells after IVS (presumably non-stimulated by the epitope and, therefore, responding predominantly to IL-2 stimulation) did not show the changes noted in tHLA+ cells (data not shown). In addition, in vitro culture of PBMC obtained from three non-tumor-bearing healthy donors in the presence or the absence of IL-2 (300 IU/ml) and no epitope-specific stimulation did not induce a preferential shift of CD8+ T cell phenotype. Although some variation in the CD8+ T cell phenotype was noted among different donors, no significant changes were noted in the percentage of CD8+ T cells expressing perforins after 10 days of in vitro culture compared with fresh PBMC (Table IV). Interestingly, in vitro culture of PBMC without IL-2 caused a marked decrease in the percentage of perforin-expressing CD8+ T cells, suggesting that IL-2 may play a role in sustaining, rather than inducing, the effector function of CTL. The percentage of CD27, CD45RA-expressing cells was not significantly affected by in vitro culture with IL-2, nor was the expression of CCR7 (Table V).
Table IV.
Percentage of CD45RA CD27 coexpression in CD8+ T cells from fresh PBMC or 10 days following in vitro culture. PBMC were obtained from non-tumor-bearing healthy donors
| Donors | CD45RA+CD27+ (naive) | CD45RA−CD27+ (memory) | CD45RA+CD27− (effector) | CD45RA−CD27− (effector/memory) | Perforin+ |
|---|---|---|---|---|---|
| PBMC | |||||
| 1 | 13 | 18 | 64 | 5 | 52 |
| 2 | 60 | 12 | 26 | 2 | 46 |
| 3 | 40 | 22 | 35 | 3 | 31 |
| Average | 38 | 17 | 42 | 3 | 43 |
| SEM | 13.6 | 2.9 | 11.5 | 0.9 | 6.2 |
| After in vitro culture with IL-2 | |||||
| 1 | 16 | 23 | 44 | 17 | 45 |
| 2 | 30 | 15 | 40 | 15 | 40 |
| 3 | 11 | 21 | 47 | 21 | 62 |
| Average | 19 | 20 | 44 | 18 | 49 |
| SEM | 5.7 | 2.4 | 2.0 | 1.8 | 6.7 |
| After in vitro culture without IL-2 | |||||
| 1 | 9 | 29 | 28 | 35 | 15 |
| 2 | 60 | 13 | 20 | 7 | 9 |
| 3 | 38 | 28 | 13 | 21 | 10 |
| Average | 36 | 23 | 20 | 21 | 11 |
| SEM | 14.8 | 5.2 | 4.3 | 8.1 | 1.9 |
Table V.
Percentage of CCR7 and CD27 coexpression in CD8+ T cells from fresh PBMC or 10 days following in vitro culture. PBMC were obtained from non-tumor-bearing healthy donors
| Donors | CCR7+CD27+ | CCR7+CD27− | CCR7−CD27+ | CCR7−CD27− |
|---|---|---|---|---|
| PBMC | ||||
| 1 | 13 | 3 | 11 | 73 |
| 2 | 41 | 4 | 14 | 42 |
| 3 | 40 | 4 | 16 | 41 |
| Average | 31 | 4 | 14 | 52 |
| SEM | 9.2 | 1.0 | 1.5 | 10.5 |
| After 10 days of in vitro culture with IL-2 | ||||
| 1 | 27 | 7 | 11 | 55 |
| 2 | 32 | 5 | 23 | 41 |
| 3 | 16 | 7 | 8 | 69 |
| Average | 25 | 6 | 14 | 55 |
| SEM | 4.7 | 1.0 | 4.6 | 8.1 |
| After 10 days of in vitro culture without IL-2 | ||||
| 1 | 26 | 9 | 22 | 42 |
| 2 | 70 | 4 | 13 | 13 |
| 3 | 62 | 12 | 11 | 16 |
| Average | 53 | 8 | 15 | 24 |
| SEM | 13.5 | 2.3 | 3.4 | 9.2 |
Kinetics of stimulation of the effector-type g209-2M-specific CTL clone P1G9 with the melanoma cell lines 624.38 (HLA-A2 +GP100+) and 624.28 (HLA-A2−GP100+)
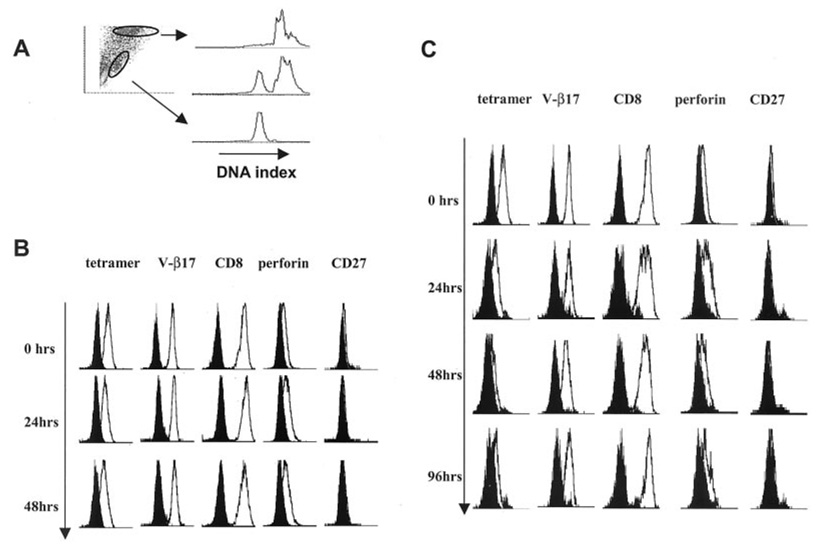
To test whether changes in CD27 expression define the status of activation of vaccine-induced T cells rather than representing a marker of their status of differentiation (naive vs memory vs effector), we stimulated in vitro the g209-2M-specific CTL clone (P1G9) derived from circulating lymphocytes of a melanoma patient who had received immunization with the same epitope (4). This clone is characterized by high affinity for the HLA/g209-2M complex, has a classical effector phenotype CD45RA+CD27−, and expresses detectable amounts of perforin when cultured in vitro in the presence of 300 IU/ml IL-2 (9). P1G9 was cocultured at a 1/4 ratio with either the HLA-A*0201-negative 624.28 or the HLA-A*0201-expressing 624.38 melanoma cell line. IL-2 (300 IU/ml) was added the following day and every 2–3 days thereafter. Before stimulation and 24, 48, and 96 h after stimulation, tHLA staining and V-β17, CD8, perforin, and CD27 expression were analyzed by FACS analysis. T cells were discriminated from cancer cells by forward and side scatter, and the accuracy of this strategy was confirmed by DNA index analysis as previously described (26) (Fig. 3A).
FIGURE 3.
Variation in phenotype of an effector-type vaccine-induced T cell clone in response to IVS with HLA-A*0201-matched tumor cells and IL-2. The effector g209-2M-specific, vaccine-induced T cell clone P1G9 (4) was cocultured with the HLA-A*0201+gp100+ melanoma cell line 624.38 (624.38-MEL) or a sister clone HLA-A*0201−gp100+ (624.28-MEL) in the presence of IL-2 (300 IU/ml). At different time points the cocultures were analyzed by FACS analysis gating melanoma and T cells according to size scatter. The accuracy of the gating was verified by DNA index analysis as we have recently described (26), with T cells being diploid and cancer cells hyperploid (A). At baseline P1G9 was CD45RA+CD27−perforin+. Upon stimulation with 624.28-MEL, no changes in phenotype were noted up to 48 h from the beginning of the coculture (B). Upon stimulation with 624.38-MEL, down-regulation of tHLA staining was noted as previously described (7), which was associated with a slight down-regulation of V-β17 and CD8 and decreased perforin expression (C). Shown are one of two experiments with similar results.
Upon IVS with the HLA-A*0201-loss variant 624.28-MEL (22, 23), no changes in tHLA staining or in the expression of CD8, V-β17, perforin, and CD27 were noted as expected due to the lack of TCR engagement with the melanoma cell line (Fig. 3B). Stimulation with the HLA-A*0201-expressing sister clone 624-38 demonstrated a decrease in tHLA staining associated with TCR/HLA-A*0201/epitope engagement as previously described (7) (Fig. 3C). This decrease was associated with no or minimal changes in the expression of other surface markers, with a slight decrease in V-β17 and CD8 expression. Thus, stimulation of a T cell effector clone using HLA-A*0201-matched cell lines induced minimal effects on the status of activation of such a clone, although these conditions are sufficient to induce IFN-γ mRNA and protein expression as well as reduction in tHLA staining as previously described (7).
Kinetics of stimulation of the effector-type g209-2M-specific CTL clone P1G9 with the melanoma cell line 624.38 (HLA-A2+gp100+) exogenously pulsed with 1 µM g209-2M peptide
The conditions used for IVS of PBMC differed from those used to stimulate P1G9 with HLA-matched tumor cells, in that PBMC were vigorously challenged with high density epitope exposure achieved by exogenous administration of 1 µM peptide. We have used this technique for quite a long time with the assumption that monocytic cells present in the PBMC preparation would contribute to Ag presentation (2, 27–29). Using this technique we have previously noted that decrements in the concentration of epitope used for cognate stimulation tightly correspond with decreasing levels of IFN-γ mRNA expression by PBMC, while down-regulation of tHLA staining is only minimally affected (4). In addition, we have previously shown that variations in epitope density on the surface of tumor cells strongly affects the intensity of T cell stimulation (22, 30), which can be greatly enhanced by incubation of cancer cells with soluble epitope before coculture (30). Indeed, analysis of a panel of CTL clones expanded from PBMC obtained from melanoma patients after immunization showed a strong correlation between the level of stimulation with tumor cells at a peptide concentration corresponding to 0.03 µM (31). Thus, we questioned whether stimulation with tumor cells expressing HLA-A*0201 and gp100 could be quantitatively comparable to the high density peptide exposure achievable with the saturating concentrations of g209-2M peptide (1 µM) used for PBMC stimulation (31).
To enhance the effects of the stimulation induced by the melanoma cell line 624.38-MEL, we therefore artificially increased its epitope density by loading tumor cells with 1 µM g209-2M peptide. Direct addition of g209-2M to the CTL clone was not feasible; preliminary experiments showed massive apoptosis of T cells (data not shown), possibly due to cross-recognition of HLA-epitope complexes on the surface of T cells or to Fas/Fas ligand interactions among activated T cells (32).
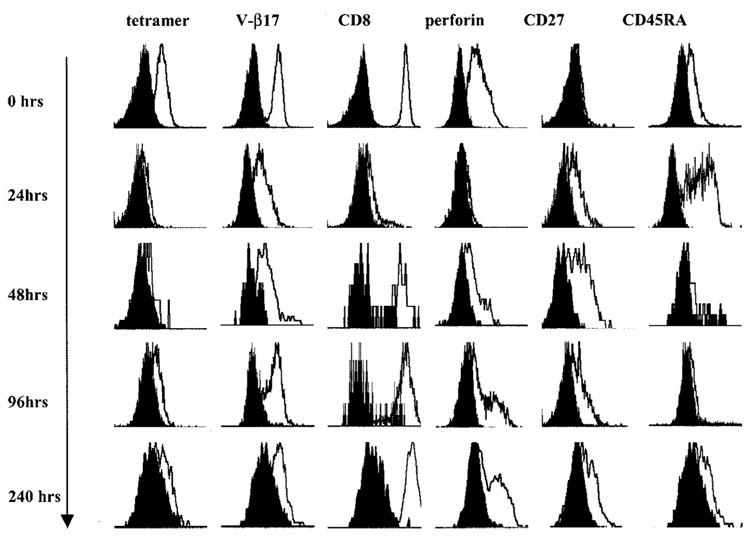
Upon exposure to g209-2M-loaded 624.38 melanoma cells, P1G9 demonstrated an immediate and profound down-regulation of the expression of CD8 and the V-β17 TCR chain expressed by this clone. The down-regulation of CD8 and the clonotypic V-β chain was associated with loss of g209-2M tHLA staining (Fig. 4). In addition, exposure to cognate stimulation decreased the expression of perforin within the first 24 h. In contrast, CD27 expression was up-regulated by 24 h after stimulation and persisted for several days following stimulation. During a period of 10 days in culture after epitope-specific stimulation in the presence of 300 IU/ml IL-2, the expression of CD8 slowly reconstituted. This reconstitution of expression was associated with restoration of V-β17 expression, followed by reappearance of tHLA staining. During the same period, perforin levels were progressively restored, while the expression of CD27 remained detectable. Thus, the P1G9 phenotype had switched from a canonical effector type (CD45RA+ CD27−perforin+) to a new hybrid phenotype CD45RA+, CD27+, and perforin+ reminiscent of a canonical naive T cell (CD45RA+CD27+). Interestingly, at a particular time point (24 h after stimulation), P1G9 had a classical naive phenotype: CD45RA+, CD27+, and perforin−. Because it is unlikely that this CTL clone had regressed to an Ag-inexperienced status of differentiation, it is most likely that switches in CD27 expression and perforin detectability reflect different levels of activation of circulating and in vitro cultured T cells. In this study we did not address in depth the differential role that cognate stimulation vs IL-2 exposure may play in affecting changes in T cell phenotype. However, we believe that the intensity of cognate stimulation may have a predominant role. In one experiment in which the same stimulatory conditions were applied to P1G9 (stimulation with 624.28 MEL or 624.38 MEL with or without exogenous loading with g209-2M) in the presence or the absence of IL-2, no demonstrable differences in expression of the previously discussed markers were noted up to 4 days following stimulation (data not shown). It should be emphasized that these data derived from a clonal population may have limited relevance to the in vivo situation, as various CTL clones may be quite heterogeneous depending on various intrinsic and extrinsic factors. However, the switch in phenotype observed in this clone in response to cognate stimulation suggests that markers such as CD27 may vary independently of the status of differentiation of individual T cells.
FIGURE 4.
Variation in phenotype of an effector-type vaccine-induced T cell clone in response to IVS with HLA-A*0201-matched tumor cells loaded with 1 µM g209-2M peptide. The effector g209-2M-specific, vaccine-induced T cell clone P1G9 (4) was cocultured with the HLA-A*0201+gp100 melanoma cell line 624.38 previously incubated with 1 µM g209-2M. Tumor and lymphocytes were gated according to side scatter as shown in Fig. 3A. A profound down-regulation of tHLA, V-β17, CD8, and perforin staining was noted. In contrast, de novo expression of CD27 was noted 24 h after stimulation and persisted up to 10 days. During the same time course, all the other markers recovered so that the originally CD45RA+ CD27− T cell clone became CD45RA+ CD27+perforin+. Shown are data from one of two experiments with similar results.
Discussion
Insights in the status of activation and/or differentiation of vaccine-induced T cells may have relevance to the interpretation of clinical results following immunization protocols. In particular, epitope-specific vaccination allows a unique opportunity to simplify the analysis of its effect onto a single HLA allele/epitope combination. This allows the study of human disease independently of HLA polymorphism and/or heterogeneity of individual tumor Ag processing and presentation efficiency. By following peptide-based immunization, we and others have repeatedly observed an enhancement of vaccine-induced Tc-pf (3, 29, 33–41). In general, these studies have shown that vaccination with minimal T cell-directed epitopic sequences can be quite effective in inducing tumor-specific T cell responses, which can be easily observed among circulating lymphocytes of immunized patients. However, with few exceptions (39–41), the identification of such immune responses could not be consistently associated with significant improvement in clinical outcome.
Several factors may be responsible for the lack of correlation between the identification of an immune response to the vaccine and the lack of cancer regression. Obviously, circulating T cells induced by vaccination may not reach the tumor site to exert their effector function. However, attempts to expand vaccine-specific T cells from biopsies through fine needle aspirates of the same lesion obtained before and after treatment were more frequently successful in postvaccination samples (42). In addition, we noted accumulation of gp100-specific lymphocytes in metastatic deposits following vaccination in 8 of 11 patients. This accumulation was associated with increased expression of IFN-γ in lesions that expressed gp100. Yet this localization was not sufficient for tumor regression despite the expression by melanoma cells of the gp100 Ag targeted by the vaccine (43). These findings suggest that vaccine-elicited T cells can localize within the target tissue and that they are engaged by tumor cells to produce IFN-γ, but this is not sufficient, and additional factors may be responsible for their lack of effect on tumor growth.
It could also be postulated that vaccine-induced Tc-pf, although clearly observable, may not be sufficient to induce tumor regression, because the number of immunization-induced T cells observed in this study may not be above the threshold that may be required to eliminate all tumor cells. In a mouse model we identified a correlation between the intensity of immune response induced by the immunogen and tumor rejection. This correlation could be easily identified in this experimental model, because the use of a clonal population of tumor cells and of inbred animals reduced tumor heterogeneity and/or variability of individual animal immune responses providing a more homogeneous target for immunotherapy (44). However, in humans a clear quantitative correlation between the extent of immune response and clinical outcome has remained elusive in most reported clinical trials, perhaps in relation to the heterogeneity of tumors in various individuals affecting their immune responsiveness (45).
T cell responses documented by IVS or by direct ex vivo enumeration of Tc-pf with vaccine-specific tHLA may not fully address their status of activation in vivo. In fact, Lee et al. (5) have shown that tumor-specific T cells identified in vaccine-naive patients by tHLA may not be able to exert effector function either through target killing or expression of cytokines such as IFN-γ. We have previously argued that this concept may not pertain to vaccine-induced CD8+ T cell responses, because in at least a percentage of them, it is possible to document the expression of IFN-γ upon cognate stimulation with vaccine-specific epitope or HLA-matched tumor cells (4–7). However, recent reports have emphasized a dichotomy between IFN-γ secretion by T cells and their killer function (8). This suggests that IFN-γ expression in itself may not be the ultimate arbiter of T cell effector function, but may represent a sensitive marker of T cell reactivity to cognate stimulation.
Hamann et al. (9) suggested a spectrum of circulating T cell phenotypes from naive to effector types based on the expression of several cellular markers of which CD45RA and CD27 appeared to best predict their status of differentiation and consequently their function. Others suggested that homing receptors might be responsible for the effector function of T cells by regulating their localization within target organs (14). The observation in this and other studies (6) that vaccine-elicited T cells express low or undetectable CCR7 suggests that homing may not be, at least based on this marker, a significant problem in the context of vaccines. This finding is consistent with an effector phenotype reported for flu- and CMV-specific T cells (6, 15) and correlates with our observation that vaccine-induced T cell responses can be detected in lesions resistant to therapy following vaccination (42, 43).
Segregation of T cell subsets into naive, memory, and effector types suggests a static and irrevocable status of differentiation that may not necessarily apply to the dynamic behavior of surface markers in response to T cell stimulation. For instance, expression of CD27 may play a role in the survival of activated T cells (46). Stimulation of the TCR complex with PHA or CD3 mAbs causes a dramatic increase in CD27, and addition of CD27 Abs to T cell cultures leads to enhanced proliferation (47). Interestingly, CD27 could be immunoprecipitated from the membrane of activated, but not resting, T cells (47). Recently, CD27 was described as a costimulatory receptor responsible for the generation and maintenance of T cell memory responses (48). Our findings suggest that CD27 expression in circulating vaccine-induced T cells may represent a marker of their status of activation rather than differentiation. Indeed, CD27 could be induced through IVS of vaccine-induced CD27− (effector) CD8+ T cells. This increase in CD27− expressing T cells after 10 days of IVS is probably the result of its de novo expression by CD27− PBMC, rather than the preferential expansion of few CD27+ T cells. In particular, the substantial number of CD45RA+CD27+ (naive) CD8+ T cells (23%) observed after IVS is unlikely to be the result of expansion of rare (below the threshold of detection of tHLA) Ag-inexperienced precursors in PBMC obtained after vaccination. We believe that all the vaccine-specific T cells identifiable through IVS after immunization must have been exposed to the vaccine epitope, because we could never expand g209-2M-specific T cells before vaccination using the exact in vitro conditions used here (2, 17). Thus, postvaccination IVS-induced T cells are most likely Ag-experienced derived from the CD45RA+CD27− PBMC pool. The CD45RA expression in Ag-experienced T cells is reminiscent of the stable CD45RA+LFAhigh memory state recently described by Faint et al. (49). Unfortunately, because of the limited number of cells available, LFA-1 as well as other potentially interesting markers such as CCR5, CXCR1, and CD62 could not be evaluated in this study. The demonstration that a vaccine-induced, effector-type T cell clone could reverse its phenotype from CD27+ to CD27− upon IVS suggests that the phenotype switch observed in IVS-expanded CTL is due to a change in functional status rather than preferential expansion of T cells in a particular status of differentiation. Thus, as suggested by others (6), CD27 may not be the most accurate surface marker to segregate naive from Ag-experienced CD8+ T lymphocytes as also reported for CD45RA (49). Indeed, expression of the CD45RA isoform has been noted to occur during the quiescent stage of memory T cell reversion to a resting state after in vivo priming by acute EBV infection (49). Interestingly, the phenotypic changes observed on P1G9 could only be induced by intense stimulation with saturating conditions of epitope (pulsed tumor cells), while exposure to tumor cells expressing natural amounts of HLA/epitope on their surface was not sufficient to induce such changes even in the presence of exogenous IL-2. This finding suggests that in vivo contact between vaccine-induced T cells and tumor cells may not be sufficient to induce productive T cell stimulation capable of sustaining a brisk immune response as suggested by others (50, 51).
Of particular significance in this study was the lack of expression of perforin in circulating vaccine-induced T cells. This in association with their small size and their lack of expression of CD27 suggests that vaccine-induced circulating T cells are in a resting status, which can be reversed by IVS. It was beyond the purpose of this study to address in depth whether this in vitro reactivation is due to re-exposure to cognate stimulation or to the 10-day culture with IL-2. Whatever the cause of the striking difference noted after 10 days of IVS, this change in phenotype may suggest a reason for the discrepancy between satisfactory function of T cells observed in vitro after IVS and their limited function in vivo. This study also did not address when and where vaccine-induced T cells turn into this postulated resting phase. All samples in this study were obtained 3 wk after immunization. Thus, it is possible that this period marks the gradual regression of vaccine-induced T cells toward a resting/memory phenotype. Analysis of vaccine-induced Tc-pf at an earlier time point might have depicted a totally different portrait. Perhaps a more intense and consistent exposure in vivo to vaccine may induce and sustain a more active and functionally rewarding immune response as suggested by others (50, 52, 53).
In summary, we hypothesize that vaccine-induced T cells belong to an effector subtype (predominantly CD27− and CCR7−) that is in a resting phase characterized by lack of perforin, small cellular size, and lack of expression of activation markers such as CD27. These cells, however, are not totally anergic and, on the contrary, are responsive to cognate stimulation, as they can produce IFN-γ upon exposure to relevant Ag (4, 7). It is possible that if the stimulus is delivered in optimal conditions their full effector function can be restored as modeled by the 10-day IVS conditions. These findings assign a limited level of functionality to circulating vaccine-induced T cells and may partially explain why increases in Tc-pf following immunization may not directly correlate with clinical cancer regression.
Footnotes
D.N. was supported by Mildred Scheel Stiftung für Krebsforschung (Deutsche Krebshilfe).
Abbreviations used in this paper: Tc-pf, T cell precursor frequency; g209, gp100: 209–217; g209-2M, g209(210 M); IVS, in vitro stimulation; tHLA, HLA/epitope tetrameric complex.
References
- 1.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwijck R, Humblet Y. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int. J. Cancer. 1995;63:883. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 2.Lee K-H, Wang E, Nielsen M-B, Wunderlich J, Migueles S, Connors M, Steinberg SM, Rosenberg SA, Marincola FM. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J. Immunol. 1999;163:6292. [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Schwartzentruber D, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dufour E, Schwartzberg L, Spiess P, et al. Immunologic and therapeutic evaluation of a synthetic tumor associated peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monsurrò V, Nielsen M-B, Perez-Diez A, Dudley ME, Wang E, Rosenberg SA, Marincola FM. Kinetics of TCR use in response to repeated epitope-specific immunization. J. Immunol. 2001;166:5817. doi: 10.4049/jimmunol.166.9.5817. [DOI] [PubMed] [Google Scholar]
- 5.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 6.Pittet MJ, Zippelius A, Speiser DE, Assenmacher M, Guillaume P, Valmori D, Lienard D, Lejune F, Cerottini J-C, Romero P. Ex vivo IFN-γ secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J. Immunol. 2001;166:7634. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen M-B, Monsurrò V, Miguelse S, Wang E, Perez-Diez A, Lee K-H, Kammula US, Rosenberg SA, Marincola FM. Status of activation of circulating vaccine-elicited CD8+ T cells. J. Immunol. 2000;165:2287. doi: 10.4049/jimmunol.165.4.2287. [DOI] [PubMed] [Google Scholar]
- 8.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina C, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann D, Baars PA, Rep MHG, Hoolbrink B, Kerkhof-Garde SR, Klein MR, van Lier RAW. Phenotype and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baars PA, Ribeiro do Couto LM, Leusen JHW, Hoolbrink B, Kuijpers TW, Lens SMA, van Lier RAW. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27− human T cells. J. Immunol. 2000;165:1910. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- 11.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RAW. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int. Immunol. 1999;11:1027. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 12.Gamadia LE, Rentenaar RJ, Baars PA, Remmerswaal EBM, Surachno S, Weel JFL, Toebes M, Schumacher TN, ten Berge JM, van Lier RAW. Differentiation of cytomegalovirus-specific CD8+ T cells in healthy and immunosuppressed virus carriers. Blood. 2001;98:754. doi: 10.1182/blood.v98.3.754. [DOI] [PubMed] [Google Scholar]
- 13.Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I provirus load. J. Infect. Dis. 2001;183:197. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:659. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 15.Champagne P, Ogg GS, King A, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 16.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201 binding residues. J. Immunol. 1996;157:2539. [PubMed] [Google Scholar]
- 17.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749. [PubMed] [Google Scholar]
- 18.Bunce M, O’Neill CM, Barnardo MC, Krausa P, Browning MJ, Morris PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 19.Dudley ME, Ngo LT, Westwood J, Wunderlich JR, Rosenberg SA. T-cell clones from melanoma patients immunized against an anchor modified gp100 peptide display discordant effector phenotypes. Cancer J. Sci. Am. 2000;6:69. [PubMed] [Google Scholar]
- 20.Riddell SR, Greenberg PD. Therapeutic reconstitution of human viral immunity by adoptive transfer of cytotoxic T lymphocyte clones. Curr. Top. Microbiol. Immunol. 1994;189:9. doi: 10.1007/978-3-642-78530-6_2. [DOI] [PubMed] [Google Scholar]
- 21.Legerski R, Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992;359:70. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- 22.Rivoltini L, Baracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective human leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J. Exp. Med. 1999;190:205. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman JD, Moss PH, Goulder PR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94. [Published erratum appears in 1998 Science 280:1821.] [PubMed] [Google Scholar]
- 25.Cormier JN, Hijazi YM, Abati A, Fetsch P, Bettinotti M, Steinberg SM, Rosenberg SA, Marincola FM. Heterogeneous expression of melanoma-associated antigens (MAA) and HLA-A2 in metastatic melanoma in vivo. Int. J. Cancer. 1998;75:517. doi: 10.1002/(sici)1097-0215(19980209)75:4<517::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Mocellin S, Fetsch PA, Abati A, Phan G, Wang E, Provenzano M, Stroncek D, Rosenberg SA, Marincola FM. Laser scanner cytometer evaluation of MART-1, gp100 and HLA-A2 expression in melanoma metastases. J. Immunother. 2001;24:447. doi: 10.1097/00002371-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Rivoltini L, Kawakami Y, Sakaguchi K, Southwood S, Sette A, Robbins PF, Marincola FM, Salgaller M, Yannelli JR, Appella E, et al. Induction of tumor reactive CTL from peripheral blood and tumor infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J. Immunol. 1995;154:2257. [PubMed] [Google Scholar]
- 28.Marincola FM, Rivoltini L, Salgaller ML, Player M, Rosenberg SA. Differential anti-MART-1/MelanA CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: evidence for in vivo priming by tumor cells. J. Immunother. 1996;19:266. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Cormier JN, Salgaller ML, Prevette T, Barracchini KC, Rivoltini L, Restifo NP, Rosenberg SA, Marincola FM. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J. Sci. Am. 1997;3:37. [PMC free article] [PubMed] [Google Scholar]
- 30.Cormier JN, Panelli MC, Hackett JA, Bettinotti M, Mixon A, Wunderlich J, Parker L, Restifo NP, Ferrone S, Marincola FM. Natural variation of the expression of HLA and endogenous antigen modulates CTL recognition in an in vitro melanoma model. Int. J. Cancer. 1999;80:781. doi: 10.1002/(sici)1097-0215(19990301)80:5<781::aid-ijc24>3.0.co;2-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudley ME, Nishimura MI, Holt AKC, Rosenberg SA. Anti-tumor immunization with a minimal peptide epitope (G9-209-2M) leads to a functionally heterogeneous CTL response. J. Immunother. 1999;22:288. doi: 10.1097/00002371-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-Mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J. Immunol. 1999;162:3273. [PMC free article] [PubMed] [Google Scholar]
- 33.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer. 1999;80:219. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Pittet MJ, Speiser DE, Lienard D, Valmori D, Guillaume P, Dutoit V, Rimoldi D, Lejeune F, Cerottini JC, Romero P. Expansion and functional maturation of human tumor antigen-specific CD8+ T cells after vaccination with antigenic peptide. Clin. Cancer Res. 2001;7:796s. [PubMed] [Google Scholar]
- 35.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 36.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, et al. Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999;190:1669. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheibenbogen C, Schmittel A, Keilholz U, Allgauer T, Hofmann U, Max R, Thiel E, Schadendorf D. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J. Immunother. 2000;23:275. doi: 10.1097/00002371-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Laforet M, Froelich N, Parissiadis A, Bausinger H, Pfeiffer B, Tongio MM. An intronic mutation responsible for a low level of expression of an HLA-A*24 allele. Tissue Antigens. 1997;50:340. doi: 10.1111/j.1399-0039.1997.tb02884.x. [DOI] [PubMed] [Google Scholar]
- 39.Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen Y-T, Ritter G, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl. Acad. Sci. USA. 2000;97:12198. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jager E, Maeurer MJ, Hohn H, Karbach J, Jager D, Zidianakis Z, Bakhshandeh-Bath A, Orth J, Neukirch C, Necker A, et al. Clonal expansion of melan A-specific cytotoxic T lymphocytes in a melanoma patient responding to continued immunization with melanoma-associated peptides. Int. J. Cancer. 2000;86:538. doi: 10.1002/(sici)1097-0215(20000515)86:4<538::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Bade E, Kuniyoshi C, Spears L, Jeffery G, Marthy V, Groshen S, Weber J. Phase I trial of a MART-1 peptide vaccine with incomplete Freund’s adjuvant for resected high-risk melanoma. Clin. Cancer Res. 1999;5:2756. [PubMed] [Google Scholar]
- 42.Panelli MC, Riker A, Kammula US, Lee K-H, Wang E, Rosenberg SA, Marincola FM. Expansion of tumor/T cell pairs from fine needle aspirates (FNA) of melanoma metastases. J. Immunol. 2000;164:495. doi: 10.4049/jimmunol.164.1.495. [DOI] [PubMed] [Google Scholar]
- 43.Kammula US, Lee K-H, Riker A, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J. Immunol. 1999;163:6867. [PubMed] [Google Scholar]
- 44.Perez-Diez A, Spiess PJ, Restifo NP, Matzinger P, Marincola FM. Intensity of the vaccine-elicited immune response determines tumor clearance. J. Immunol. 2002;168:338. doi: 10.4049/jimmunol.168.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marincola FM, Jaffe EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 2000;74:181. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 46.Camerini D, Walz G, Loenen WAM, Borst J, Seed B. The T cell activation antigen CD27 is a member of the nerve growth factor/tumor necrosis factor receptor gene family. J. Immunol. 1991;147:3165. [PubMed] [Google Scholar]
- 47.van Lier RAW, Borst J, Vroom TM, Klein H, van Mourik P, Zeijlemaker WP, Melief CJ. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J. Immunol. 1987;139:1589. [PubMed] [Google Scholar]
- 48.Hendriks J, Gravestein LA, Tesselaar K, van Lier RAW, Schumacher M, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 49.Faint JM, Annels NE, Curnow SJ, Shields P, Pilling D, Hislop AD, Wu L, Akbar AN, Buckley CD, Moss PA, et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J. Immunol. 2001;167:212. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system. Semin. Immunol. 1996;8:271. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 51.Matzinger P. An innate sense of danger. Semin. Immunol. 1998;10:399. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 52.Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 1997;186:645. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Semin. Immunol. 2000;12:163. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]