Photodynamic therapy (PDT) is gaining acceptance as a technique for cancer treatment in recent years.1-3 PDT utilizes a photosensitizer, which works as a light-sensitive drug, to cause damage to the target cells and/or tissue locally upon the illumination of light with appropriate wavelengths.4 It is generally accepted that the therapeutic effect of PDT is based on the interaction between the excited photosensitizer and the surrounding molecules, generating reactive oxygen species (ROS), such as singlet oxygen (1O2). ROS can cause oxidative damage to biological substrates and ultimately lead to cell death. As a key component in effective and efficient PDT, the photosensitizers, also called PDT drugs, should ideally be: (1) specific to the target; (2) highly effective in producing ROS when exposed to appropriate illumination; (3) excitable by a wavelength close to the near infrared region (800 nm to 1 μm), where tissue penetration of the illumination is at a maximum. Regarding the last desired feature, single photons with infrared wavelengths are usually too weak energetically to generate ROS. Thus, multiphoton excitation would be needed for infrared light to be used as illumination source.4-8 Here we report the synthesis and characterization of a type of nanomaterial capable of generating 1O2 under continuous wave infrared excitation, based on photon upconverting nanoparticles (PUNPs). The results demonstrate that such nanoparticles have great potential for becoming a new type of versatile PDT drugs for photodynamic therapy.
Photon upconverting materials convert lower-energy light to higher-energy light through excitation with multiple photons. They have been known and used for some time in a number of different applications.9 Materials with photon upconverting properties, usually containing lanthanide ions, are much less common than those with down-converting properties. They typically have narrower absorption and line emission spectra, are not susceptible to photobleaching, and are chemically stable. Although they do not directly produce ROS, we utilize the fact that they adsorb infrared photons and emit visible ones to further excite the photosensitizing molecules, thus indirectly causing the photosensitizing molecules to generate 1O2 under infrared excitation.
The design and synthesis of the PUNPs-based photosensitizers we proposed are schematically shown in Figure 1. The core was a NaYF4:Yb3+,Tm3+ nanoparticle, a photon upconverting material capable of emitting blue light (∼477 nm) upon excitation by an infrared light source (∼975 nm). The nanoparticle was then coated by a thin layer of tris(bipyridine)ruthenium(II)-doped silica. Ru(bpy)32+ (bpy, bipyridine) had long been known to generate 1O2 with unit efficiency in a number of solvents when properly excited (absorbance maximum at ∼450 nm).10 Our hypothesis was that the embedded Ru(bpy)32+ in the silica layer would adsorb the blue emission from PUNPs, and 1O2 would then be produced in the reaction between the excited state of Ru(bpy)32+ and triplet oxygen in solution.
Figure 1.
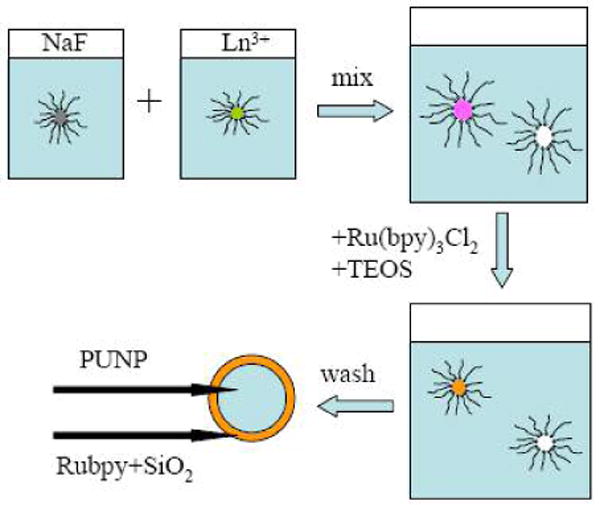
Schematic of PUNPs-based photosensitizer.
The NaYF4:Yb3+,Tm3+ nanoparticles were synthesized by a microemulsion method. The synthesis was carried out in a water/cetyl trimethylammonium bromide (CTAB)/hexanol microemulsion system. In brief, 0.2 M aqueous solutions of YCl3, YbCl3 and TmCl3, and 0.415 M aqueous solutions of NaF were prepared and used as the water components in preparing two separate microemulsion solutions of the same H2O/CTAB/hexanol ratio (16/24/60 by weight), one containing all lanthanide ions and one containing NaF. The two microemulsion solutions were mixed under rigorous stirring and the reaction was allowed to proceed overnight.
The nanoparticles were then coated by a thin layer of Ru(bpy)32+-doped silica through a variation of the well-known Stöber process. Calculated amounts of tetraethoxysilane (TEOS) and 1 mM Ru(bpy)3Cl2 aqueous solutions were added to the microemulsion solution under continuous stirring for 24 hours at room temperature. The resulting nanoparticles were collected by adding acetone to break down the microemulsion system and washed a few times with methanol.
The final product was characterized spectroscopically and microscopically. The photoluminescence spectra of the NaYF4:Yb3+,Tm3+ nanoparticles before and after being coated with Ru(bpy)32+-doped silica, under 975 nm excitation, are shown in Figure 2. One can see that the coating does not affect the photon upconverting property of the NaYF4:Yb3+,Tm3+ nanoparticles. Figure 3 is an FESEM image of the photon upconverting nanoparticles coated with Ru(bpy)32+-doped silica. Most of the nanoparticles were in the range of 30 to 60 nm, with good monodispersity.
Figure 2.
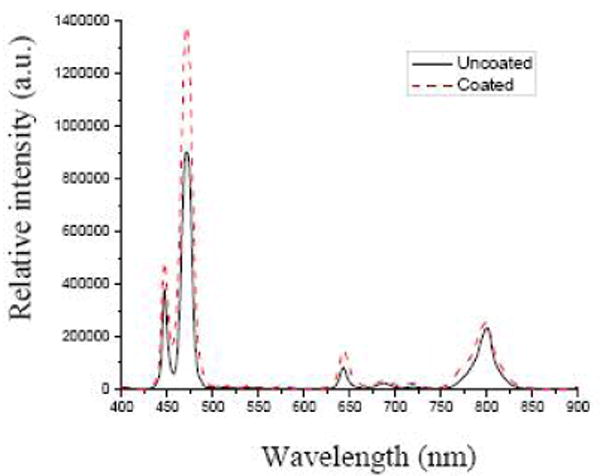
Photoluminescence spectra of NaYF4:Yb3+,Tm3+ nanoparticles before and after being coated with Ru(bpy)32+-doped silica.
Figure 3.
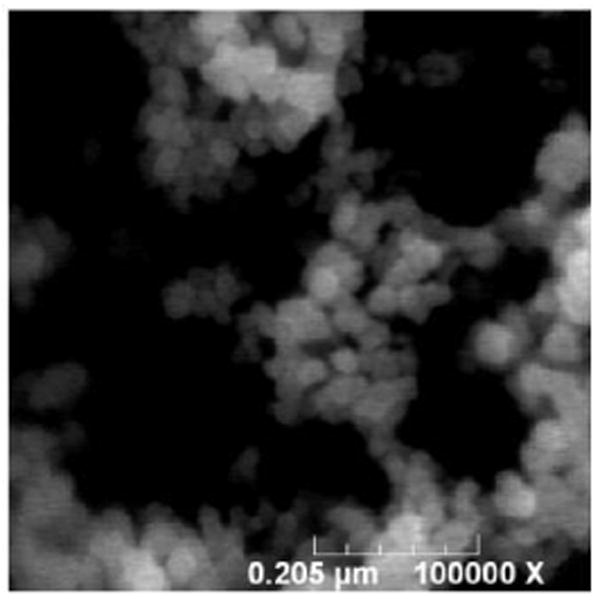
FESEM image of the final PUNPs-based photosensitizers.
The generation of 1O2 by these PUNPs-based photosensitizers was detected chemically, by using the disodium salt of 9,10-anthracenedipropionic acid (ADPA) as a probe molecule.11 ADPA would be bleached by 1O2 to its nonfluorescent endoperoxide. By measuring the decrease of the strong fluorescence emissions of ADPA, when excited at ∼375 nm, one could monitor the generation of 1O2.
Experimentally, 1 μl of 0.2 M ADPA solution was added to 3 ml phosphate buffer solution (pH = 7.2) containing 0.7 mg of nanoparticles, in each of three vials. The measurements were carried out under three sets of different conditions at different time intervals. The first vial was kept in the dark throughout the experiment, except for the light exposure when taking fluorescence measurements. The second vial was illuminated for 2 minutes by a 450 nm light from a 70W xenon lamp through a monochromator before every fluorescence measurement was taken. The third vial was illuminated for 2 minutes by a 975 nm diode laser of 20 mW output before every fluorescence measurement was taken. All fluorescence measurements of ADPA were set at 420 nm emission/378 nm excitation.
The results of the fluorescence measurements are shown in Figure 4. With no illumination, the ADPA fluorescence intensity barely decreased over time. When the solution was illuminated by 450-nm and 975-nm light sources, the fluorescence intensity decreased appreciably. Previous reports had shown that the intensity decrease of ADPA emission followed an exponential decay over time, as ADPA was being quenched by the generated 1O2.12-15 The data points in Figure 4 for the two runs that were illuminated by 450 nm and 975 nm light, respectively, could also be fit into an exponential decay function, as indicated by the lines. This suggested that the kinetics of the Ru(bpy)32+-coated PUNPs generating 1O2 are very similar to those of other silica-based nanoparticles described in the literature.12-14 The major difference was that the Ru(bpy)32+-coated PUNPs were able to be excited by both IR (975 nm) and visible (450 nm) illuminations, while the others in the literature could be excited only by visible illumination. This difference would allow the penetration depth to increase several times for the PUNPs-based photosensitizers, a highly desired feature in clinical applications.
Figure 4.
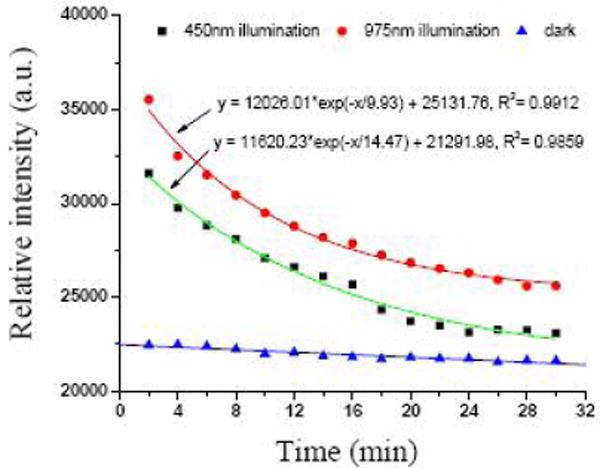
Change of intensity of ADPA emission at 420 nm, excited at 378 nm, for the respective solutions. The lines represent the fitting of the data points.
We would point out the versatility of such PUNPs-based photosensitizers. There are many Ru-compounds, or other metal complexes, absorbing at ∼470 nm and generating 1O2 that can be readily used in this format. Furthermore, there are other PUNPs (such as NaYF4:Yb3+,Er3+ nanoparticles) available and/or being developed. They can also be adopted in this design, as long as appropriate compounds that absorb in the corresponding wavelengths and generate 1O2 are included in the coating. We believe photosensitizers of such design hold great promise in photodynamic therapy.
Acknowledgments
This work was partially supported by Grant Number RR-016480 from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH), and by the start-up fund to P. Z. from New Mexico Tech. Dr. Hong Tang's help in SEM imaging is greatly appreciated.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J Natl Cancer Inst. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharman WM, Allen CM, van Lier JE. Drug Discovery Today. 1999;4:507. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown SB, Brown EA, Walker I. Lancet Oncol. 2004;5:497. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 4.Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Photochem Photobiol Sci. 2006;5:727. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- 5.Stiel H, Teuchner K, Paul A, Freyer W, Leupold D. J Photochem Photobiol A. 1994;80:289. doi: 10.1111/j.1751-1097.1993.tb02320.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhawalkar JD, Kumar ND, Zhao CF, Prasad PN. J Clin Laser Med Surg. 1997;15:201. doi: 10.1089/clm.1997.15.201. [DOI] [PubMed] [Google Scholar]
- 7.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. J Am Chem Soc. 2003;125:7860. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 8.Gao D, Agayan RR, Xu H, Philbert MA, Kopelman R. Nano Lett. 2006;6:2383. doi: 10.1021/nl0617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auzel F. Chem Rev. 2004;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 10.Zahir KO, Haim A. J Photochem Photobiol A: Chem. 1992;63:167. [Google Scholar]
- 11.Lindig BA, Rodgers MAJ, Schaap AP. J Am Chem Soc. 1980;102:5590. [Google Scholar]
- 12.Moreno MJ, Monson E, Reddy RG, Rehemtulla A, Ross BD, Philbert M. Sensors and Actuators B. 2003;90:82. [Google Scholar]
- 13.Tang W, Xu H, Kopelman R, Philbert MA. Photochem Photobiol. 2005;81:242. doi: 10.1562/2004-05-24-RA-176.1. [DOI] [PubMed] [Google Scholar]
- 14.Yan F, Kopelman R. Photochem Photobiol. 2003;78:587. doi: 10.1562/0031-8655(2003)078<0587:teomis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Steelant W, Kumar M, Scholfield M. J Am Chem Soc. 2007;129:4526. doi: 10.1021/ja0700707. [DOI] [PMC free article] [PubMed] [Google Scholar]


