Abstract
Nonmuscle myosin light-chain kinase (MYLK) mediates increased lung vascular endothelial permeability in lipopolysaccharideinduced lung inflammatory injury, the chief cause of the acute respiratory distress syndrome. In a lung injury model, we demonstrate here that MYLK was also essential for neutrophil transmigration, but that this function was mostly independent of myosin II regulatory light chain, the only known substrate of MYLK. Instead, MYLK in neutrophils was required for the recruitment and activation of the tyrosine kinase Pyk2, which mediated full activation of β2 integrins. Our results demonstrate that MYLK-mediated activation of β2 integrins through Pyk2 links β2 integrin signaling to the actin motile machinery of neutrophils.
Neutrophils are the first line of defense against invading micro-pathogens. To kill pathogens, neutrophils must first attach to the blood vessel wall, then transmigrate into tissue to reach the site of infection and consume pathogens by phagocytosis1. Therefore, pathogen-induced adhesion and migration of neutrophils are critical events in the innate immune response2,3. In contrast, inappropriate sequestration of neutrophils in tissue coupled with their activation can also cause profound injury, such as that in sepsis-induced acute respiratory distress syndrome4,5.
Myosin light-chain kinase (MYLK; A000026) is a calcium-calmodulin–dependent kinase that phosphorylates myosin II regulatory light chain (MLC), the substrate for MYLK6,7. MYLK has two isoforms: smooth muscle (short form; 108–130 kilodaltons) and nonmuscle (long form; 210 kilodaltons)8,9. Mice deficient in nonmuscle MYLK (Mylk−/− mice) are protected from endotoxin-induced lung injury and also have much better survival10. The protection in Mylk−/− mice may result from inhibition of the endothelial hyper-permeability response and impaired neutrophil transmigration and activation due to the loss of nonmuscle MYLK function. Nonmuscle MYLK has been shown to be pivotal in endothelial barrier disruption through the regulation of actomyosin contractility in endothelial cells11,12. However, the function of nonmuscle MYLK in regulating neutrophil transmigration in the innate immune response has not been investigated.
Integrins are a family of transmembrane receptors for extracellular matrix proteins13. The β2 integrins, the main integrins expressed in neutrophils and other leukocytes, are key effectors mediating the adhesion of neutrophils to endothelial cells during inflammation and other immune responses14. Integrins function not only as adhesive proteins but also as signaling molecules13–16. Intracellular signals such as those stimulated by chemokine receptors activate integrins to an intermediate active state and induce the binding of integrins to their ligands in the extracellular matrix17. Integrin-matrix interactions in turn induce ‘outside-in’ signals that activate the tyrosine kinases c-Src, Syk and Pyk2 (also called Ptk2b; A001952)18, which promote actin polymerization, thereby reinforcing the integrin-cytoskeleton connection. This interaction leads to the full activation of integrins18–21. Therefore, actin dynamics regulated by tyrosine kinases are important for β2 integrin–mediated adhesion of neutrophils to the endothelium14,22–24.
MYLK is involved in integrin-mediated attachment of the leading edge to the matrix in T cells and adhesion disassembly in fibroblasts through the regulation of myosin II (refs. 25,26). In addition to its kinase activity, MYLK interacts with cytoskeletal components such as actin, microtubules and cortactin27–30, which raises the possibility that MYLK can regulate integrin function by organizing the cytoskeletal architecture9. In this study, we demonstrate that the nonmuscle MYLK was required for neutrophil adhesion to and migration across the endothelium in a lipopolysaccharide (LPS)-induced model of lung injury. Unexpectedly, this function of MYLK was mostly independent of myosin II activity. Instead, we found that MYLK was required for actin polymerization and the full activation of β2 integrins and thus mediated neutrophil transendothelial migration. Our results demonstrate that MYLK’s binding to and activation of the tyrosine kinases c-Src and Pyk2 was crucial for the recruitment of Pyk2 to β2 integrins. These observations suggest that MYLK mediates a regulatory pathway in neutrophils during innate immune responses. This pathway may provide a therapeutic target for the prevention and treatment of inappropriate neutrophil migration and sequestration in conditions such as sepsis-induced lung inflammation and injury.
RESULTS
MYLK is required for neutrophil transmigration
Mylk−/− mice are less susceptible to LPS-induced acute lung injury10. We found that after injection of LPS, Mylk−/− mice had a smaller increase in lung vascular permeability and edema formation than did wild-type mice (Supplementary Fig. 1 online). As nonmuscle MYLK is expressed both in endothelial cells and neutrophils (Supplementary Fig. 2 online), the protective effect in Mylk−/− mice could have been the result of either less permeability of the endothelial barrier or impaired migratory ability of neutrophils. To distinguish the function of nonmuscle MYLK in neutrophil transmigration from that in endothelial barrier regulation, we used an ex vivo lung injury assay, perfusing isolated wild-type mouse lungs with neutrophils from Mylk−/− or wild-type mice31. We measured the lung microvessel filtration coefficient and gain of lung wet weight to assess the vascular permeability of lungs and edema formation, respectively. The group with Mylk−/− neutrophil perfusion had significantly lower microvessel filtration coefficient values and wet weight gain (by about 50% in each case) than did the group perfused with wild-type neutrophils in the presence of LPS (Fig. 1a,b). As we used wild-type lungs as recipients in both groups, the difference represents the impaired ability of Mylk−/− neutrophils to cause lung injury. These functional studies demonstrate that MYLK in neutrophils is a critical factor mediating LPS-induced lung injury.
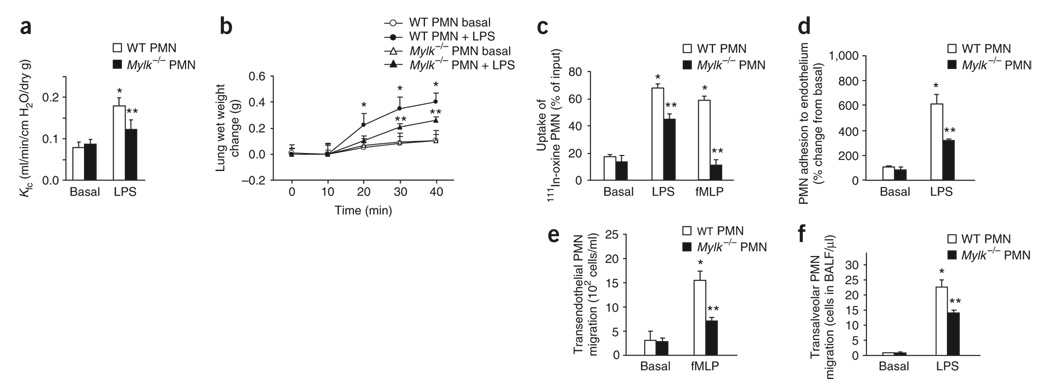
Figure 1. Ex vivo LPS-induced lung injury and edema formation.
(a) Change in microvessel permeability in wild-type lungs after perfusion of wild-type (WT) or Mylk−/− neutrophils (PMN), followed by challenge for 16 h with LPS (10 mg/kg given intraperitoneally), assessed as the pulmonary microvessel filtration coefficient (Kfc) and presented as per gram of dry lung weight (dry g). (b) Time-dependent change in pulmonary edema formation, as assessed by the increase in wet weight of lungs treated as described in a. (c) Sequestration of 111In-oxine–labeled PMNs in lungs after 111In-labeled PMNs were stimulated with LPS or fMLP and then added to perfusates of lung preparations. (d) Adhesion of wild-type and Mylk−/− PMNs to cultured mouse lung vascular endothelial cells. (e) Transmigration of LPS-primed wild-type and Mylk−/− PMNs across endothelial cells toward fMLP in the lower chamber of a Transwell. (f) Migration of PMNs into the airspace of wild-type and Mylk−/− mice challenged with LPS. *, P < 0.05, compared with basal; **, P < 0.05, compared with wild type after LPS stimulation. Data are the mean of five (a,b), four (c–e) or six (f) independent experiments (error bars, s.e.m.).
To examine lung neutrophil sequestration in these studies, we perfused wild-type lungs with 111In-oxine–labeled neutrophils along with LPS, the bacterial chemoattractant fMLP (N-formyl-methionylleucyl-phenylalanine) or saline (control). After washing out unbound neutrophils from vessels, we measured the γ-radioactivity of lung tissue, which represented the resident neutrophils. Although both LPS and fMLP stimulated the uptake of wild-type neutrophils in lungs, the uptake of Mylk−/− neutrophils was significantly lower (by about 40% and 90% in response to each stimulus, respectively; Fig. 1c).
Neutrophil adhesion and migration are two critical events during the transendothelial migration of neutrophils; thus, we addressed by in vitro assay whether either step was affected for Mylk−/− neutrophils. We purified wild-type and Mylk−/− neutrophils and added them to cultured wild-type mouse lung vascular endothelial cells. After washing away nonadherent cells, we counted residual neutrophils to determine adhesive activity. With LPS stimulation, the number of adherent wild-type neutrophils was sixfold higher than without (Fig. 1d), whereas the number of adherent Mylk−/− neutrophils was about 50% less than wild type (Fig. 1d). We analyzed neutrophil migration by Transwell assay with two chambers separated by a monolayer of endothelial cells. We applied LPS-primed wild-type or Mylk−/− neutrophils to the upper chamber and the chemoattractant fMLP to the lower chamber. We assessed cells that had migrated into the lower chamber and attached to the basal side of the filter after 4 h of incubation. The transendothelial migration of LPS-primed wild-type neutrophils was sixfold higher after fMLP stimulation relative to that with LPS only. However, the fMLP-induced migration of LPS-primed Mylk−/− neutrophils was about 55% that of wild-type neutrophils (Fig. 1e). We also consistently noted less LPS-induced transalveolar migration of neutrophils in Mylk−/− mice than in wildtype mice (Fig. 1f). These data demonstrate that both adhesion to endothelial cells and migration across the pulmonary endothelium are substantially impaired in Mylk−/− neutrophils.
We then compared the migratory activity of wild-type and Mylk−/− neutrophils after adherence to a fibrinogen-coated surface in vitro. Wild-type neutrophils after fMLP stimulation migrated in a more linear way with fewer turns. Mylk−/− neutrophils were still able to polarize and migrate, but they frequently changed direction (Supplementary Fig. 3a,b online), which suggested involvement of MYLK in stabilizing the neutrophil leading edge. The MYLK-deficient neutrophils also had migrated faster (Supplementary Fig. 3c). These data collectively show that MYLK is an important determinant of neutrophil migration both in vivo and in vitro.
Myosin II is activated in the absence of MYLK
As MLC is the only known substrate for MYLK (MYLK phosphorylates MLC at Thr18 and Ser19)6,7, we next determined whether MYLK regulates the adhesion and migration of neutrophils secondary to activation of MLC. We assessed MLC activation with an antibody that recognizes the phosphorylated subunit of MLC (p19-MLC). In wild-type lungs and neutrophils, p19-MLC was 1.5-fold higher after LPS stimulation (Fig. 2a,b). Although basal p19-MLC was slightly lower in Mylk−/− lungs (Fig. 2a) and neutrophils (Fig. 2b), MLC was activated after LPS stimulation to amounts similar to those of wildtype. We obtained similar results with fMLP stimulation (Supplementary Fig. 4 online). The question arose of whether MLC is activated by the smooth-muscle MYLK isoform, which is also present in Mylk−/− mice. However, we noted that Mylk−/− neutrophils showed little MYLK activity after LPS stimulation (Supplementary Fig. 5 online). Thus, MYLK was not required for LPS- or fMLP-induced MLC phosphorylation, and the phenotype resulting from MYLK deletion was not mediated by MLC signaling. These results indicate alternative functions for MYLK in mediating neutrophil adhesion and migration.
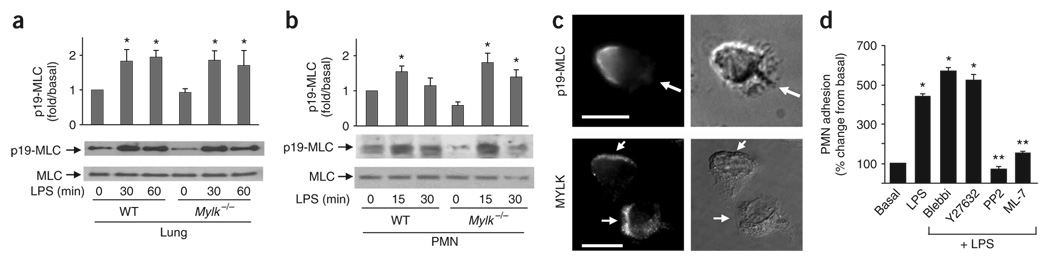
Figure 2. Loss of MYLK function fails to prevent myosin II activation.
(a,b) MLC phosphorylation in wild-type and Mylk−/− lungs (a) or PMNs (b). Bottom blots, confirmation of equal protein loading by analysis with anti-MLC. Above, densitometry analysis. *, P < 0.05, compared with basal (no LPS stimulation). Data are from one experiment representative of three (error bars, s.e.m.). (c) Subcellular distribution of activated myosin II (top; anti-p19-MLC) and MYLK (bottom; anti-MYLK) in polarized PMNs. Arrows indicate leading edges. Scale bars, 10 µm. Results are representative of three experiments. (d) Adhesion of wild-type PMNs preincubated with LPS (1 µg/ml), then cultured for 30 min at 37 °C together with mouse lung vascular endothelial cells with or without inhibitors (horizontal axis). Blebbi, blebbistatin. *, P < 0.05, compared with basal; **, P < 0.05, treatment with inhibitor plus LPS compared with LPS alone. Data are the mean of four independent experiments (error bars, s.e.m.).
That hypothesis was supported by morphological and functional studies of myosin II and MYLK. In polarized neutrophils, active myosin II (as visualized by staining with antibody to p19-MLC (anti-p19-MLC)) accumulated in the rear of cells32 (Fig. 2c). In contrast, MYLK was located mainly at the leading edge of polarized neutrophils (Fig. 2c). In addition to the distinct subcellular distribution of MYLK, loss of function of myosin II or MYLK resulted in different cell migration. Blocking myosin II activity with the inhibitor blebbistatin33 impaired the neutrophil rear contraction, as shown by noncontracted long tails32. However, Mylk−/− neutrophils had normal rear contraction with accumulation of MLC at the trailing edges during migration (Supplementary Figs. 3 and 6 online). Moreover, both Mylk−/− neutrophils and wild-type neutrophils treated with the MYLK inhibitor ML-7 showed less adhesion to the endothelium (Fig. 1d and Fig. 2d). In contrast, the myosin II inhibitor blebbistatin and the Rho kinase inhibitor Y27632 (which blocks myosin II activation34,35) resulted in enhanced neutrophil adhesion (Fig. 2d).
Myosin II is known to mediate the ‘backness’ signal regulated by the RhoA and Rho kinase pathway, which prevents lateral pesudopod formation during neutrophil migration32. Inhibition of the ‘backness’ signal can enhance the ‘frontness’ signal, such as F-actin formation32,36. Wild-type neutrophils treated with blebbistatin or Y27632 had much more actin polymerization before and after LPS stimulation (Supplementary Fig. 7a online). In contrast, F-actin content was lower in Mylk−/− neutrophils than in wild-type neutrophils (40% less; Supplementary Fig. 7b) and was lower in wild-type neutrophils after ML-7 treatment (40% less than no treatment; Supplementary Fig. 7a), which suggested that MYLK does not act as a ‘backness’ signal and has a function opposite to that of myosin II in cytoskeleton assembly. Thus, MYLK and myosin II have distinct subcellular localizations and different functions during neutrophil migration, which indicates an important function for MYLK in regulating neutrophil migration by a pathway other than through MLC phosphorylation.
MYLK is required for β2 integrin activation in neutrophils
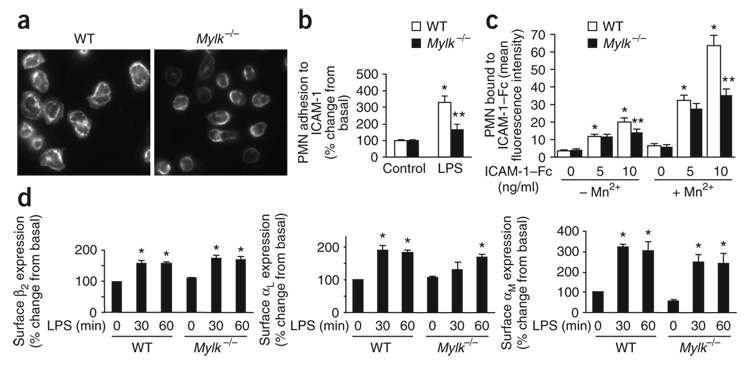
As MYLK regulation of neutrophil adhesion and migration is independent of MLC phosphorylation, we next explored a previously unknown function for MYLK. The β2 integrins are the main cell surface adhesion molecules required for the adhesion of neutrophils to the endothelium14. Because loss MYLK function led to impaired adhesive ability and migration as well as less tissue sequestration of neutrophils (Fig. 1c–e), we examined the possibility that β2 integrin function was disrupted in Mylk−/− neutrophils. On surfaces coated with ICAM-1 (a ligand for β2 integrins), wild-type neutrophils adhered and spread with clustering of β2 integrin, as detected with antibody to αM, an α-subunit of β2 integrins (Fig. 3a,b). In contrast, 50% fewer Mylk−/− neutrophils adhered to ICAM-1 in the presence of LPS with much less spreading (Fig. 3a,b), which indicated that β2 integrin function was impaired in Mylk−/− neutrophils. To assess the activation of β2 integrins, we used a soluble ICAM-1 binding assay. We incubated freshly isolated wild-type and Mylk−/− neutrophils with Fc-tagged ICAM-1 and analyzed them by flow cytometry. LPS-induced ICAM-1 binding in the presence of Mn2+ was significantly lower in Mylk−/− neutrophils than in wild-type neutrophils (Fig. 3c), which indicated that MYLK is required for the activation of β2 integrin. To determine whether the lower integrin activation was due to less surface expression of integrin, we assessed LPS-induced cell surface expression of αL, αM and β2 on wild-type and Mylk−/− neutrophils. LPS increased the expression of all three integrin molecules at the surface of Mylk−/− neutrophils; such expression was similar to that of wild-type neutrophils (Fig. 3d).
Figure 3. Activation of β2 integrin in neutrophils.
(a) Microscopy of wild-type and Mylk−/− PMNs spread on ICAM-1–coated surfaces, then fixed with methanol and stained with anti-aM. Original magnification, ×60. (b) Adhesion to ICAM-1–coated surfaces of wild-type and Mylk−/− PMNs challenged with LPS or not. *, P < 0.05, compared with no LPS; **, P < 0.05, compared with wild type after LPS stimulation. (c) Analysis of β2 integrin activation by soluble ICAM-1 binding assay with (+) or without (−) 10 mM Mn2+. ICAM-1–Fc, Fc-tagged ICAM-1. *, P < 0.05, compared with no stimulation; **, P < 0.05, compared with wild type after Mn2+ stimulation. (d) Flow cytometry of the surface expression of β2 integrins on circulating wild-type and Mylk−/− PMNs. *, P < 0.05, compared with no LPS. Data are from one experiment representative of three (a) or are the mean of four independent experiments (b–d; error bars, s.e.m.).
MYLK is required for activation of Src and Pyk2
Integrin activation can be divided into two stages18–21. ‘Inside-out’ signals activate integrins to an intermediate active state that promotes integrin-ligand binding. After integrin-ligand binding, ‘outside-in’ signals then activate tyrosine kinases such as c-Src, Syk and Pyk2, thereby fully activating integrins, possibly through additional actin polymerization and reinforced integrin-cytoskeleton interaction18–21. To explore how MYLK functions in the mechanism of integrin activation, we first studied whether crosstalk of MYLK with these tyrosine kinases regulated actin polymerization. We assessed activation of c-Src, Syk and Pyk2 with phosphorylation-specific antibodies in wild-type and Mylk−/− neutrophils. All three tyrosine kinases were phosphorylated in wild-type neutrophils after LPS challenge (Fig. 4a). However, phosphorylation of c-Src and Pyk2 was about 80% and 60% lower, respectively, in Mylk−/− neutrophils, whereas phosphorylation of Syk was only slightly lower (Fig. 4a), which indicated that MYLK is required for the activation of c-Src and Pyk2. Furthermore, our functional data showed that blocking c-Src activity with its inhibitor, PP2, resulted in less adhesion of neutrophils to the endothelium (Fig. 2d) and less F-actin formation (Supplementary Fig. 7a), consistent with the results obtained with Mylk−/− neutrophils (Fig. 1d and Supplementary Fig. 7b).
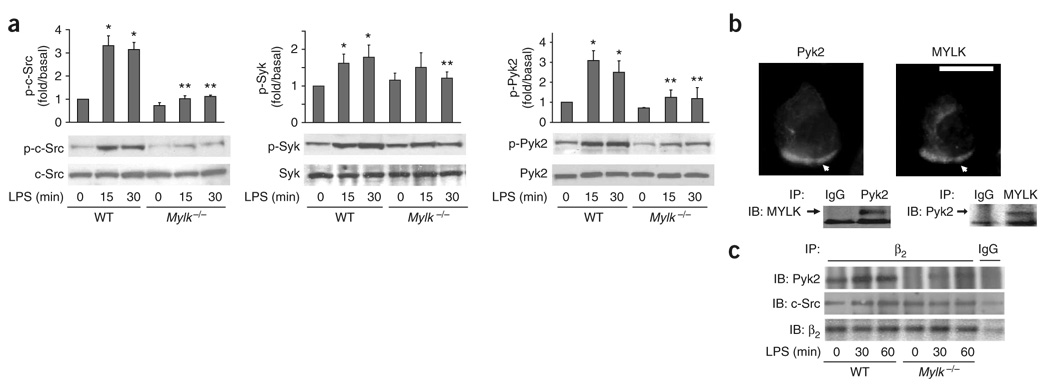
Figure 4. Tyrosine kinase activation and interaction with β2 integrin.
(a) Phosphorylation (p-) of c-Src, Syk and Pyk2 in wild-type and Mylk−/− PMNs. Bottom blots, confirmation of equal protein loading by analysis with anti-c-Src, anti-Pyk2 and anti-Syk. Above, densitometry analysis. *, P < 0.05, compared with no LPS; **, P < 0.05, compared with other groups after LPS stimulation. Data are the mean of four independent experiments (error bars, s.e.m.). (b) Colocalization of MYLK and Pyk2 in wild-type PMNs stimulated with fMLP and stained with anti-MYLK and anti-Pyk2 (above). Arrows indicate leading edges. Scale bar, 10 µm. Below, coimmunoprecipitation of MYLK and Pyk2 in wild-type PMNs. IP, immunoprecipitation; IB, immunoblot. Data are from one experiment representative of three. (c) Association of β2 integrin with c-Src and Pyk2 in wild-type and Mylk−/− PMNs stimulated for 0, 30 or 60 min with LPS (1 µg/ml); equal amounts of protein immunoprecipitated from lysates with anti–β2 integrin are analyzed by immunoblot with anti-Pyk2, anti-c-Src or anti–β2 integrin. IgG, immunoglobulin G (control antibody). Data are representative of three independent experiments.
MYLK has been shown to bind to c-Src37, and here we detected that MYLK was also associated with Pyk2 in both coimmunostaining and coimmunoprecipitation assays (Fig. 4b). Notably, although both c-Src and Pyk2 bound to β2 integrins in wild-type neutrophils (Fig. 4c), the Pyk2–β2 integrin interaction but not the c-Src–β2 integrin interaction was disrupted in Mylk−/− neutrophils (Fig. 4c). We further explored how MYLK regulates Pyk2 activation by in vitro assay. We found that MYLK interacted with Pyk2 directly and phosphorylated Pyk2 in vitro (Supplementary Fig. 8a,b online) and that ML-7 partially blocked Pyk2 activation (Supplementary Fig. 8c), which indicated that the kinase activity of MYLK and the MYLK-Pyk2 interaction may both be required for Pyk2 activation. These results collectively demonstrate that MYLK is critical for the activation both c-Src and Pyk2 and is required for the recruitment of Pyk2 to the β2 integrin complex.
MYLK regulates the β2 integrin–cytoskeleton interaction
The interaction of integrin with F-actin through actin-associated proteins such as talin is critical for strong integrin-cytoskeleton connections and integrin activation18. Pyk2 has been shown to activate the small Rho GTPase Cdc42 by inhibiting PSGAP (a Rho GTPase–activating protein), thus promoting actin polymerization38. We found less F-actin formation in Mylk−/− neutrophils (Supplementary Fig. 7b), which suggested that Pyk2-mediated actin polymerization and β2 integrin–cytoskeleton interactions could be a chief signaling mechanism ‘downstream’ of MYLK responsible for neutrophil transmigration.
To test that hypothesis, we determined whether MYLK participates in mediating β2 integrin–cytoskeleton assembly. Although β2 integrins bound to talin in wild-type neutrophils15, this binding was much lower in Mylk−/− neutrophils (Fig. 5a). We obtained similar results for the interaction of β2 integrin with another actin-associated protein, α-actinin (Fig. 5b). However, we did not detect any interaction of MYLK with β2 integrins, talin or α-actinin in wild-type neutrophils (data not shown). Thus, MYLK may indirectly regulate the interaction of β2 integrins and actin-associated proteins, possibly through Pyk2-mediated actin polymerization. Our results have collectively shown that MYLK is required for the activation of c-Src and Pyk2 during the transendothelial migration of neutrophils and for the recruitment of Pyk2 to the β2 integrin complex. The activation of tyrosine kinases in turn is essential in the mechanism of actin polymerization; it reinforces the β2 integrin–cytoskeleton connection and fully activates β2 integrins, a crucial requirement for the adhesion and transmigration of neutrophils (Supplementary Fig. 9 online).
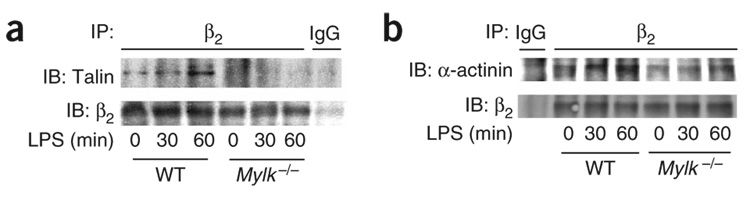
Figure 5. The interaction of β2 integrin and the cytoskeleton in neutrophils.
Association of β2 integrin with the actin-associated proteins talin (a) and α-actinin (b) in wild-type and Mylk−/− PMNs stimulated for 0, 30 or 60 min with LPS (1 µg/ml); equal amounts of protein immunoprecipitated from lysates with anti–β2 integrin are analyzed by immunoblot with anti-talin or anti-α-actinin. IgG, control antibody. Data are representative of three independent experiments.
DISCUSSION
The nonmuscle isoform of MYLK is fundamental in the regulation of endothelial barrier function11,12. Studies have also shown that Mylk−/− mice are less susceptible to endotoxin-induced lung injury10. However, such studies have not distinguished the function of MYLK in neutrophils from that in endothelial cells. In our study here, the use of an ex vivo sepsis model and isolated neutrophils allowed us to address the function of MYLK in the mechanism of neutrophil transmigration in lungs. We noted that loss of MYLK function inhibited neutrophil sequestration and transalveolar migration in wild-type lungs and, moreover, the lower sequestration of Mylk−/− neutrophils resulted in less of a neutrophil-mediated increase in pulmonary vascular permeability and lung edema formation. Our findings have shown that activation of MYLK in neutrophils resulting in the adhesion of neutrophils to pulmonary vascular endothelium is essential in neutrophil-mediated inflammatory lung injury.
Although MLC is the only known substrate of MYLK, our findings have indicated that the function of MYLK in regulating neutrophil adhesion and migration is not mediated by MLC phosphorylation.We found that MLC could still be phosphorylated in Mylk−/− neutrophils. Although the smooth-muscle isoform of MYLK is also present in Mylk−/− mice, we found that Mylk−/− neutrophils showed little MYLK activity after LPS stimulation, which suggests that activation of MLC in Mylk−/− mice is not mediated by the smooth-muscle isoform MYLK. It is possible, however, that MLC phosphorylation in neutrophils is regulated by other kinases such as Rho kinase and Zip kinase34,35,39. MLC and MYLK also showed distinct subcellular localizations and different functions during neutrophil transmigration, consistent with the biochemical data. MYLK was localized to the leading edge of polarized neutrophils, whereas active MLC accumulated at the trailing edge. Furthermore, blocking myosin II and MYLK produced opposite effects on both neutrophil adhesion and actin polymerization. These data collectively indicate that MYLK has a MLC phosphorylation–independent function that mediates neutrophil adhesion and migration during LPS-induced lung injury.
MYLK, in addition to having a kinase domain, contains sites that bind to cytoskeletal proteins and tyrosine kinases27,29,37, which suggests that MYLK may function as an adaptor protein through these interactions. We have provided evidence here that in addition to binding to the tyrosine kinase c-Src, MYLK also bound to Pyk2. MYLK interacted with Pyk2 directly and phosphorylated Pyk2 in vitro, and ML-7 partially blocked Pyk2 activation, which indicates that the kinase activity of MYLK and the MYLK-Pyk2 interaction may be both required for Pyk2 activation. Nevertheless, it remains unclear whether MYLK can directly phosphorylate Pyk2 in vivo. It has been reported that activation of Pyk2 activates Cdc42 and subsequently induces actin polymerization38. We noted that loss of MYLK also abolished Cdc42 activation (data not shown) as well as actin polymerization stimulated by LPS. Thus, MYLK may be required for the activation of a tyrosine kinase–Cdc42–actin signaling pathway mediating neutrophil adhesion and migration.
The β2 integrins are the main adhesion molecules expressed in neutrophils and are responsible for mediating the adhesion of neutrophils to the endothelium. Our data showed lower activation of β2 integrins in Mylk−/− neutrophils. In the two-step model of integrin activation18–21, both c-Src and Pyk2 are the ‘outside-in’ signals activated after integrin-matrix binding and thereby promote actin polymerization24,38,40. The additional actin polymerization reinforces the integrin-cytoskeleton interaction, which sustains β2 integrins in the high-affinity and high-avidity state, reflecting the full activation of integrins18–21. Here we have shown that in addition to being required for integrin-matrix binding, MYLK is also required for the activation of c-Src and Pyk2, and it thereby contributes to a later stage of β2 integrin activation through the recruitment and activation of Pyk2 to the integrin complex. In this model, it remains unclear how MYLK regulates Src, although our data have shown that Src activation depended on the presence of MYLK. Src is not a substrate for MYLK, as ML-7 did not inhibit Src activation (data not shown). Src has been shown to directly phosphorylate MYLK37, but it is also possible, as evident from our results, that MYLK can itself regulate Src through a feedback mechanism.
Although MYLK is known as myosin II regulatory light-chain kinase, we have identified here an MLC phosphorylation–independent function for MYLK in complex with c-Src and Pyk2. This function of MYLK was essential for neutrophil transmigration during sepsis-induced lung injury. In contrast to myosin II, which is known as a ‘backness’ molecule responsible for rear contraction, MYLK functions as a ‘frontness’ molecule that accumulates at the front of neutrophils, where it is responsible for actin polymerization and the full activation of β2 integrins.
METHODS
Mice, cell cultures and reagents
Wild-type C57BL/6 mice were from Charles River Laboratories. Mice deficient in MYLK were gifts from D.M. Watterson10. All mice were bred and housed in pathogen-free conditions with free access to food and water in the Animal Care Facility. All experimental procedures complied with institutional and National Institutes of Health guidelines for animal use. Mouse neutrophils were purified from mouse bone marrow and peripheral venous blood with a discontinuous Percol gradient as described with some modifications41. This procedure yielded polymorphonuclear neutrophil (PMN) purity of over 95% and viability of over 95%, as assessed by Trypan blue exclusion and analysis of cytospin preparations (Shandon) stained with the Hema 3 system (Fisher)31. Culture of neutrophil-like dHL60 cells and transfection were done as described32. MYLK cDNA was from Open Biosystems. Pyk2 cDNA was a gift from X. Zhu. Antibodies to MYLK were from Sigma (M7905) and Abgent (AP79669). Antibodies to p19-MLC (3671) and to phosphorylated c-Src (2113), Pyk2 (3291) and Syk (2715), as well as purified glutathione S-transferase–MYLK (Gly1425–Ser1776), were from Cell Signaling. Pyk2 protein was from Millipore.
Lung injury and neutrophil sequestration ex vivo
The pulmonary microvessel filtration coefficient and increases in lung wet weight were assessed as described31. For analysis of neutrophil sequestration, isolated bone marrow neutrophils were labeled with 111In-labeled oxine (Amersham) as described42. Neutrophils (1 × 106) were infused over 5 min with a syringe pump into the pulmonary artery line of the lung preparation, followed by a 30-minute washout period. Venous effluent samples were collected at 5-minute intervals and radioactivity was measured. The number of accumulating radiolabeled neutrophils per gram of dry lung was calculated at the end of the washout period.
MYLK activity assay
The activation of MYLKwas measured in total cell lysates as described43. Cells were suspended in ice-cold kinase buffer (40 mM HEPES, pH 7.0, 5 mM Mg acetate, 0.55 mM CaCl2 and 0.1% (vol/vol) Tween-80) containing freshly added 1 mM Na3VO4, 5 mM NaF, 1 mM phenylmethyl-sulfonyl fluoride, 10 µg/ml of pepstatin, 10 µg/ml of leupeptin, 10 µg/ml of aprotinin and 100 µg/ml of soybean trypsin inhibitor, then were disrupted by a cycle of freezing and thawing. Samples were sonicated with a probe twice for 12 s each on ice and were centrifuged with a microfuge. MYLK activity was assessed in supernatants of cell lysates in the presence or absence of substrate. A saturating concentration of 300 µM substrate was used for the MYLK activity assay. Reaction mixture containing substrate peptide or buffer plus 5 µCi [γ-32P]ATP and 0.5 mM unlabeled ATP was added to each tube and samples were incubated for 10 min at 30 °C. Reactions were stopped by filtration through Whatman P81 paper. After being washed, filters were added to scintillation fluid and radioactivity was measured in a scintillation counter (Wallac). ‘Blanks’ were assay samples without substrate.
F-actin content of neutrophils
F-actin was measured in neutrophils as described with some modifications44. Cells were stimulated for various times at 37 °C with LPS (Escherichia coli 0111:B4; EMD Chemicals) or fMLP (Sigma). Cells were immediately fixed for 30 min at 25 °C with 3.7% (vol/vol) paraformaldehyde in PBS and were washed with PBS. Cells were simultaneously made permeable and stained for 30 min at 37 °C in the dark with a fresh mixture of l-α-lysophosphatidylcholine palmitoyl (0.5 mg/ml; Sigma) and N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-phallacidin (0.1 µg/ml; Molecular Probes). After being stained, samples were washed and resuspended in PBS. F-actin was measured with fluorescein phalloidin (Invitrogen) with a Triad LT plate reader at excitation of 495 nm and an emission of 520 nm (Dynex Technologies).
Immunostaining and confocal microscopy
Freshly isolated neutrophils (2 × 106) added to coverslips coated with fibrinogen (10 µg/ml) were challenged for various times at 37 °C with LPS (1 µg/ml; E. coli 0111:B4; EMD Chemicals). Cells were then fixed and made permeable and were stained sequentially with the appropriate antibodies. Images for confocal microscopy were obtained with a Zeiss LSM 510 microscope with excitation laser lines of 488 nm and 543 nm.
Neutrophil adhesion to endothelial cells and transendothelial migration
Mouse lung vascular endothelial cells were isolated45 and grown to confluence in 96-well gelatin-coated plates. Neutrophils loaded with calcein (acetoxy-methyl ester) were added to mouse lung vascular endothelial cells and PMN adhesion to and migration on endothelial cells were assayed as described45.
Immunoprecipitation and immunoblot analysis
For immunoblot analysis, lysates were prepared as described45. Phosphorylation of MLC was measured as described46. For densitometry, the optical density of bands on autoradiograms was assessed with scanned autoradiograph films and the National Institutes of Health Image program.
Statistical analysis
Statistical comparisons were made with the two-tailed Student’s t-test. Differences in mean values were considered significant at a P value of less than 0.05.
Supplementary Material
Note: Supplementary information is available on the Nature Immunology website.
ACKNOWLEDGMENTS
We thank D.M. Watterson (Northwestern University) for Mylk−/− mice; X. Zhu (University of Chicago) for Pyk2 cDNA; X. Zhu, X. Du, R. Ye and Y. Li for suggestions and reading the manuscript; R.A. Skidgel and T. Sharma (Department of Pharmacology Molecular Core Facility) for making glutathione S-transferase–Pyk2; and G. Liu, C. Gilbert, S. Debra and K. Javaid for technical assistance. Supported by the University of Illinois (J.X.) and the US National Institutes of Health (HL77806 and HL46350 to A.B.M.).
Footnotes
Accession codes. UCSD-Nature Signaling Gateway (http://www.signaling-gateway.org): A000026 and A001952.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Cohen MS. Molecular events in the activation of human neutrophils for microbial killing. Clin. Infect. Dis. 1994;18 Suppl 2:S170–S179. doi: 10.1093/clinids/18.supplement_2.s170. [DOI] [PubMed] [Google Scholar]
- 2.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L419–L422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, et al. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 2004;172:7–13. doi: 10.4049/jimmunol.172.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Simpson SQ, Casey LC. Role of tumor necrosis factor in sepsis and acute lung injury. Crit. Care Clin. 1989;5:27–47. [PubMed] [Google Scholar]
- 5.Crockett-Torabi E, Ward PA. The role of leukocytes in tissue injury. Eur. J. Anaesthesiol. 1996;13:235–246. doi: 10.1046/j.1365-2346.1996.00982.x. [DOI] [PubMed] [Google Scholar]
- 6.Adelstein RS. Regulation of contractile proteins by phosphorylation. J. Clin. Invest. 1983;72:1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 8.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 9.Kudryashov DS, et al. Unique sequence of a high molecular weight myosin light chain kinase is involved in interaction with actin cytoskeleton. FEBS Lett. 1999;463:67–71. doi: 10.1016/s0014-5793(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright MS, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc. Natl. Acad. Sci. USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J. Cell. Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 12.Yuan SY, et al. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ. Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 14.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J. Leukoc. Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr. Opin. Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol. Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 18.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 19.van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol. 2000;12:542–547. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 20.Totani L, et al. Src-family kinases mediate an outside-in signal necessary for β2 integrins to achieve full activation and sustain firm adhesion of polymorphonuclear leucocytes tethered on E-selectin. Biochem. J. 2006;396:89–98. doi: 10.1042/BJ20051924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamri R, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 22.Advani A, Marshall SM, Thomas TH. Impaired neutrophil actin assembly causes persistent CD11b expression and reduced primary granule exocytosis in type II diabetes. Diabetologia. 2002;45:719–727. doi: 10.1007/s00125-002-0802-0. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SI, Hotchin NA, Nash GB. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent downregulation in neutrophils. J. Cell Sci. 2000;113:2737–2745. doi: 10.1242/jcs.113.15.2737. [DOI] [PubMed] [Google Scholar]
- 24.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 25.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MYLK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 26.Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MYLK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 27.Kudryashov DS, et al. Myosin light chain kinase (210 kDa) is a potential cytoskeleton integrator through its unique N-terminal domain. Exp. Cell Res. 2004;298:407–417. doi: 10.1016/j.yexcr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Vilitkevich EL, Kudriashev DS, Stepanova OV, Shirinskii VP. A new actin-binding area of the myosin light chains’ high-molecular kinase. Ross. Fiziol. Zh. Im. I. M. Sechenova. 2004;90:577–585. [PubMed] [Google Scholar]
- 29.Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 30.Smith L, et al. Properties of long myosin light chain kinase binding to F-actin in vitro and in vivo. J. Biol. Chem. 2002;277:35597–35604. doi: 10.1074/jbc.M206483200. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, et al. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J. Immunol. 2001;167:2895–2901. doi: 10.4049/jimmunol.167.5.2895. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 33.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 34.Sutton TA, Mang HE, Atkinson SJ. Rho-kinase regulates myosin II activation in MDCK cells during recovery after ATP depletion. Am. J. Physiol. Renal Physiol. 2001;281:F810–F818. doi: 10.1152/ajprenal.2001.281.5.F810. [DOI] [PubMed] [Google Scholar]
- 35.Ueda K, Murata-Hori M, Tatsuka M, Hosoya H. Rho-kinase contributes to diphosphorylation of myosin II regulatory light chain in nonmuscle cells. Oncogene. 2002;21:5852–5860. doi: 10.1038/sj.onc.1205747. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Wang F, Van Keymeulen A, Rentel M. Neutrophil microtubules suppress polarity and enhance directional migration. Proc. Natl. Acad. Sci. USA. 2005;102:6884–6889. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birukov KG, et al. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60Src. J. Biol. Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 38.Ren XR, et al. Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J. Cell Biol. 2001;152:971–984. doi: 10.1083/jcb.152.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata-Hori M, Suizu F, Iwasaki T, Kikuchi A, Hosoya H. ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells. FEBS Lett. 1999;451:81–84. doi: 10.1016/s0014-5793(99)00550-5. [DOI] [PubMed] [Google Scholar]
- 40.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakur ML, et al. Indium-111-labeled cellular blood components: mechanism of labeling and intracellular location in human neutrophils. J. Nucl. Med. 1977;18:1022–1026. [PubMed] [Google Scholar]
- 43.Mansfield PJ, Hinkovska-Galcheva V, Carey SS, Shayman JA, Boxer LA. Regulation of polymorphonuclear leukocyte degranulation and oxidant production by ceramide through inhibition of phospholipase D. Blood. 2002;99:1434–1441. doi: 10.1182/blood.v99.4.1434. [DOI] [PubMed] [Google Scholar]
- 44.Betsuyaku T, et al. A functional granulocyte colony-stimulating factor receptor is required for normal chemoattractant-induced neutrophil activation. J. Clin. Invest. 1999;103:825–832. doi: 10.1172/JCI5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao XP, et al. Inactivation of CD11b in a mouse transgenic model protects against sepsis-induced lung PMN infiltration and vascular injury. Physiol. Genomics. 2005;21:230–242. doi: 10.1152/physiolgenomics.00291.2004. [DOI] [PubMed] [Google Scholar]
- 46.Vogel SM, et al. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol. Genomics. 2000;4:137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Immunology website.