Abstract
The dental follicle (DF) differentiates into the periodontal ligament. In addition, it may be the precursor of other cells of the periodontium, including osteoblasts and cementoblasts. We hypothesized that stem cells may be present in the DF and be capable of differentiating into cells of the periodontium. Stem cells were identified in the DF of the rat first mandibular molar by Hoechst staining, alkaline phosphatase staining, and expression of side-population stem cell markers. These cells were shown to be able to differentiate into osteoblasts/cementoblasts, adipocytes, and neurons. Treating the DF cell population with doxorubicin, followed by incubation in an adipogenesis medium, suggested that the adipocytes originated from stem cells. Thus, a possibly puripotent stem cell population is present in the rat DF.
Keywords: stem cells, dental follicle, differentiation
INTRODUCTION
The dental follicle (DF), a loose connective tissue sac that surrounds the unerupted tooth, plays different roles in the life of a tooth. Its presence is required for eruption (Cahill and Marks, 1980; Marks and Cahill, 1984), whereby it appears to regulate the osteoclastogenesis and osteogenesis needed for eruption (e.g., see review by Wise et al., 2002, 2005; Wise and Yao, 2006). As the tooth pierces the gingiva, the DF differentiates into the periodontal ligament (PDL) to anchor the tooth in its socket to the surrounding alveolar bone. In addition to this differentiation of the DF to form the fibroblasts of the PDL, it has been postulated that some DF cells may differentiate into the cementoblasts of the tooth, as well as perhaps some of the osteoblasts of the alveolar bone (e.g., see review by Bosshardt and Schroeder, 1996). Although some studies suggest that some of the cementoblasts may arise from Hertwig’s epithelial root sheath, as well as from the DF (Zeichner-David et al., 2003), other studies indicate that all of the cementoblasts arise from the DF (Diekwisch, 2001).
If the DF cells are differentiating into cell types other than the fibroblasts of the PDL, perhaps it is stem cells within the DF that are forming these other cell types. As is well-known, adult stem cells from a variety of sources have the capacity to differentiate into a limited number of cell types (i.e., they are pluripotent), whereas embryonic stem cells are totipotent and likely have the capacity to differentiate into any cell type that would originate from the endoderm, mesoderm, or ectoderm.
Stem cells appear to be present in the DF of human wisdom teeth (Morsczeck et al., 2005) and in the mouse DF (Luan et al., 2006). Regarding the latter, the authors cloned 3 DF cell lines, one of which likely was the fibroblastic DF cells that form the PDL, another that remained undifferentiated, and a third that exhibited mineralization behavior as revealed by von Kossa staining. Injection of bovine dental follicle cells into immunodeficient mice results in the formation of cementum, as detected by an anti-cementum attachment protein (Handa et al., 2002). More recently, a cementoblast progenitor cell line from bovine DF cells has been isolated and immortalized (Saito et al., 2005).
Given the above background, the objectives of this study were three-fold: First, are stem cells present in the rat DF, as appears to be the case in humans, mice, and cows? Second, what are the differentiation capabilities of these stem cells? For example, can they differentiate only into cells derived from mesoderm, or can they differentiate into cell types that originate from other germ layers? Third, what are the properties of the stem cells such that they might be isolated in a cost-effective and efficient manner?
MATERIALS & METHODS
Establishment of Cell Cultures from the Rat Dental Follicle
Dental follicles were surgically removed from the first mandibular molars of rat pups at days 5–7 post-natally, and the isolated DF cells were then suspended in MEM medium (normal growth medium for DF cells) containing 10% newborn calf serum (NCS), 1 mM sodium pyruvate, and antibiotics as previously described (Wise et al., 1992). Next, a 5-mL quantity of the cell suspension was transferred into a T-25 cell culture flask and incubated overnight to allow the cells to adhere. The non-adherent cells were then removed by aspiration, and the remaining adherent cells were either grown as described previously (Wise et al., 1992), referred to here as DF cells, or grown in a stem cell growth medium consisting of α-MEM and 20% heat-inactivated fetal bovine serum (FBS), referred to here as DF stem cells. For either culture, upon confluence, the cells were trypsinized and passaged into new flasks until the desired passages were obtained. The animal use protocol was approved by the Institutional Animal Care and Use Committee of Louisiana State University.
Expression of the ABC Transport Gene
Total RNA was extracted from the DFs of the post-natal rat first mandibular molars (day 5 to day 11) and from the cultured DF cells and DF stem cells of passage 3 with a TRI REAGENT protocol (Molecular Research Center, Cincinnati, OH, USA). The RNA samples were digested with DNase I to remove any possible DNA. RNA concentration was measured by OD260 with the OD260/OD280 ratio greater than 1.9.
Gene expression of a side-population stem cell marker, BCRP, was determined by conventional RT-PCR. For this, the RNA was reverse-transcribed into cDNA with reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The PCR was conducted by the mixing of cDNA with gene-specific primers, dNTP, PCR buffer, and Taq DNA polymerase, followed by the running of 30 thermal cycles at (denaturing) 94°C for 45 sec, (annealing) 58°C for 1 min, and (extension) 72°C for 1 min. PCR was also conducted to amplify in parallel the β-actin gene that served as the control. The primer sequences for BCRP were as follows: 5′ AGTCCGGAAAACAGCTGAGA 3′ (forward), and 5′ CCCATCACAACGTCATCTTG 3′ (reverse). PCR products underwent electrophoresis in a 1% agarose gel and were visualized under UV light.
Cell Growth and Differentiation Experiments
To characterize the cell growth in culture, we seeded DF cells and DF stem cells at passage 3 and passage 9 in 3-cm-diameter tissue culture Petri dishes and cultured them with appropriate media (i.e., DF cells in MEM + 10% NCS + sodium pyruvate ; DF stem cells in α-MEM + 20% FBS).
To monitor for potential stem cells, we fixed cultures of either DF cells or DF stem cells in neutral-buffered formalin after culturing them for 1, 3, 5, 7, 9, 11, 13, and 15 days and then stained them for alkaline phosphatase (ALP) activity by incubating them in ALP substrate solution containing 0.01% Naphthol AS-Mx phosphate for 15 min at room temperature. To determine if the cells could differentially pump out Hoechst, the substrate of BCRP, we added Hoechst 33342 to the culture medium at a final concentration of 0.2 µg/mL and then examined it under an inverted fluorescence microscope for blue nuclear staining after 20 min of incubation.
To determine the differentiation capabilities of the putative stem cells, we subjected DF stem cells to various differentiation procedures. For osteogenesis, cells were cultured for 2 wks in medium (Hung et al., 2002) consisting of DMEM-LG, 10% FBS, 50 µg/mL ascorbate-2 phosphate, 10−8 M dexamethasone , and 10 mM β-glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA), followed by von Kossa and Alizarin Red staining to assess for mineral deposition. In the absence of antibodies, which are specific for cementoblasts in the rat, and rat cDNA sequences for CAP or CEM-1, our method to detect osteoblasts may also be detecting cementoblasts. For adipogenesis, cells were cultured for 2 wks in medium (Hung et al., 2002) consisting of DMEM-LG, 10% FBS (Invitrogen Corporation, Carlsbad, CA, USA), 50 µg/mL ascorbate-2 phosphate (Sigma-Aldrich), 10−7 M dexamethasone (Sigma-Aldrich), and 50 µg/mL indomethacin (Sigma-Aldrich), with a medium change every 3 days. We then stained the cells with Oil Red O to evaluate adipogenesis.
To induce neurogenesis, we initially treated DF stem cells in a transition medium of DMEM-LG with 10% FBS and 10 ng/mL basic fibroblast growth factor for 24 hrs. Next, the cells were incubated in a neuronal induction medium of DMEM-LG, 2% DMSO, 200 µM butylated hydroxyanisole, 25 mM KCl, 2 mM valporic acid, 10 µM forskolin, 1 µM hydroxycortisone, 5 µg/mL insulin, and 2 mM L-glutamine. After 24 hrs of incubation, the cells were fixed and immunostained for neurofilament-200. Briefly, cells were fixed in cold methanol for 5 min, and incubated with anti-neurofilament-200 (Chemicon, Temecula, CA, USA) overnight at 4°C. Immunostaining controls were incubated with rabbit IgG instead of primary antibody. Next, they were incubated with secondary antibody labeled with horseradish peroxidase, followed by 3 washes with PBS. The horseradish peroxidase was detected by incubation with a DAB substrate.
In another experiment to determine if the DF stem cells were indeed the adipocyte precursors, we treated DF stem cells with doxorubicin (DOX), a substrate of BCRP, at concentrations of 0.5, 1, or 2 µM for 1, 2, 4, and 6 hrs. The surviving cells were placed in an adipogenesis differentiation medium, as previously described.
RESULTS
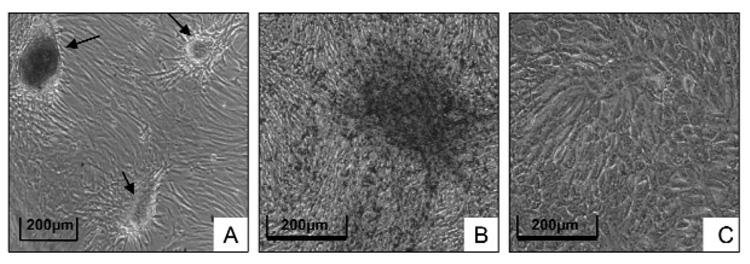
When the DF cells were grown in a stem cell growth medium, clusters of cells formed after 2 wks (Fig. 1A) that were not seen when the cells were grown in normal MEM medium (Fig. 1C). In turn, these clusters stained for ALP (Fig. 1B), which, among other things, is a potential marker for stem cells when it is expressed on the cell membrane. No ALP staining was seen in DF cells grown in normal MEM medium (Fig. 1C).
Figure 1.
DF cells grown in a stem cell medium form cell clusters after 2 wks of culture (A), and the clusters stained positive for alkaline phosphatase (B). In contrast, DF cells grown in the normal MEM medium did not form cell clusters, and no alkaline phosphatase staining was seen (C). (This Figure appears in color in the online version.)
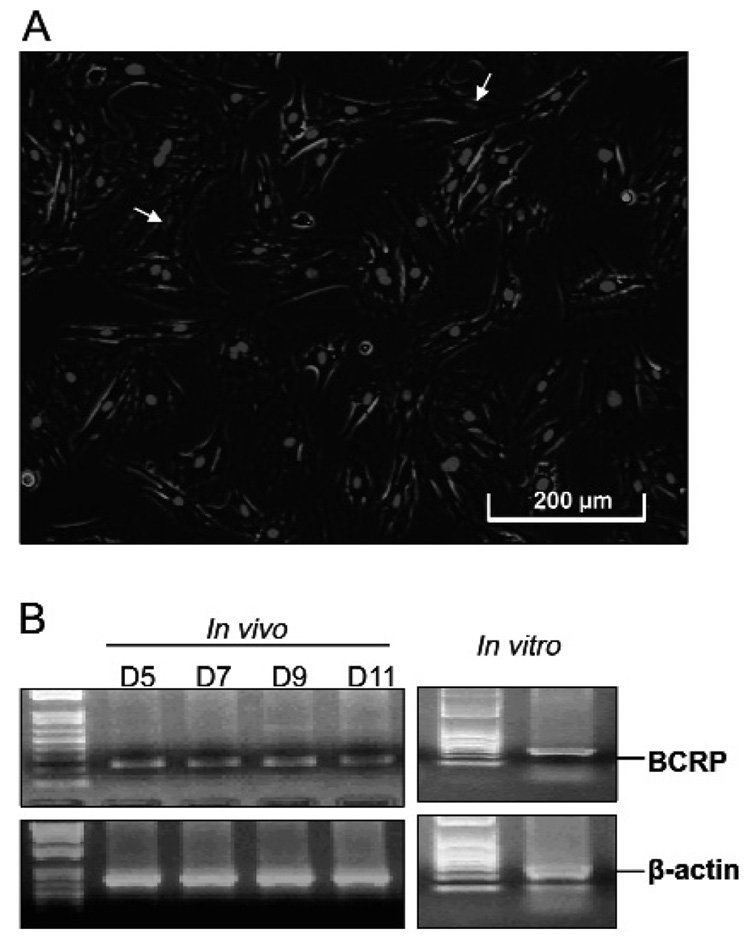
Growing the DF cells in stem cell medium and then staining with Hoechst 33342 to detect side-population stem cells showed that some 2–4% of the cells weakly fluoresced (Fig. 2A). Such cells likely are stem cells, because side-population stem cells cause the efflux of the dye with BCRP, an ABC membrane transporter. Conventional RT-PCR confirmed that the BCRP gene was expressed by the cells in the DF both in vivo and in vitro (Fig. 2B).
Figure 2.
Fluorescent staining of DF cells with Hoechst 33342. Note that the fluorescence staining of nuclei was weaker in some cells (arrows), putative stem cells, as compared with the majority with bright fluorescence (A). RT-PCR to detect gene expression of BCRP. Note that BCRP expression was detected in the DF of the rat first mandibular molar at post-natal days 5, 7, 9, and 11 in vivo, as well as in the cultured DF cells (B).
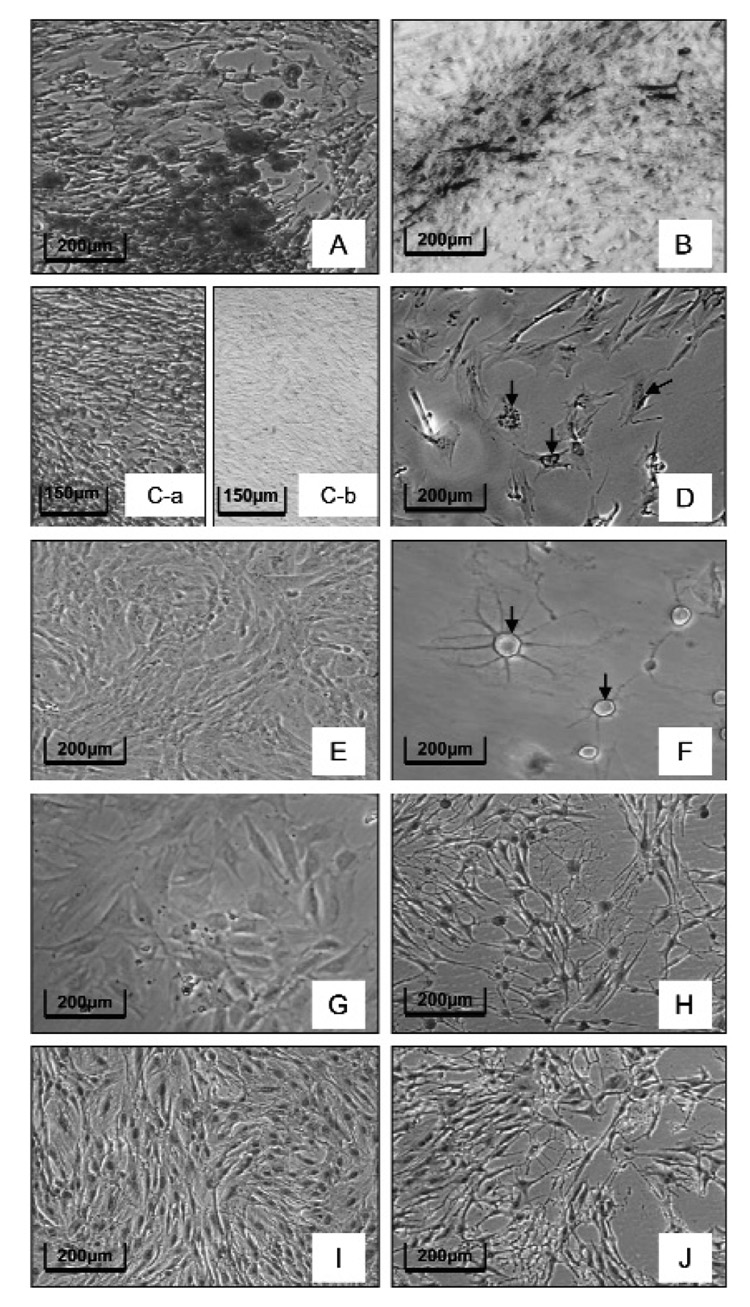
To determine the differentiation capabilities of the putative stem cells, we placed cells grown in the stem cell medium in different differentiation media. When placed in an osteogenic differentiation medium for 2 wks, osteoblasts/cementoblasts formed and developed mineralization nodules (clusters), as revealed by Alizarin red staining (Fig. 3A) and von Kossa staining (Fig. 3B). No staining was seen if the cells were not placed in the osteogenic medium (Figs. 3C-a, 3C-b). When cells grown in stem cell medium were placed in an adipogenic medium for 2 wks and then stained with Oil Red O, stained adipocytes were observed (Fig. 3D), whereas none was observed if the cells were not placed in the adipogenic medium (Fig. 3E). Finally, placing cells into a neuronal induction medium for 24 hrs resulted in the development of cells that resembled multipolar neurons (Fig. 3F). No neurons were seen in the controls in which the cells were not in the neuronal induction medium (Fig. 3G). The use of a marker for late neuron differentiation, neurofilament 200, resulted in immunostaining of the neurons in the induction medium (Fig. 3H), with no immunostaining seen in the controls not placed in the neuron induction medium (Fig. 3I). The controls in which primary antibody was absent did not stain (Fig. 3J).
Figure 3.
Differentiation of DF stem cells into various cell types. Alizarin red staining (A) and von Kossa staining (B) to determine osteogenic differentiation: The red staining in Alizarin red and black staining in von Kossa indicate the deposition of mineralization (A,B). No such staining was seen in the controls not subjected to osteogenic induction (C-a, control for Alizarin red staining; C-b, control for von Kossa staining). Oil Red O stain to detect adipogenesis (D). Note the stained adipocytes (arrow) in the induction treatment (D) vs. no adipocytes in the control without adipogenic induction (E). Multipolar neurons (arrow) were seen in the neuron induction treatment (F), while no neurons were seen in the control maintained in the stem cell medium (G). Immunostaining for neurofilament 200, a late neuron differentiation marker (H). Note that the heavy staining was seen in the induced neurons (H), while no staining appeared in the control cells maintained in the growth medium only (I). No immunostaining was seen when primary antibody was substituted with IgG in the immunostaining controls (J).
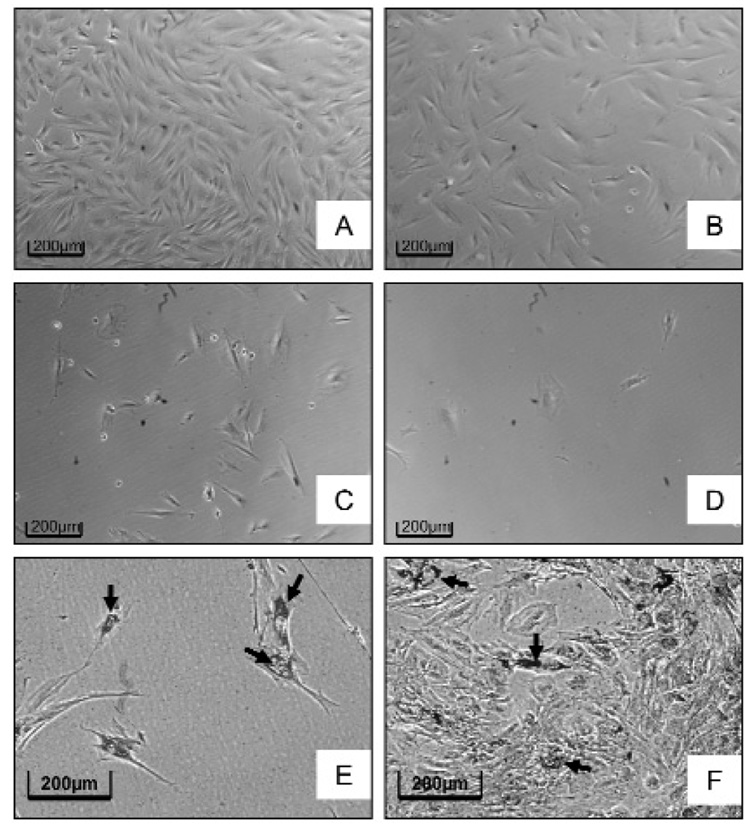
As a method to show that the differentiated cells were derived from stem cells, we treated the DF cells in stem cell medium with DOX for 1, 2, 4, or 6 hrs. DOX, a substrate of BCRP, is an anthracycline cancer drug that is effluxed from certain types of stem cells and thus should kill the non-stem cells, but not the stem cells. The results indicated that, after 4 hrs of incubation in 1 µM DOX, less than 20% of the cells had survived, and by 6 hrs, almost all of the cells were dead (Figs. 4A–4D). Thus, removing the cells from DOX at either 2 or 4 hrs and incubating them in adipogenic medium resulted in the majority of the cells that had been treated for 4 hrs in DOX differentiating into adipocytes (Fig. 4E), in contrast to the controls (Fig. 4F).
Figure 4.
Treatment of the DF cells with doxorubicin (DOX). The number of surviving cells was greatly reduced as the duration of DOX (1 µM) incubation was increased from 2 hrs (B), to 4 hrs (C), and to 6 hrs (D), as compared with the control without DOX treatment (A). The placement of cells treated with DOX for 4 hrs in an adipogenesis induction medium resulted in the majority of the cells forming Oil Red O-positive adipocytes (arrow) (E), whereas in the controls not treated with DOX, but placed in adipogenesis induction medium, the majority of the cells remained undifferentiated, with only a few forming adipocytes (arrow) (F). The results suggest that DOX kills the non-stem cells, and that adipocytes are derived from the stem cells. (This Figure appears in color in the online version.)
DISCUSSION
This study demonstrates the presence of stem cells in the DF of the rat and extends the finding of such cells in the DF to another species in addition to their presence in the DF of human (Morsczeck et al., 2005) and mouse molars (Luan et al., 2006). The ability of these stem cells to differentiate into osteoblasts is likely, given that, after cells in stem cell medium are placed in an osteogenic induction medium, the various clusters of cells that form stain with Alizarin Red and von Kossa staining. Such staining was also observed with human DF stem cells (Morsczeck et al., 2005).
The above staining measures a calcification process and thus may be detecting cementum, as well as osteoid. Unfortunately, a specific antibody to detect cementum in the rat is not available. A 55-kDa cementum attachment protein (CAP) has been isolated from human and bovine teeth (McAllister et al., 1990; Narayanan et al., 1995). In turn, bovine DF cells, when transferred into immunodeficient mice, form cementum-like matrix that is positive for CAP by immunostaining (Handa et al., 2002). Recently, a cementoblast progenitor cell line has been developed from bovine DF cells, and these cells form cementum when transplanted into immunodeficient mice (Saito et al., 2005).
The above studies by others certainly indicate that at least some of the dental follicle cell population becomes cementoblasts. Although they do not specifically prove that these cementoblasts arise from the stem cells in the follicle, once a specific antibody to rat cementum is developed, experiments could be conducted to determine this. Given that BMP-2 may induce mouse dental follicle cells toward a cementoblast/osteoblast phenotype (Zhao et al., 2002), perhaps this would be a method to induce DF stem cells to form cementoblasts, which could then be detected by immunostaining. Furthermore, once rat cDNA sequences become available for CAP or CEM-1, a RT-PCR approach might be used for identification.
Of particular interest in this study is the fact that the DF stem cells can differentiate into adipocytes and neurons. Regarding the former, we believe that this is the first report that adipocytes can be formed from DF stem cells. Although this likely does not normally occur in vivo in the DF or subsequent PDL, it does demonstrate the ability of the stem cells to differentiate into several cell types.
The ability of the DF stem cells to differentiate into neurons may be, in effect, a reflection of the origin of the DF. The DF originates from the neural crest, and among the many derivatives of the neural crest are the dorsal root ganglia and ganglia of the autonomic nervous system. Thus, given the ancestry of the DF, it is likely that the stem cells in the DF also may arise from the neural crest and thus be easily induced to form neurons.
For future studies, particularly those involving tissue engineering, the DF stem cells will need to be isolated. The finding that DOX appears to eliminate the non-stem cells from the population may be a means of efficiently and inexpensively isolating side-population stem cells, provided that the cells maintain their pluripotent phenotype.
ACKNOWLEDGMENT
This work was supported by NIH grant DE008911-16 to G.E.W.
REFERENCES
- Bosshardt DD, Schroeder HE. Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec. 1996;245:267–292. doi: 10.1002/(SICI)1097-0185(199606)245:2<267::AID-AR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Cahill DR, Marks SC., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, et al. Cementum matrix formation in vivo by cultured dental follicle cells. Bone. 2002;31:606–611. doi: 10.1016/s8756-3282(02)00868-2. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Luan X, Ito Y, Dangaria S, Diekwisch TGH. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Jr, Cahill DR. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch Oral Biol. 1984;29:311–322. doi: 10.1016/0003-9969(84)90105-5. [DOI] [PubMed] [Google Scholar]
- McAllister B, Narayanan AS, Miki Y, Page RC. Isolation of a fibroblast attachment protein from cementum. J Periodontal Res. 1990;25:99–105. doi: 10.1111/j.1600-0765.1990.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Narayanan AS, Ikezawa K, Wu D, Pitaru S. Cementum specific components which influence periodontal connective tissue cells. Connect Tissue Res. 1995;33:19–21. doi: 10.3109/03008209509016976. [DOI] [PubMed] [Google Scholar]
- Saito M, Handa K, Kiyono T, Hattori S, Yokoi T, Tsubakimoto T, et al. Immortalization of cementoblast progenitor cells with Bmi-1 and TERT. J Bone Miner Res. 2005;20:50–57. doi: 10.1359/JBMR.041006. [DOI] [PubMed] [Google Scholar]
- Wise GE, Yao S. Regional differences of expression of bone morphogenetic protein-2 and RANKL in the rat dental follicle. Eur J Oral Sci. 2006;114:512–516. doi: 10.1111/j.1600-0722.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Wise GE, Lin F, Fan W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992;267:483–492. doi: 10.1007/BF00319370. [DOI] [PubMed] [Google Scholar]
- Wise GE, Frazier-Bowers S, D’Souza RN. Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med. 2002;13:323–334. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84:837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen L-S, Arzate H, et al. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn. 2003;228:651–663. doi: 10.1002/dvdy.10404. [DOI] [PubMed] [Google Scholar]
- Zhao M, Xiao G, Berry JE, Franceschi RT, Reddi A, Somerman MJ. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–1451. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]