Abstract
The criteria for controlling the regioselectivity of Staudinger reduction of azides have been investigated. These findings enable a convenient direct N-1 modification of the perazidoneamine and perazidoribostamycin resulting in the synthesis of aminoglycoside antibiotics with activity against drug resistant bacteria.
Introduction
Ever since the discovery of aminoglycoside antibiotics, their challenging synthesis has attracted much effort. Pioneered by Umezawa and others in the 1970s, a vast amount of aminoglycoside derivatives along with various modification methods have been created aiming to improve the activity of aminoglycoside against resistant bacteria.1-3 A wide range of structural modifications have been documented in the literature, among which attaching functionalities at the N-1 position of the 2-deoxystreptamine among kanamycin or neomycin class antibiotics, is one of the most effective methods of reviving the activity against aminoglycoside resistant bacteria.4 This strategy has led to the development of semi-synthetic amikacin that has an (S)-4-amino-2-hydroxybutyryl (AHB) group at N-1 position.
In order to implement an AHB group at N-1 position, a regioselective differentiation of amino groups is essential. The regioselective manipulation of the amino groups on aminoglycosides has been extensively studied, especially in neomycin, kanamycin, and neamine. In general, these methods utilize metal-mediated chelation,5-9 cyclization between the selected carbamate-protected amino group and its vicinal hydroxyl group10-12 or enzymatic approaches.13 However, selectivity of these strategies closely depends on the scaffolds of parent aminoglycosides. In addition, there is no applicable method for regioselective differentiation of the azido groups on perazidoaminoglycosides.
Results and Discussion
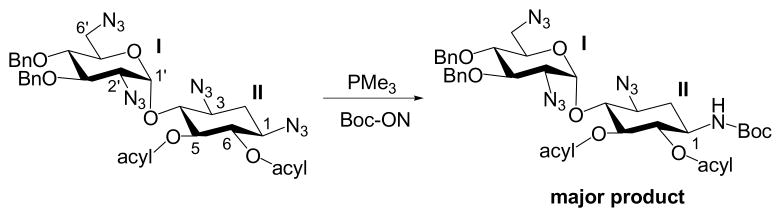
Our group has devoted efforts into the synthesis of novel aminoglycosides using glycodiversification strategy based on tetraazidoneamine scaffold.14,15 With the advantages of better solubility and accessibility for more structural variations, perazidoaminoglycosides have been the recent focus for generating new aminoglycoside derivatives. We have been actively engaging in the studies for the regioselective reduction of azido group via a Staudinger reaction. It has been suggested that the electron-deficient azides can be reduced more rapidly than the electron-rich azides.16 We have developed a strategy that utilizes the electron-donating protecting groups at O-3′ and O-4′ positions (ring I) and the electron-withdrawing protecting groups at O-5 and O-6 positions (ring II) of tetraazidoneamine to enable a regioselective Staudinger reduction of azido group at N-1 position (Scheme 1).17
Scheme 1. Strategy for Regioselective Reduction of Azido Group.
It has also been reported by Wong and co-workers that the regioselectivity of the azide reduction can be achieved for several perazido compounds.18 The observed regioselectivity was interpreted with the electronic factors and the chemical shifts of ipso-protons of azides were used to correlate to the predicted site of azide reduction. In general, the higher the chemical shift, the higher the reactivity toward the Staudinger reduction (Scheme 2).
Scheme 2. Reported Regioselective Reduction of Azido Group18.
We are intrigued by the above reported results for the distinct reactivity of N-2′ azido groups. Although the reported chemical shifts of both ipso-protons can be used to explain the observed selectivity, the unusual upfield shift of the H-2′ of per-azido per-benzyl tobramycin needs to be further clarified. We have observed similar upfield shift for the H-2′ in the presence of acyl protecting group at O-5 and an anisotropic effect has been proposed to offer the shielding of H-2′ based on molecular modeling.17
However, it is still uncertain to us that such an up-field shift of H-2′ resulting from the anisotropic effect of the O-5 protecting group can be accountable for the observed regioselectivity. The upfield shift of H-2′ caused by anisotropic effect is different from the one caused by inductive effect. The latter can be correlated to the electron-deficiency of azido group while the former may not. The difference raises the issue for the role of electron-withdrawing group at the O-5 position. Is the selectivity of Staudinger reaction controlled solely by electron-withdrawing (electronic) effect or, perhaps, steric effect may have influence as well? Clearly, electron-withdrawing effect is important which explains that no reduction from the N-6′ azido group on several known azidoneamine derivatives has yielded identifiable product. On the other hand, the electron-withdrawing effect from the O-5 acyl group seems to be too far away from N-1 azido group to have a significant contribution to the observed regioselectivity.
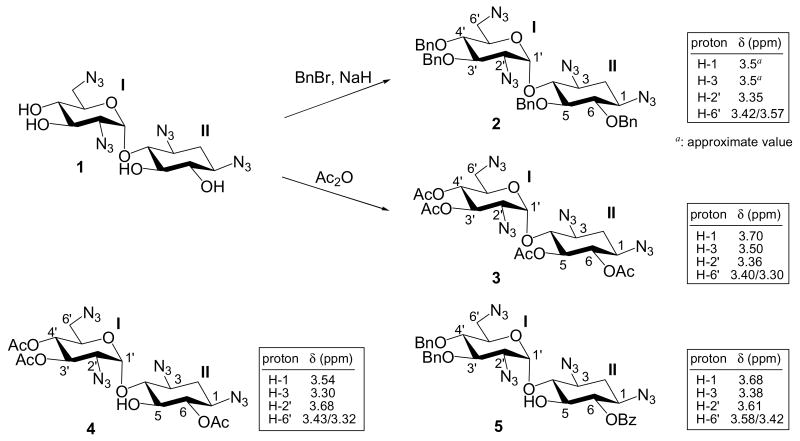
To unravel the role of O-5 protecting group, four tetraazidoneamine derivatives, 228, 3, 421 and 517 were studied (Scheme 3). Compound 3 has four acetyl groups at O-5, O-6, O-3′ and O-4′ positions. If electron-withdrawing effect is the only factor for the selectivity of Staudinger reaction, the N-2′ azido group should be the most reactive one since it is the closest to the anomeric center and should be the most electron deficient azide. If N-1 azido group is the most reactive one, the primary role for the acyl group at O-5 should be the provider of steric hindrance that prevent the reduction of N-2′ azido group. Interestingly, H-2′ appears to have the lower chemical shift (δ = 3.36 ppm) as compared to H-1 and H-3 of 3. This discrepancy suggests that the upfield shift of H-2′ is a result of anisotropic effect from the O-5 protecting group and cannot be interpreted as the electron-deficiency for N-2′ azido group.
Scheme 3. Synthesis of Azidoneamine Derivatives.
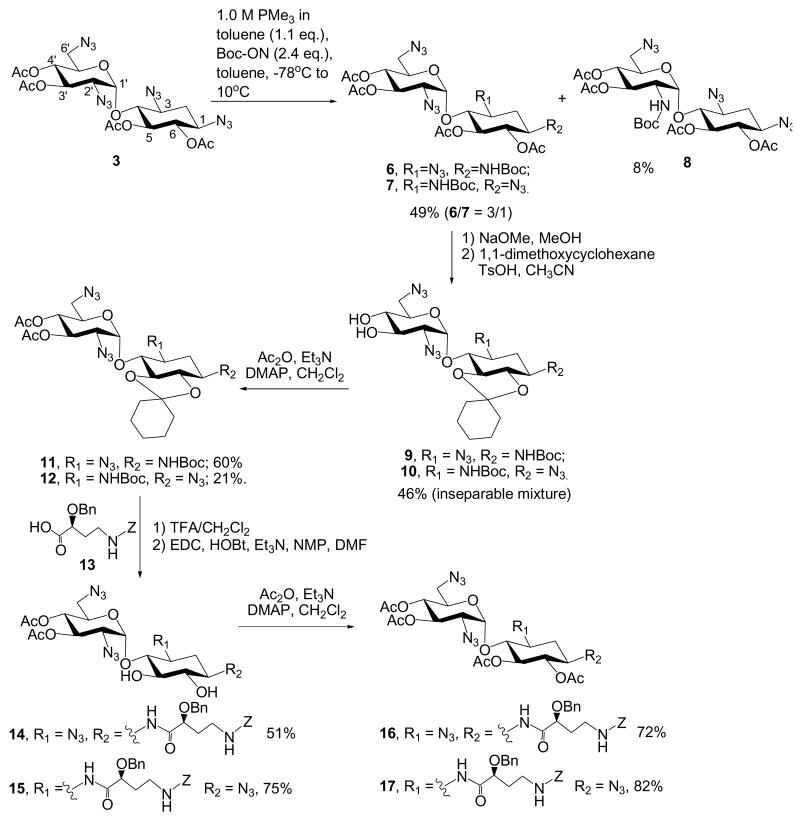
When compound 3 was subjected to the treatment of one-pot Staudinger reduction/Boc-protection following the reported condition,17,19 three components were obtained (Scheme 4). The reaction mixture was purified as a two-component mixture in 49% yield and compound 8 (8% yield). Compound 8 was determined to be N-2′ Boc-protected adduct by 1H-1H COSY. Further derivatization of the two-component mixture (6 and 7) via deacetylation, diol-protection and acetylation furnished compounds 11 and 12, which were separated by flash column chromatography. Unfortunately, the regioselectivity of compounds 11 and 12 cannot be identified due to the complex overlapping of their proton signals. Deprotection of the Boc group on compounds 11 and 12 followed by coupling with the AHB derivative, 13,20 led to the synthesis of neamine derivatives, 14 and 15 with AHB at N-1 and N-3, respectively. However, the signals for H-1 and H-3 necessary for confirming the regioselectivity were still indiscernible. Finally, acetylation at O-5 and O-6 created sufficient difference in chemical shifts allowing the determination of regioselectivity. Compound 16 was verified to have an AHB group at N-1 while 17 was determined to have an AHB group at N-3.
Scheme 4. Regioselective Staudinger Reduction of Azidoneamine Derivatives.
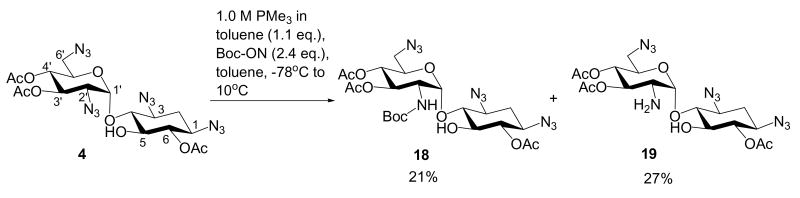
Compound 2 was designed to have no electron-withdrawing advantage as a comparison to compound 3. Compound 5 was designed to have electron-withdrawing effect for N-1 azide but not for N-2′ azide. Meanwhile, there is no O-5 protecting group for blocking the access to the N-2′ azido group. Staudinger reduction of compounds 2 and 5 produced complex mixture with no dominant identifiable product. Compound 4 was designed to have electron-withdrawing effect for both N-1 and N-2′ azido groups. However, N-2′ azido group is expected to be more reactive than N-1 since it is closer to the anomeric center. When compound 4 was treated in the identical condition, two dominant products were isolated (Scheme 5). Spectroscopic characterization confirmed that these were N-2′ Boc-protected amino (18, 21%) and N-2′ amino (19, 27%) neamine derivatives.
Scheme 5. Staudinger Reduction of Compound 4.
By combining the results from the regioselective Staudinger reduction, the role of O-5 protecting group and the factors that govern the regioselective azide-amino transformation can be established. First: the electronic effect (electron-deficiency) is the dominant factor in controlling the regioselectivity of azide reduction. However, the steric hindrance may play an auxiliary but essential role in controlling the regioselectivity. Comparing the results from Staudinger reaction of 3 and 4 clearly demonstrates that the steric hindrance of O-5 protecting group prevents the reduction of N-2′ azido group. Electron-withdrawing group at O-6 is pivotal in making the N-1 azido group the most reactive one. In order to achieve the favored regioselectivity i.e. reduction of N-1 azido group, it is preferable to make ring I electron-rich by employing electron-donating protecting groups, such as Bn, at O-3′ and O-4′, and ring II electron-deficient by introducing electron-withdrawing protecting groups, such as Ac or Bz, at both O-5 and O-6. Second: although the chemical shifts of ipso-protons can provide guidance in the favored site of azide reduction, their significance in regioselectivity needs to be carefully interpreted. The anisotropic effect resulting from the aromatic ring (Bn or Bz) and/or carbonyl functionality of the O-5 protecting groups that causes the upfield shift of H-2′ cannot be correlated with the lower reactivity of N-2′ azido group. Third: even in the optimal designs (electron-rich ring I and electron-deficient ring II), minor reduction at sites other than N-1 azido group could be inevitable, making only modest yield for the desired N-1 reduced product. In the event of lacking dominant electronic and steric effects, the Staudinger reduction is expected to occur at multiple sites creating a complex mixture and, hence, difficulties in isolating identifiable product.
These findings can now be used to explain the observed selectivity reported by Wong's and our group. For example, for 6,3′,4′-tri-O-benzyltetraazidoneamine, there is no protecting group at O-5 to provide steric hindrance for blocking the reduction of N-2′ azide. Meanwhile, there is no electron-withdrawing protecting group at O-6 to favor the reduction at N-1 azide. Thus, it is conceivable that N-2′ will be the dominant site of Staudinger reduction. For per-azido per-benzyl tobramycin, the upfield shift of H-2′ is the result of anisotropic effect from O-5 Bn. The potential reduction of N-2′ azide is blocked by the presence of O-5 Bn group. The N-3″ azide is most reactive (or most electron-deficient), which can be correlated to the observed chemical shift.
For compound 2, there is no O-6 electron-withdrawing group to favor the reduction of N-1 azido group despite having an O-5 Bn for blocking the reduction of N-2′ azide. Thus, random reduction at multiple sites may occur, creating complex mixture and no identifiable product. The lack of steric hindrance from O-5 protecting group in compound 5 causes the competitive reductions between N-2′ and N-1 azido groups. Therefore, the desired N-1 reduced product could not be produced predominantly and then isolated. Finally, with the designs like compound 3 and those shown in Scheme 1, a regioselective reduction at N-1 azide can be achieved by a collaboration of both electron-withdrawing and steric effects.
Synthesis of Butirosin B and Neokacin
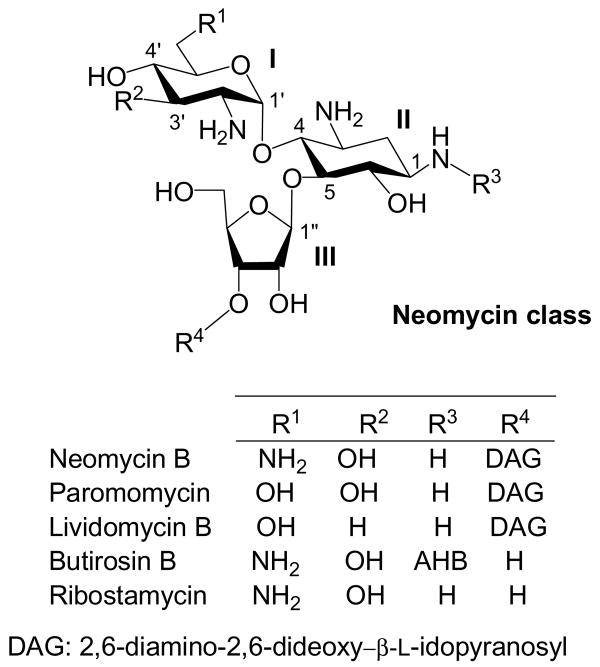
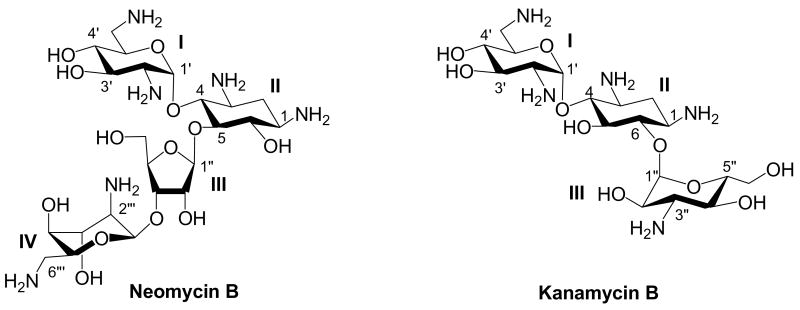
After revealing the criteria regarding the regioselectivity of Staudinger reaction of per-azido neamine, we began to explore the possibility of introducing AHB group via a regioselective reduction of the N-1 azido group for structurally more complicated per-azido neomycin class derivatives. Neomycin class aminoglycosides contain a characteristic 4,5-disubstituted neamine core (ring II) (Figure 1).
Figure 1. Structures of Neomycin Class Aminoglycosides.
Our study shows that protecting group at O-5 is essential in providing steric hindrance for favoring the regioselective reduction of N-1 azido group. Thus, it is conceivable that the furanose ring (ring III) at the O-5 of the neomycin class antibiotics may exert the same effect. Making ring II electron-deficient by introducing O-6 acyl protecting group is important as well. Rather than incorporating electron-donating group, such as Bn, at ring I, it is easier synthetically to acylate all the hydroxy groups.
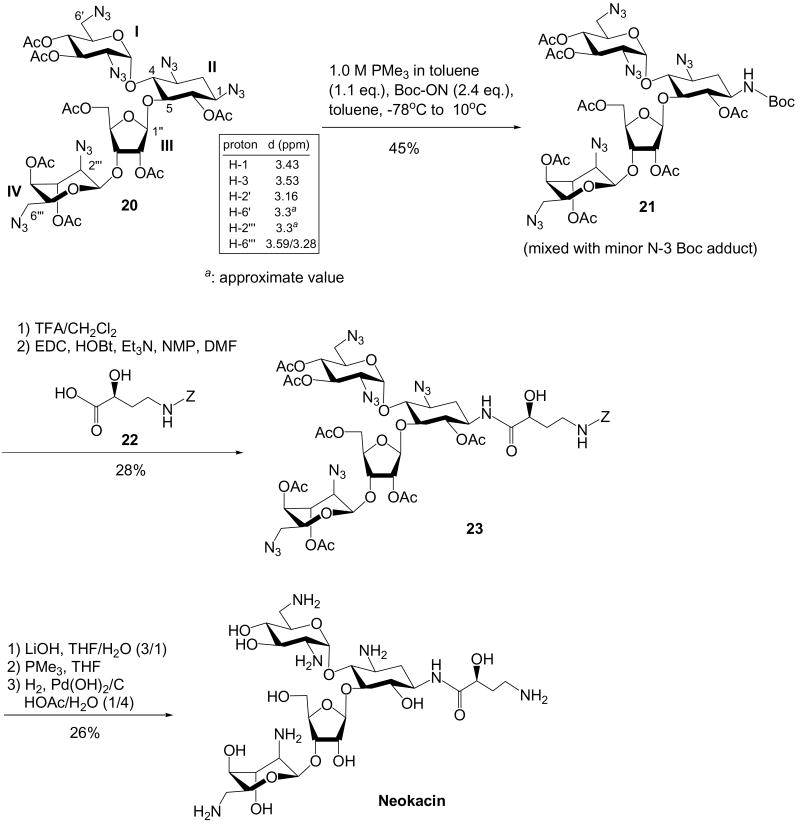
Per-acetylated per-azido neomycin, such as 20,21 contains two additional N-2‴ and N-6‴ azido groups at ring IV (Scheme 6). The N-6‴ azido group, which is transformed from a primary amino group that is analogous to the N-6′ azido group at ring I, should not affect the outcome of selectivity. Since ring IV adapts an 1C4 conformation, the N-2‴ azido group resides at an axial position. Substituent at the axial position is more electron rich than that of the equatorial position.23 Additionally, the reactivity of N-2‴ azido group was expected to be decreased due to a 1, 3-diaxial interaction exerted by C-4‴ acetyloxy group. Thus, we expected the N-2‴ azido group should be less reactive than the N-1 azido group. Finally, despite having acetyl groups at O-3′ and O-4′ making ring I electron-deficient, the steric hindrance from the furanose ring (ring III) is expected to hamper the reduction of N-2′ azido group. Although chemical shifts of the ipso-protons of N-3 vs. N-1 indicate that the former should be more reactive, we were expecting that the reactivity of N-3 could be decreased by the steric effect of ring I.
Scheme 6. Regioselective Staudinger Reduction of Azidoneomycin.
After treating 20 in the same condition, a mixture of N-1 and N-3 Boc-protected adducts, 21, was obtained although the N-1 Boc-protected adduct appeared to be the dominant product by COSY analysis. Deprotection of the Boc followed by the coupling with Cbz-protected AHB, 2220 furnished a major product as 23. Characterization of the major product confirms the attachment of AHB group at the desired N-1 position. Global deprotection of the protecting groups furnished the desired neomycin derivative with AHB at N-1 position, which we called neokacin.
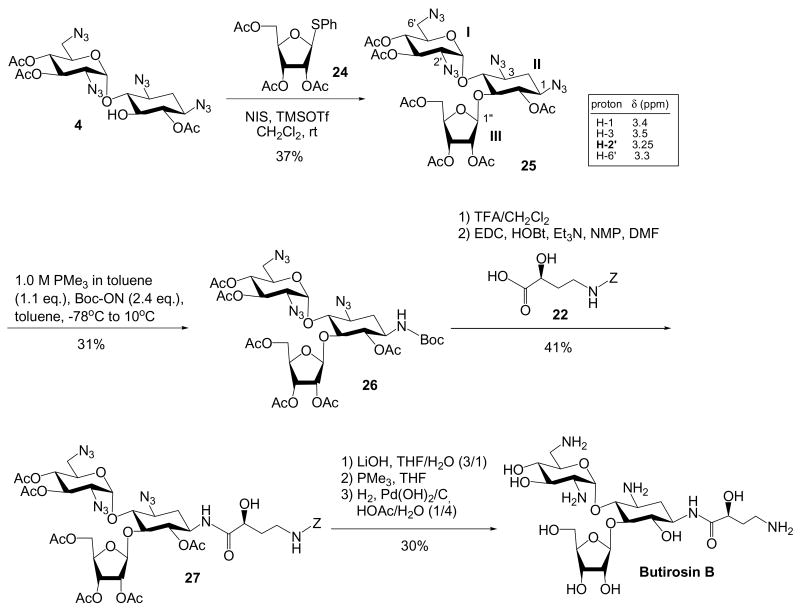
Because the commercially available ribostamycin is too expensive to be used as the starting material, a glycosylation strategy for the synthesis of per-azido per-O-acetylated ribostamycin has been developed (Scheme 7). Glycosylation of 4 with 2422 provided the ribostamycin-based adduct, 25. Interestingly, the chemical shifts of the ipso-protons of 25 also suggest that the N-3 azido group should be the most reactive. However, after treating 25 in the same condition, a regioselective Staudinger reduction can be achieved. Incorporation of Cbz-protected AHB followed by the global deprotection procedures yielded the ribostamycin adducts with N-1 AHB group, also known as butirosin B.
Scheme 7. Regioselective Staudinger Reduction of Azidoribostamycin.
Synthesis of Amikacin Derivatives
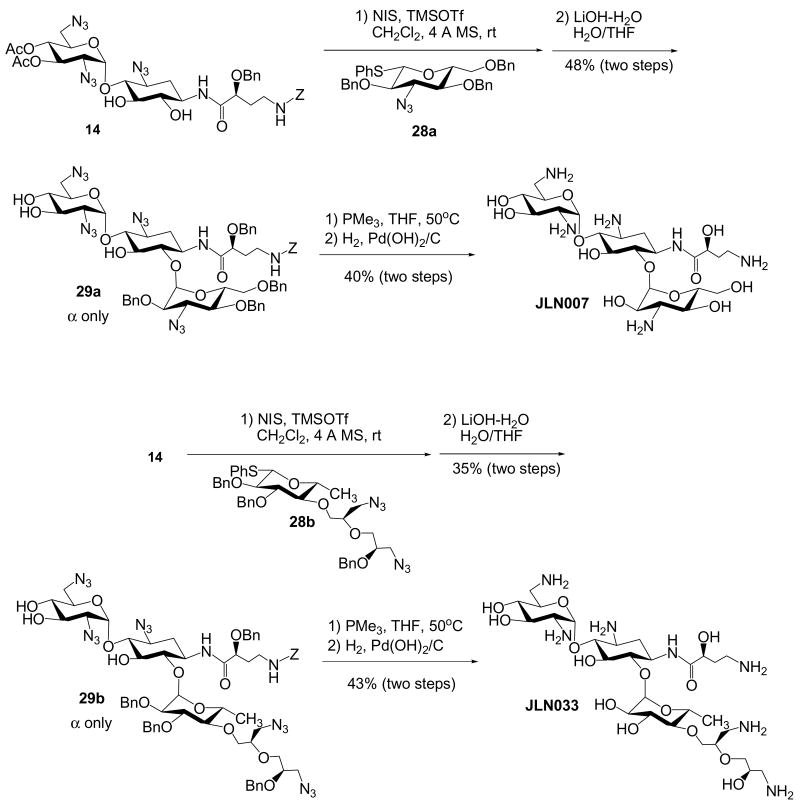
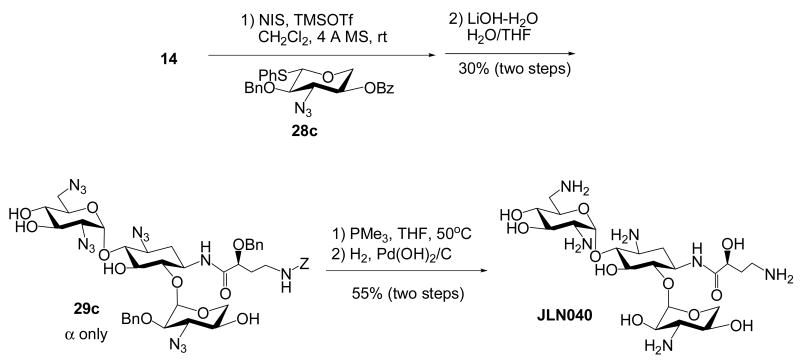
The newly developed regioselective Staudinger reaction can also be applied for the synthesis of kanamycin class aminoglycosides with N-1 AHB group or amikacin analogs. Although we have previously reported the synthesis of two amikacin analogs,17 JLN005 and JLN027, the newly developed protocol provides more convergent and efficient route for the construction of a library of amikacin analogs. Similar to the previous synthesis17, the glycosyl acceptor 14 was regiospecifically and stereoselectively glycosylated with thiophenylglycosyl donors, 28a-c15 that we have prepared. The afforded glycosylated trisaccharides were often mixed with inseparable impurities. Nevertheless, after the hydrolysis of all the acyl protecting groups, three members of the kanamycin derivatives, 29a-c were obtained as more pure form and improved α/β ratio (Scheme 8). Global deprotection via Staudinger reduction and hydrogenation procedures led to the synthesis of three amikacin analogues, JLN007, JLN033 and JLN040.
Scheme 8. Synthesis of Kanamycin Class Aminoglycosides with N-1 AHB.
Confirming the Regioselectivity by COSY Experiment
As mentioned previously, the regioselectivity of Staudinger reaction can be confirmed by 2D COSY experiment. Two diagnostic spectroscopic signatures can be utilized to unambiguously assign the site of azido reduction/Boc protection: a) the ipso-proton (geminal proton) where azido reduction/Boc protection occur or where the AHB group is attached will have a significant (ca. 0.5 ppm) downfield shift, b) a COSY signal between the ipso-proton (geminal proton) and the vicinal AHB-NH proton. The chemical shifts for several selected compounds in d-chloroform are summarized in Table 1 using the widely adapted numbering system for neomycin and kanamycin class aminoglycosides (Figure 2).
Table 1. Chemical Shifts of Selected Aminoglycoside Derivatives.
| Structure | 3 | 8 | 16 | 17 | 4 | 18 | 19 | 25 | 26 | 20 | 21 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1 | 3.7 | 3.6 | 4.1 | 3.5 | 3.54 | 3.5 | 3.5 | 3.4 | 3.7 | 3.43 | 3.7 | 4.09 |
| H-2 (eq./ax.) | 2.45/1.65 | 2.44/1.65 | 2.3/1.40 | 2.1/1.52 | 2.41/1.62 | 2.36/1.63 | 2.35/1.58 | 2.39/1.60 | 2.39/1.5 | 2.39/1.63 | 2.3/1.63 | 2.35/1.56 |
| H-3 | 3.5 | 3.5 | 3.5 | 4.0 | 3.4 | 3.4 | 3.3 | 3.5 | 3.51 | 3.53 | 3.5 | 3.57 |
| H-4 | 3.67 | 3.69 | 3.6 | 3.7 | 3.4 | 3.4 | 3.44 | 3.7 | 3.66 | 3.7 | 3.65 | 3.71 |
| H-5 | 5.16 | 5.13 | 5.21 | 5.11 | 3.65 | 3.6 | 3.58 | 3.87 | 3.91 | 3.9 | 3.93 | 3.98 |
| H-6 | 4.94 | 4.94 | 4.81 | 4.95 | 4.93 | 4.86 | 4.94 | 4.97 | 4.78 | 4.96 | 4.79 | 4.91 |
| H-1′ | 5.21 | 5.1 | 5.18 | 4.82 | 5.35 | 5.1 | 5.27 | 5.9 | 5.9 | 5.95 | 5.93 | 5.91 |
| H-2′ | 3.36 | 4.0 | 3.3 | 3.40 | 3.68 | 3.9 | 3.03 | 3.25 | 3.22 | 3.16 | 3.16 | 3.17 |
| H-3′ | 5.44 | 5.09 | 5.43 | 5.24 | 5.49 | 5.17 | 5.12 | 5.45 | 5.44 | 5.47 | 5.46 | 5.46 |
| H-4′ | 5.03 | 5.04 | 5.02 | 4.99 | 5.05 | 5.02 | 4.96 | 5.01 | 4.99 | 4.99 | 4.96 | 4.97 |
| H-5′ | 4.5 | 4.4 | 4.4 | 4.0 | 4.34 | 4.3 | 4.2 | 4.4 | 4.52 | 4.4 | 4.45 | 4.46 |
| H-6′ | 3.3/3.4 | 3.3-3.4 | 3.1-3.4 | 3.4/3.11 | 3.43/3.32 | 3.2-3.3 | 3.38/3.28 | 3.3 | 3.2-3.3 | 3.3 | n.d. | 3.2-3.3 |
| H-1″ | - | - | - | - | - | - | - | 5.42 | 5.43 | 5.35 | 5.37 | 5.37 |
| H-2″ | - | - | - | - | - | - | - | 5.1 | 5.08 | 4.89 | 4.88 | 4.9 |
| H-3″ | - | - | - | - | - | - | - | 5.19 | 5.2 | 4.4 | n.d. | 4.42 |
| H-4″ | - | - | - | - | - | - | - | 4.26 | 4.2 | 4.32 | n.d. | 4.3 |
| H-5″ | - | - | - | - | - | - | - | 4.44/4.16 | 4.38/4.15 | 4.26 | 4.1 | 4.28 |
| H-1‴ | - | - | - | - | - | - | - | - | - | 4.87 | 4.87 | 4.87 |
| H-2‴ | - | - | - | - | - | - | - | - | - | 3.3 | n.d. | 3.3 |
| H-3‴ | - | - | - | - | - | - | - | - | - | 5.04 | 5.02 | 5.02 |
| H-4‴ | - | - | - | - | - | - | - | - | - | 4.7 | 4.7 | 4.69 |
| H-5‴ | - | - | - | - | - | - | - | - | - | 4.09 | n.d. | 4.1 |
| H-6‴ | - | - | - | - | - | - | - | - | - | 3.59/3.28 | n.d. | 3.58/3.27 |
n.d.: not distinguishable due to the overlap of multiple signals
Figure 2. Numbering System for Neomycin and Kanamycin Classes Aminoglycosides.
Antibacterial Activity Study
The most common mode bacterial resistance against aminoglycosides arises from the overexpression of aminoglycoside-modifying enzymes (AME). These enzymes are grouped as aminoglycoside phosphotransferases (APHs), aminoglycoside acetyltransferases (AACs), and aminoglycoside nucleotidyltransferases (ANTs). Our newly synthesized aminoglycosides were assayed against a panel of bacteria and the minimum inhibitory concentration (MIC) was determined using amikacin, neomycin, butirosin A, gentamicin and kanamycin as the controls (Tables 2 and 3).24 Aminoglycoside susceptible Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Klebsiella pneumoniae (ATCC 13883, resistant to ampicillin, susceptible to aminoglycosides) were used as standard reference strains. E. coli (pSF815) and E. coli (pTZ19U-3) are laboratory resistant strains using E. coli (TG1) as the host. K. pneumoniae (ATCC 700603) is a clinical isolate that is resistant to ceftazidime, other β-lactams, and several aminoglycosides (ANT(2″)).25Pseudomonas aeruginosa (ATCC 27853) that expresses several AME including APH(3′)-IIb that render the bacterium manifests modest resistance toward aminoglycosides.26 Methicillin-resistant S. aureus (ATCC 33591) (MRSA) is the leading cause of bacterial infections.27 Many MRSA strains contain genes encoded for APH(3′), ANT(4′), and AAC(6′)/APH(2″), which render the bacteria resistant to many aminoglycosides.
Table 2. Minimum Inhibitory Concentrations (MIC) for Neomycin Class Aminoglycosidesa.
| entry | strains | neomycin | ribostamycin | butirosin A | neokacin | butirosin B | JT00517 |
|---|---|---|---|---|---|---|---|
| 1 | E. colib | 4-8 | 8 | 4-8 | 8-16 | 8 | 8 |
| 2 | E. coli (TG1)c | 4-8 | 4 | 2 | 8 | 2 | 4 |
| 3 | E. coli (pSF815) d | 4 | 4 | 1 | 4 | 2 | 2-4 |
| 4 | E. coli (pTZ19U-3) e | Inactivek | Inactive | 0.5-1 | 2 | 2 | 4 |
| 5 | K. pneumoniaef | Inactive | Inactive | 1 | 4 | 2-4 | 4 |
| 6 | K. pneumoniaeg | 4-8 | 2-4 | 2 | 2 | 2-4 | 1-2 |
| 7 | S. aureush | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| 8 | S. aureusi | 1-2 | 8 | 4 | 2-4 | 4 | 4 |
| 9 | P. aeruginosaj | Inactive | Inactive | Inactive | 16 | Inactive | Inactive |
Unit: μg/mL, ND: Not Determined,
Escherichia coli (ATCC 25922),
E. coli (TG1) (aminoglycoside susceptible strain),
E. coli (TG1) (pSF815 plasmid encoded for (AAC(6′)/APH(2″)),
E. coli (TG1) (pTZ19U-3 plasmid encoded for APH(3′)-I),
Klebsiella pneumoniae (ATCC 700603),
K. pneumoniae (ATCC 13883),
Staphylococcus aureus (ATCC 33591) (MRSA),
S. aureus (ATCC 25923),
Pseudomonas aeruginosa (ATCC 27853),
Inactive is defined as MIC ≥ 32 μg/mL.
Table 3. Minimum Inhibitory Concentrations (MIC) for Kanamycin Class Aminoglycosidesa.
| entry | strains | amikacin | kanamycin B | gentamicinl | JLN00517 | JLN007 | JLN02717 | JLN033 | JLN040 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | E. colib | 2-4 | 4-8 | 2 | 8-16 | 4-8 | 2-4 | 8-16 | 4 |
| 2 | E. coli (TG1)c | 1 | 4 | 2 | 4 | 4 | 1 | 4 | 4 |
| 3 | E. coli (pSF815) d | 0.5 | inactivek | Inactive | 2 | 2 | 1 | 2 | 1 |
| 4 | E. coli (pTZ19U-3) e | 0.25 | Inactive | 1 | 2 | 0.5-1 | 0.25-0.5 | 0.5 | 0.5 |
| 5 | K. pneumoniaef | 1 | Inactive | 8-16 | ND | 2-4 | 1-2 | 4 | 2 |
| 6 | K. pneumoniaeg | 1-2 | 1-2 | 1 | ND | 2 | 1 | 4 | 1-2 |
| 7 | S. aureush | 16 | Inactive | 4 | ND | Inactive | Inactive | Inactive | Inactive |
| 8 | S. aureusi | 2 | 1-2 | 0.5 | 2-4 | 2 | 1-2 | 2 | 1-2 |
| 9 | P. aeruginosaj | 0.5-1 | Inactive | 0.5-1 | 4-8 | 4 | 2-4 | 4-8 | 1-2 |
Unit: μg/mL, ND: Not Determined,
Escherichia coli (ATCC 25922),
E. coli (TG1) (aminoglycoside susceptible strain),
E. coli (TG1) (pSF815 plasmid encoded for (AAC(6′)/APH(2″)),
E. coli (TG1) (pTZ19U-3 plasmid encoded for APH(3′)-I),
Klebsiella pneumoniae (ATCC 700603),
K. pneumoniae (ATCC 13883),
Staphylococcus aureus (ATCC 33591) (MRSA),
S. aureus (ATCC 25923),
Pseudomonas aeruginosa (ATCC 27853),
Inactive is defined as MIC ≥ 32 μg/mL,
gentamicin consists of 33% gentamicin C1, 19% gentamicin C1a, and 48% gentamicin C2a and C2. All these compounds have 3′,4′-dideoxygenation.
Based on the antibacterial activity of the neomycin class antibiotics, attaching AHB at N-1 is clearly an effective modification to revive the antibacterial activity as shown in the results from synthetic neokacin, butirosin B and JT005 against susceptible and resistant E. coli (entries 2, 3 and 4, Table 2). More significantly, these synthetic aminoglycosides are active against K. pneumoniae (ATCC 700603), which causes severe pneumonia and renders multi-drug resistance by harboring extended-spectrum β-lactamases (ESBLs) (entry 5, Table 2). All the synthesized kanamycin class aminoglycoside with N-1 AHB group also manifest prominent against this clinically isolated K. pneumoniae.
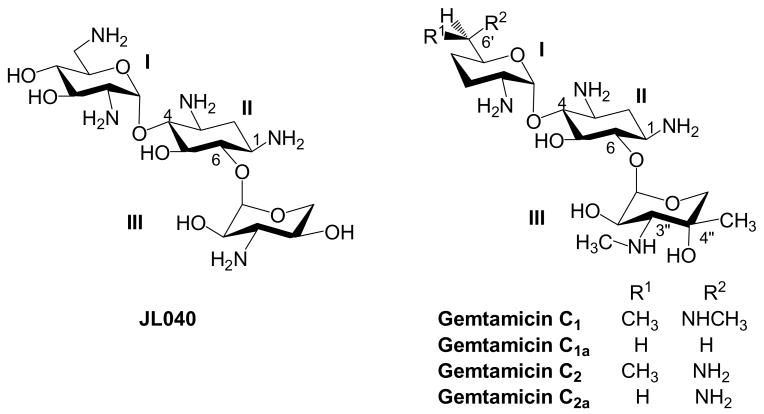
P. aeruginosa is an opportunistic human pathogen and the leading cause of nosocomial infection. With the exception of neokacin, which shows only modest antibacterial activity, the attachment of N-1 AHB cannot revive the activity of neomycin class aminoglycosides against P. aeruginosa (entry 9, Table 2). Similar observation has been observed previously and it has been suggested that attaching N-1 AHB group may deter the acetylation at N-6′ (AAC(6′)) for kanamycin class aminoglycosides but have no effect against the action of AAC(6′) for the neomycin class aminoglycosides.28 In contrast, kanamycin class aminoglycosides bearing N-1 AHB group while still having 3′ and 4′ hydroxyl groups appear to have regained activity (entry 9, Table 3). However, gentimicin that contain 3′,4′-deoxygenation but no N-1 AHB group also displays prominent activity against P. aeruginosa. We have recently synthesized a kanamycin (gentamicin) analogs, JL040,29 which differs from JLN040 only at the absence of N-1 AHB group (Figure 3). JL040 is inactive against P. aeruginosa. The result demonstrates the importance of having AHB group at N-1 for the activity of kanamycin class aminoglycoside against P. aeruginosa.
Figure 3. Structures of JL040 and Gentamicin.
MRSA is another formidable drug resistant pathogen, which harbor multiple AMEs. Without surprise, no newly synthesized aminoglycosides show activity against MRSA. Interestingly, gentimicin is active against MRSA while structurally similar JL040 and JLN040 are inactive. The results suggest that methyl groups at C-6′, N-3″ and C-4″ on the carbohydrate scaffold may play crucial role(s) in the activity of kanamycin class aminoglycoside against MRSA.
Conclusion
We have completed the investigation on the regioselectivity of Staudinger reaction of perazidoaminoglycosides. Electron-withdrawing effect is the dominant factor in controlling the regioselectivity, however, steric factor can have significant impact as well. It is the combination of these two factors that a selective Staudinger reaction can occur preferably at the N-1 azido group of per-azido neamine and neomycin derivatives. Chemical shift of ipso-proton can provide valuable prediction regarding the preference of reduction site(s). Nonetheless, such information cannot be interpreted as the absolute factor in controlling the regioselectivity. Although in some cases, the yield is moderate, it can be attributed to the non-regioselective reduction of azido group at other positions. In general, the method we have developed a novel way of directly introducing structural modifications at the N-1 of neomycin class aminoglycosides. In addition, this method can lead to the synthesis of kanamycin class aminoglycosides with N-1 modifications. Finally, the results from the antibacterial studies reveal drastic difference in the activity between the neomycin and kanamycin class aminoglycosides among various bacteria.
Experimental Section
Proton magnetic resonance spectra were recorded using 400 MHz spectrometers. Chemical shifts were reported as parts per million (ppm) downfield from tetramethylsilane in δ unit, and coupling constants were given in cycles per second (Hz). Splitting patterns were designed as s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. 13C NMR spectra were obtained by broad-band WALTZ decoupling using 100 MHz spectrometer. All NMR spectra were recorded at ambient temperature unless otherwise noted. Chemical reagents and starting materials were purchased and used without purification unless otherwise noted. Dichloromethane was distilled over CaH2. Other solvents were used without purification.
5,6,3′,4′-tetra-O-acetyl-1,3,2′,6′-tetraazidoneamine (3)
To a solution of 1,3,2′,6′-tetraazidoneamine (7.6 g, 17.83 mmol),17 Et3N (26.3 mL, 0.19 mol) and DMAP (0.50 g, catalyst) in anhydrous CH2Cl2 (200 mL), Ac2O (17.7 mL, 0.19 mol) was added slowly. The reaction mixture was stirred at room temperature until the completion of the reaction (monitored by TLC, Rf = 0.46, EtOAc: Hexane = 35: 65). The reaction was then quenched by addition of saturated NaHCO3, and extracted with EtOAc. The organic solution was washed with 1N HCl(aq), water, saturated NaHCO3(aq), brine and dried over anhydrous Na2SO4. After removal of the solvent followed by purification with a gradient column chromatography (Hexane: EtOAc = 80: 20 to 40: 60), the product was obtained as white crystals (4.88 g, 8.21 mmol, 46%). 1H NMR (400 MHz, CDCl3) δ 5.44 (dd, J = 10.8, 9.2 Hz, 1H, H-3′), 5.21 (d, J = 3.8 Hz, 1H, H-1′), 5.16 (dd, J = 9.7, 9.7 Hz, 1H, H-5), 5.03 (dd, J = 10.2, 9.3 Hz, 1H, H-4′), 4.94 (dd, J = 10.0, 9.9 Hz, 1H, H-6), 4.5 (m, 1H, H-5′), 3.67 (dd, J = 9.8, 9.7 Hz, 1H, H-4), 3.7 (m, 1H, H-1), 3.5 (m, 1H, H-3), 3.3 - 3.4 (m, 3H), 2.45 (ddd, J = 13.4, 4.6, 4.5 Hz, 1H, H-2eq), 2.09 (s, 6H), 2.08 (s, 3H), 2.06 (s, 3H), 1.65 (ddd, J = 13.3, 12.5, 12.5 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 170.1, 170.0, 169.9, 169.5, 99.0, 78.9, 74.3, 73.8, 70.0, 69.8, 69.6, 60.8, 58.6, 57.8, 50.9, 31.9, 20.8 (2C), 20.7 (2C); HRESI Calcd for C20H30N13O10 [M+NH4]+m/z 612.2238; measure m/z 612.2213.
5,6,3′,4′-Tetra-O-acetyl-1-N-tert-butoxycarbonyl-3,2′,6′-triazidoneamine (6) and 5,6,3′,4′-Tetra-O-acetyl-3-N-tert-butoxycarbonyl-1,2′,6′-triazidoneamine (7)
Please refer to the general procedure for selective Staudinger reaction. Compound 6 and 7 were inseparable and were obtained with a combined yield of 49%.
5,6,3′,4′-Tetra-O-acetyl-2′-N-tert-butoxycarbonyl-1,3,6′-triazidoneamine (8)
Please refer to the general procedure for selective Staudinger reaction. Compound 8 was obtained in 8% yield. 1H NMR (400 MHz, CDCl3) δ 5.13 (dd, J = 9.6, 9.1 Hz, 1H, H-5), 5.0 - 5.1 (m, 2H), 5.04 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.94 (dd, J = 9.9, 9.9 Hz, 1H, H-6), 4.55 (d, J = 10.3 Hz, 1H, NH), 4.40 (ddd, J = 9.9, 4.3, 4.1 Hz, 1H, H-5′), 4.00 (ddd, J = 10.5, 10.4, 3.7 Hz, 1H, H-2′), 3.69 (dd, J = 9.7, 9.7 Hz, 1H, H-4), 3.6 (m, 1H, H-1), 3.5 (m, 1H, H-3), 3.3 - 3.4 (m, 2H), 2.44 (ddd, J = 13.4, 4.6, 4.5 Hz, 1H, H-2eq), 2.07 (s, 3H), 2.04 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H), 1.65 (ddd, J = 13.1, 12.7, 12.6 Hz, 1H, H-2ax), 1.43 (s, 9H, C(O)(CH3)3); 13C NMR (100 MHz, CDCl3) δ 170.9, 169.8 (2C), 169.6, 155.3, 98.9, 80.3, 77.7, 74.2, 73.7, 71.3, 70.2, 69.4, 58.8, 57.9, 53.0, 51.2, 32.0, 28.3, 20.8 (2C), 20.7 (2C); HRESI Calcd for C25H36N10O12Na [M+Na]+m/z 691.2412; measure m/z 691.2390.
1-N-tert-butoxycarbonyl-3,2′,6′-triazido-5,6-O-cyclohexylideneneamine (9) and 3-N-tert-butoxycarbonyl-1,2′,6′-triazido-5,6-O-cyclohexylideneneamine (10).17 3′,4′-Di-O-acetyl-1-N-tert-butoxycarbonyl-3,2′,6′-triazido-5,6-O-cyclohexylideneneamine (11)
Please refer to the procedure for the preparation of compound 3. From a mixture of 9 and 10 (1.97 g, 3.40 mmol), two components were isolated with gradient column chromatography (Hexane: EtOAc = 60: 40 to 0: 100) and characterized individually. The major product was compound 11 (1.36 g, 2.05 mmol, 60%). 1H NMR (400 MHz, CDCl3) δ 5.54 (d, J = 3.5 Hz, 1H, H-1′), 5.45 (dd, J = 10.6, 9.4 Hz, 1H, H-3′), 4.99 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.8 (m, 1H, NH), 4.3 (m, 1H, H-5′), 3.74 (dd, J = 9.4, 9.4 Hz, 1H, H-4), 3.7 (m, 1H, H-1), 3.56 (dd, J = 9.5, 9.3 Hz, 1H, H-5), 3.5 (m, 1H, H-3), 3.2 - 3.3 (m, 3H), 3.24 (dd, J = 10.1, 3.5 Hz, 1H, H-2′), 2.6 (m, 1H, H-2eq), 2.05 (s, 3H), 2.03 (s, 3H), 1.5 - 1.6 (m, 10H), 1.42 (s, 9H, C(O)(CH3)3), 1.36 (ddd, J = 13.9, 12.8, 12.1 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 170.0, 169.9, 155.4, 113.9, 96.7, 80.2, 80.0, 78.2 (2C), 69.9, 69.6, 69.5, 60.8, 60.6, 51.1, 48.9, 36.6, 36.1, 35.3, 28.5, 25.0, 23.8, 20.8; HRESI Calcd for C27H40N10O10Na[M+Na]+m/z 687.2827; measure m/z 687.2806.
3′,4′-Di-O-acetyl-3-N-tert-butoxycarbonyl-1,2′,6′-triazido-5,6-O-cyclohexylideneneamine (12)
Please refer to the procedure for the preparation of compound 3. From a mixture of 9 and 10 (1.97 g, 3.40 mmol), two components were isolated with gradient column chromatography (Hexane: EtOAc = 60: 40 to 0: 100) and characterized individually. The minor product was compound 12 (0.47 g, 0.71 mmol, 21%). 1H NMR (400 MHz, CDCl3) δ 5.47 (d, J = 3.5 Hz, 1H, H-1′), 5.39 (dd, J = 9.9, 9.9 Hz, 1H), 4.97 (d, J = 9.8, 9.6 Hz, 1H), 4.7 (m, 1H), 4.2 (m, 1H, H-5′), 3.7 - 3.8 (m, 2H), 3.7 (m, 1H, H-1), 3.60 (dd, J = 9.4, 9.3 Hz, 1H), 3.41 (dd, J = 9.7, 9.7 Hz, 1H), 3.4 (m, 2H), 3.25 (dd, J = 10.7, 3.6 Hz, 1H, H-2′), 2.4 (m, 1H, H-2eq), 2.08 (s, 3H), 2.03 (s, 3H), 1.6 - 1.7 (m, 11H), 1.48 (s, 9H, C(O)(CH3)3); 13C NMR (100 MHz, CDCl3) δ 169.9, 169.7, 155.1, 113.7, 97.2, 79.9, 79.4, 78.7, 69.9, 69.8, 69.0, 60.8, 57.7, 51.3, 50.8, 36.6, 36.1, 35.6, 28.5, 25.1, 23.9, 20.8, 20.7; HRESI Calcd for C27H40N10O10Na[M+Na]+m/z 687.2827; measure m/z 687.2800.
3′,4′-Di-O-acetyl-1-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-3,2′,6′-triazidoneamine (14)
A solution of compound 11 (0.60 g, 0.90 mmol) in dichloromethane was added with 99% trifluoroacetic acid (3 mL) and water (0.02 mL), and the reaction mixture was stirred for 7 hrs at room temperature. The reaction mixture was quenched with triethylamine and concentrated to dryness and used without purification. A mixture of (S)-2-O-benzyl-4-(benzyloxycarbonylamino)butyric acid (0.34 g, 0.99 mmol), EDC (0.26 g, 1.35 mmol), HOBt (0.18 g, 1.35 mmol), Et3N (1 ml) and the above crude amine in anhydrous DMF (5 mL) was stirred overnight under nitrogen at room temperature. After completion of the reaction (monitored by TLC, Hexane: EtOAc = 35: 65), the reaction mixture was concentrated and re-dissolved in EtOAc. The organic solution was washed with water, brine and dried over anhydrous Na2SO4. Removal of the solvent followed by purification with gradient column chromatography (Hexane: EtOAc = 60: 40 to 0: 100) afforded the product as white crystals (0.37 g, 0.45 mmol, 51%). 1H NMR (400 MHz, CDCl3) δ 7.3 - 7.4 (m, 10H), 6.7 (m, 1H), 5.5 (m, 2H), 5.0 (m, 4H), 4.60 (d, J = 11.5 Hz, 1H), 4.49 (d, J = 11.9 Hz, 1H), 4.4 (m, 1H, H-5′), 4.03 (dd, J = 4.6, 4.6 Hz, 1H), 3.8 (m, 1H, H-1), 3.2 - 3.5 (m, 9H), 2.3 (m, 1H, H-2eq), 2.10 (s, 3H, CH3), 2.07 (s, 3H, CH3), 2.0 (m, 2H), 1.3 (m, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 172.8, 172.2, 169.0, 156.8, 136.9, 136.8, 129.0, 128.7, 128.3, 128.2, 127.9, 98.5, 81.8, 78.7, 75.0, 73.2, 70.7, 69.7, 69.4, 66.9, 61.4, 59.1, 51.1, 48.7, 37.1, 32.7, 32.2, 29.8, 20.8, 20.8; HRESI Calcd for C35H44N11O12 [M+H]+m/z 810.3170; measure m/z 810.3159.
3′,4′-Di-O-acetyl-3-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-1,2′,6′-triazidoneamine (15)
Please refer to the procedure for the preparation of compound 14. 1H NMR (400 MHz, CDCl3) δ 7.3 - 7.4 (m, 10H), 6.7 (d, J = 8.3 Hz, 1H), 5.39 (dd, J = 10.2, 9.5 Hz, 1H, H-3′), 5.1 (m, 2H), 5.02 (dd, J = 9.9, 9.7 Hz, 1H, H-4′), 5.0 (d, J = 2.9 Hz, 1H, H-1′), 4.64 (d, J = 11.1 Hz, 1H), 4.55 (d, J = 11.2 Hz, 1H), 4.1 (m, 1H, H-5′), 3.98 (dd, J = 5.5, 5.4 Hz, 1H), 3.9 (m, 1H, H-3), 3.66 (dd, J = 10.4, 3.5 Hz, 1H, H-2′), 3.56 (dd, J = 8.8, 8.8 Hz, 1H), 3.2 - 3.5 (m, 6H), 3.16 (dd, J = 13.5, 4.4 Hz, 1H, H-6′), 2.08 (s, 3H), 2.0 - 2.1 (m, 3H), 2.01 (s, 3H), 1.45 (ddd, J = 12.5, 12.4, 12.3 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 172.2, 170.1, 169.9, 156.5, 137.0, 136.7, 129.0, 128.7, 128.3, 128.2, 98.3, 85.4, 78.0, 75.9, 75.8, 72.7, 71.7, 69.6, 69.1, 66.9, 62.7, 59.9, 50.7, 48.1, 37.4, 33.2, 31.7, 20.8, 20.8; HRESI Calcd for C35H44N11O12 [M+H]+m/z 810.3170; measure m/z 810.3145.
3′,4′,5,6-Tetra-O-acetyl-1-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-3,2′,6′-triazidoneamine (16)
Please refer to the procedure for the preparation of compound 3. 1H NMR (400 MHz, CDCl3) δ 7.3 - 7.4 (m, 10H), 6.78 (d, J = 7.9 Hz, 1H), 5.43 (dd, J = 10.4, 9.5 Hz, 1H, H-3′), 5.21 (dd, J = 10.5, 9.5 Hz, 1H, H-5), 5.18 (d, J = 3.9 Hz, 1H, H-1′), 5.1 (m, 2H), 5.02 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.97 (dd, J = 5.5, 5.5 Hz, 1H, NH), 4.81 (dd, J = 10.2, 10.2 Hz, 1H, H-6), 4.59 (d, J = 11.6 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 4.4 (m, 1H, H-5′), 4.1 (m, 1H, H-1), 3.9 (m, 1H, H-5′), 3.6 (dd, J = 9.8, 9.3 Hz, 1H, H-4), 3.5 (m, 1H, H-3), 3.2 - 3.4 (m, 5H), 2.3 (m, 1H, H-2eq), 2.07 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 1.97 (s, 3H), 1.9 (m, 1H), 1.8 (m, 1H), 1.4 (m, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 172.4, 171.3, 169.9 (2C), 169.0, 156.5, 136.8, 136.7, 129.0, 128.7, 128.5, 128.2, 98.9, 79.2, 78.3, 73.5, 73.1, 70.0, 69.7, 69.6, 66.8, 60.8, 58.6, 50.9, 47.3, 37.5, 32.8, 32.5, 29.8, 20.8, 20.6; HRESI Calcd for C39H48N11O14 [M+H]+m/z 894.3382; measure m/z 894.3366.
3′,4′,5,6-Tetra-O-acetyl-3-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-1,2′,6′-triazidoneamine (17)
Please refer to the procedure for the preparation of compound 3. 1H NMR (400 MHz, CDCl3) δ 7.3 - 7.4 (m, 10H), 6.85 (d, J = 8.8 Hz, 1H), 5.24 (dd, J = 10.1, 9.6 Hz, 1H, H-3′), 5.11 (dd, J = 9.5, 9.1 Hz, 1H, H-5), 5.0 (m, 3H), 4.99 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.95 (dd, J = 9.5, 9.4 Hz, 1H, H-6), 4.82 (d, J = 2.5 Hz, 1H, H-1′), 4.64 (d, J = 11.6 Hz, 1H), 4.51 (d, J = 12.1 Hz, 1H), 4.0 (m, 1H, H-3), 3.9 - 4.0 (m, 2H), 3.7 (dd, J = 9.0, 8.9 Hz, 1H, H-4), 3.5 (m, 1H, H-1), 3.3 - 3.4 (m, 2H), 3.2 - 3.3 (m, 2H), 3.11 (dd, J = 13.5, 4.2 Hz, 1H, H-6′), 2.1 (m, 1H, H-2eq), 2.08 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 1.99 (s, 3H), 1.9 - 2.0 (m, 2H), 1.52 (ddd, J = 12.4, 12.3, 12.2 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 172.3, 170.1, 169.8 (2C), 169.7 (2C), 156.5, 137.0, 136.7, 129.0, 128.7, 128.3 (2C), 128.2, 98.1, 80.3, 77.8, 73.6, 72.7, 72.5, 70.6, 69.5, 69.0, 66.8, 61.9, 58.0, 50.6, 48.4, 37.2, 32.5, 31.4, 20.7 (4C); HRESI Calcd for C39H48N11O14 [M+H]+m/z 894.3382; measure m/z 894.3377.
6,3′,4′-Tri-O-acetyl-2′-N-tert-butoxycarbonyl-1,3,6′-triazidoneamine (18)
Please refer to the general procedure for selective Staudinger reaction. Compound 18 was obtained with a 21% yield. 1H NMR (400 MHz, CDCl3) δ 5.17 (dd, J = 10.1, 10.0 Hz, 1H, H-3′), 5.1 (m, 1H, H-1′), 5.02 (dd, J = 9.9, 9.6 Hz, 1H, H-4′), 4.86 (dd, J = 9.8, 9.8 Hz, 1H, H-6), 4.3 (m, 1H, H-5′), 3.8 (m, 1H, H-2′), 3.4 - 3.6 (m, 2H), 3.2 - 3.4 (m, 4H), 2.36 (ddd, J = 13.2, 4.5, 4.4 Hz, 1H, H-2eq), 2.14 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H), 1.63 (ddd, J = 12.9, 12.6, 12.6 Hz, 1H, H-2ax), 1.39 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.3, 169.8, 163.1, 155.6, 98.4, 80.8, 80.2, 76.3, 74.7, 71.4, 69.7, 69.5 (2C), 58.9, 58.7, 51.2, 31.9, 28.4 (3C), 21.0, 20.9, 20.8; HRESI Calcd for C23H34N10O11Na[M+Na]+m/z 649.2306; measure m/z 649.2285.
6,3′,4′-Tri-O-acetyl-2′-amino-1,3,6′-triazidoneamine (19)
Please refer to the general procedure for selective Staudinger reaction. Compound 19 was obtained with a 27% yield. 1H NMR (400 MHz, CDCl3) δ 5.27 (d, J = 4.1 Hz, 1H, H-1′), 5.12 (dd, J = 10.2, 9.7 Hz, 1H, H-3′), 4.96 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.94 (dd, J = 9.9, 9.9 Hz, 1H, H-6), 4.2 (m, 1H, H-5′), 3.58 (dd, J = 9.6, 9.1 Hz, 1H, H-5), 3.5 (m, 1H, H-1), 3.44 (dd, J = 9.9, 9.0 Hz, 1H, H-4), 3.38 (dd, J = 13.3, 2.7 Hz, 1H, H-6′), 3.3 (m, 1H, H-3), 3.28 (dd, J = 13.5, 5.3 Hz, 1H, H-6′), 3.03 (dd, J = 10.5, 3.9 Hz, 1H, H-2′), 2.35 (ddd, J = 13.2, 4.5, 4.2 Hz, 1H, H-2eq), 2.12 (s, 3H), 2.06 (s, 3H), 2.01 (s, 3H), 1.58 (ddd, J = 12.8, 12.6, 12.5 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 171.6, 170.5, 169.8, 101.6, 85.0, 74.7, 74.4, 74.0, 69.6, 69.4, 58.7, 58.3, 54.1, 51.1, 32.1, 21.1, 21.0, 20.8; HRESI Calcd for C18H27N10O9 [M+H]+m/z 527.1962; measure m/z 527.1972.
1-N-tert-butoxycarbonyl-hepta-O-acetyl-pentaazido neomycin (21)
Please refer to the general procedure for selective Staudinger reaction. Compound 21 was obtained with a 45% yield. 1H NMR (400 MHz, CDCl3) δ 5.93 (d, J = 3.9 Hz, 1H), 5.46 (dd, J = 10.6, 9.3 Hz, 1H), 5.37 (d, J = 2.4 Hz, 1H), 5.02 (dd, J = 2.9, 2.8 Hz, 1H), 4.96 (dd, J = 10.2, 9.4 Hz, 1H), 4.88 (dd, J = 2.8, 2.4 Hz, 1H), 4.87 (d, J = 1.8 Hz, 1H), 4.79 (dd, J = 9.5, 9.5 Hz, 1H), 4.70 (dd, J = 1.8, 1.8 Hz, 1H), 4.5 (m, 1H), 4.45 (dd, J = 11.8, 1.8 Hz, 1H), 4.41 (dd, J = 6.5, 5.1 Hz, 1H), 4.3 (m, 1H), 4.25 (dd, J = 11.4, 6.4 Hz, 1H), 4.1 (m, 1H), 3.93 (dd, J = 9.0, 8.8 Hz, 1H), 3.7 (m, 1H, H-1), 3.65 (dd, J = 9.6, 8.7 Hz, 1H), 3.59 (dd, J = 12.9, 9.6, 8.1 Hz, 1H), 3.5 (m, 1H), 3.2 - 3.3 (m, 4H), 3.16 (dd, J = 10.7, 3.8 Hz, 1H, H-2′), 2.3 (m, 1H, H-2eq), 2.18 (s, 3H), 2.14 (s, 3H), 2.10 (s, 6H), 2.08 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 1.63 (ddd, J = 12.9, 12.8, 12.4 Hz, 1H, H-2ax), 1.42 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.1, 170.9, 170.3, 170.2, 169.9 (2C), 168.7, 155.2, 107.4, 99.3, 96.6, 82.8, 80.5, 79.5, 77.4, 76.8, 75.9, 75.1, 73.7, 70.2, 69.7, 69.5, 68.9, 65.9, 63.5, 61.0, 59.5, 56.7, 51.3, 50.8, 49.1, 33.2, 28.4, 21.0, 20.9 (2C), 20.8 (3C), 20.7; MALDI Calcd for C42H58Cl2N16O22Na ([M+Na]+) m/z 1161.3809; measure m/z 1161.3801.
1-N-[(S)-4-(benzyloxycarbonylamino)-2-hydroxybutanoyl]-hepta-O-acetyl-pentaazido neomycin (23)
Please refer to the procedure for the preparation of compound 14. Compound 23 was obtained with a 28% yield. 1H NMR (400 MHz, CDCl3) δ 7.3 (m, 5H), 7.10 (d, J = 9.1 Hz, 1H, NH), 5.91 (d, J = 3.7 Hz, 1H, H-1′), 5.46 (dd, J = 10.6, 9.3 Hz, 1H, H-3′), 5.37 (d, J = 2.5 Hz, 1H, H-1″), 5.2 (m, 1H, NH), 5.11 (d, J = 13.0 Hz, 1H), 5.08 (d, J = 12.8 Hz, 1H), 5.02 (dd, J = 2.7, 2.7 Hz, 1H, H-3‴), 4.97 (dd, J = 9.8, 9.7 Hz, 1H, H-4′), 4.91 (dd, J = 5.0, 3.7 Hz, 1H, H-6), 4.90 (dd, J = 2.5, 2.5 Hz, 1H, H-2″), 4.87 (d, J = 1.8 Hz, 1H, H-1‴), 4.69 (dd, J = 1.6, 1.6 Hz, 1H, H-4‴), 4.4 - 4.5 (m, 3H), 4.2 - 4.3 (m, 2H), 4.0 - 4.1 (m, 3H), 3.98 (dd, J = 8.6, 8.4 Hz, 1H, H-5), 3.71 (dd, J = 9.4, 8.2 Hz, 1H, H-4), 3.5 - 3.6 (m, 3H), 3.2 - 3.3 (m, 3H), 3.27 (dd, J = 12.9, 4.3 Hz, 1H, H-6‴), 3.17 (dd, J = 10.8, 3.8 Hz, 1H, H-2′), 3.1 (m, 1H), 2.35 (ddd, J = 13.5, 4.4, 4.4 Hz, 1H, H-2eq), 2.16 (s, 3H), 2.13 (s, 3H), 2.10 (s, 3H), 2.08 (s, 6H), 2.06 (s, 3H), 2.05 (s, 3H), 1.5 - 1.6 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.5, 170.8, 170.3, 170.2, 169.9 (2C), 168.6, 158.4, 136.2, 128.8, 128.6, 128.5, 128.4, 128.2, 107.2, 99.3, 96.6, 82.3, 79.6, 76.7, 75.9, 75.2, 75.1, 73.6, 70.1, 69.6, 69.4, 68.9, 67.4, 65.8, 63.6, 61.0, 59.3, 56.6, 51.3, 50.8, 47.2, 37.1, 35.4, 32.1, 29.8, 21.0, 20.9 (2C), 20.8 (3C), 20.6; HRESI Calcd for C49H64N17O24 ([M+H]+) m/z 1274.4310; measure m/z 1274.4295.
1-N-[(S)-4-amino-2-hydroxybutanoyl]neomycin (Neokacin)
Compound 23 (0.20 g, 0.16 mmol) was dissolved in tetrahydrofuran (3 mL) and water (1 mL), and lithium hydroxide monohydrate (0.06 g, 1.54 mmol) was added. The reaction mixture was stirred at room temperature till the completion of the reaction (ca. 24 hours, monitored by TLC, Rf = 0, EtOAc: Hexane = 65: 35). The reaction mixture was filtered through Celite and the solvent was removed and the crude product was directly used for next step. To a starting material/THF solution in a reaction vial equipped with a reflux condenser, 0.1 M NaOH(aq) (0.5 mL ) and PMe3 (1M in THF, 5 - 7 equivalents) were added. The reaction mixture was stirred at 50 °C for 2 hrs. The product has a Rf of 0 when eluted with EtOAc/MeOH (9/1) solution and a Rf of 0.6 when eluted with iPrOH/1M NH4OAc (2/1) solution. After completion of the reaction, the solvents were removed, and the crude benzylated aminoglycoside was added with catalytic amount of Pd(OH)2/C (20% Degussa type) and 5 mL of degassed HOAc/H2O (1/3). After being further degassed, the reaction mixture was stirred at room temperature under atmospheric H2 pressure. After being stirred for 1 day, the reaction mixture was filtered through Celite. The residue was washed with water, and the combined solutions were concentrated. The crude product was purified with Amberlite CG50(NH4+) eluted with a gradient of NH4OH solution (0% – 20%). The final product with Cl- salt can be prepared with an ion-exchange column packed with Dowex 1X8-200 (Cl- form) and eluting with water. After collection of the desired fractions and removal of solvent, the final products (35.2 mg, 0.04 mmol, 26%) are subjected to bioassay directly. The reported final products are characterized by 1H and 13C NMR at this stage. 1H NMR (400 MHz, D2O) δ 5.94 (d, J = 3.8Hz, 1H), 5.39 (d, J = 1.6 Hz, 1H), 5.27 (s, 1H), 4.53 (dd, J = 6.9, 4.9 Hz, 1H), 4.39 (dd, J = 4.7, 2.0 Hz, 1H), 4.3 (m, 1H), 4.29 (dd, J = 8.1, 3.7 Hz, 1H), 4.20 (dd, J = 3.1, 3.0 Hz, 1H), 4.1 (m, 1H), 3.8 - 4.0 (m, 6H), 3.75 (dd, J = 12.4, 4.9 Hz, 1H), 3.63 (dd, J = 10.2, 8.8 Hz, 1H), 3.5 (m, 2H), 3.45 (dd, J = 10.2, 9.7 Hz, 1H), 3.3 - 3.4 (m, 5H), 3.25 (dd, J = 13.7, 6.9 Hz, 1H), 3.1 (m, 2H), 2.1 (m, 2H), 1.9 (m, 1H), 1.67 (ddd, J = 12.7, 12.5, 12.4 Hz, 1H, H-2ax); 13C NMR (100 MHz, D2O) δ 175.8, 110.4, 95.8, 95.5, 86.0, 81.3, 77.7, 75.3, 74.1, 73.8, 71.0, 70.4, 69.8, 69.3, 68.7, 67.9, 67.6, 60.4, 53.9, 51.1, 49.2, 49.1, 40.7, 40.4, 36.9, 31.4, 31.1; HRESI Calcd for C27H54N7O15 ([M+H]+) m/z 716.3677; measure m/z 716.3695.
5-O-(2,3,5-Tri-O-acetyl-β-D-ribofuranosyl)-6,3′,4′-tri-O-acetyl-1,3,2′,6′-tetraazidoneamine (25)
A mixture of compounds 24 (0.32 g, 0.89 mmol) and 4 (0.41 g, 0.74 mmol), and activated 4 Å molecular sieves was stirred in anhydrous CH2Cl2 (15 mL) at room temperature for 5 hrs. N-Iodosuccinimide (0.20 g, 0.89 mmol) was quickly added into the above solution, and the reaction mixture was cooled to -60 °C. After the solution was warmed to -50 °C, trimethylsillyl triflate (20 μL, 0.13 mmol) was added. The solution was stirred overnight and TLC of the reaction showed the complete consumption of glycosyl donor (Rf = 0.27, monitored by TLC, Rf = 0.27, Hexane: EtOAc = 65: 35). The reaction was quenched by addition of NaHCO3(s), Na2SO4 -10H2O(s), Na2S2O3(s) then filtered through Celite. After removal of solvents, the crude product was re-dissolved in EtOAc. The organic solution was washed with water, brine, and dried over anhydrous Na2SO4. Removal of solvent and purification with a gradient column chromatography (Hexane: EtOAc = 80: 20 to 30: 70) afforded the desired product (0.22 g, 0.27 mmol, 37%). 1H NMR (400 MHz, CDCl3) δ 5.90 (d, J = 3.8 Hz, 1H, H-1′), 5.45 (dd, J = 10.6, 9.2 Hz, 1H, H-3′), 5.42 (d, J = 3.7 Hz, 1H, H-1″), 5.19 (dd, J = 4.9, 4.6 Hz, 1H, H-3″), 5.10 (dd, J = 4.8, 3.8 Hz, 1H, H-2″), 5.01 (dd, J = 9.9, 9.4 Hz, 1H, H-4′), 4.97 (dd, J = 9.8, 8.7 Hz, 1H, H-6), 4.4 (m, 1H, H-5′), 4.44 (dd, J = 12.1, 3.0 Hz, 1H, H-5″), 4.26 (ddd, J = 4.3, 3.8, 3.3 Hz, 1H, H-4″), 4.16 (dd, J = 12.2, 3.9 Hz, 1H, H-5″), 3.87 (dd, J = 9.2, 9.1 Hz, 1H, H-5), 3.70 (dd, J = 9.7, 8.9 Hz, 1H, H-4), 3.5 (m, 1H, H-3), 3.4 (m, 1H, H-1), 3.3 (m, 1H, H-6′), 3.25 (dd, J = 10.7, 3.8 Hz, 1H, H 2′), 2.39 (ddd, J = 13.3, 4.6, 4.5 Hz, 1H, H-2eq), 2.23 (s, 3H), 2.13 (s, 3H), 2.12 (s, 3H), 2.10 (s, 3H), 2.06 (s, 6H), 1.60 (ddd, J = 12.9, 12.8, 12.7 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 170.8, 170.2, 169.8, 169.7, 169.6, 169.5, 106.5, 96.5, 81.0, 79.8, 76.6, 74.8, 74.3, 71.1, 70.2, 69.4, 69.4, 63.3, 61.1, 59.2, 58.3, 51.1, 31.7, 21.0, 20.9 (2C), 20.6 (2C), 20.6; HRESI Calcd for C29H38N12O16Na ([M+Na]+) m/z 833.2426; measure m/z 833.2434.
5-O-(2,3,5-Tri-O-acetyl-β-D-ribofuranosyl)-1-N-tert-butoxycarbonyl-6,3′,4′-tri-O-acetyl-3,2′,6′-triazidoneamine (26)
Please refer to the general procedure for selective Staudinger reaction. Compound 26 was obtained with a 31% yield. 1H NMR (400 MHz, CDCl3) δ 5.90 (d, J = 3.7 Hz, 1H, H-1′), 5.44 (dd, J = 10.7, 9.3 Hz, 1H, H-3′), 5.43 (d, J = 3.7 Hz, 1H, H-1″), 5.20 (dd, J = 4.9, 4.9 Hz, 1H, H-3″), 5.08 (dd, J = 4.6, 3.9 Hz, 1H, H-2″), 4.99 (dd, J = 10.0, 9.5 Hz, 1H, H-4′), 4.78 (dd, J = 10.1, 10.0 Hz, 1H, H-6), 4.64 (d, J = 9.1 Hz, 1H), 4.52 (ddd, J = 10.1, 5.1, 2.9 Hz, 1H, H-5′), 4.38 (dd, J = 11.4, 3.0 Hz, 1H, H-5″), 4.2 (m, 1H, H-4″), 4.15 (dd, J = 12.1, 3.8 Hz, 1H, H-5″), 3.91 (dd, J = 9.1, 9.0 Hz, 1H, H-5), 3.7 (m, 1H, H-1), 3.66 (dd, J = 9.8, 8.9 Hz, 1H, H-4), 3.51 (ddd, J = 12.1, 9.8, 4.4 Hz, 1H, H-3), 3.2 - 3.3 (m, 2H, H-6′), 3.22 (dd, J = 10.8, 3.8 Hz, 1H, H-2′), 2.39 (ddd, J = 13.1, 4.4, 4.3 Hz, 1H, H-2eq), 2.14 (s, 3H), 2.10 (s, 3H), 2.08 (s, 3H), 2.08 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H),1.5 (m, 1H , H-2ax), 1.42 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.1, 170.7, 170.1, 169.9, 169.6 (2C), 155.3, 106.6, 96.5, 81.7, 80.3, 79.4, 75.3, 74.5, 71.0, 70.2, 69.5, 69.3, 63.3, 61.2, 59.4, 51.2, 49.2, 33.2, 29.8, 28.4 (3C), 21.0, 20.8 (3C), 20.6, 20.5; HRESI Calcd for C34H48N10O18Na ([M+Na]+) m/z 907.3045; measure m/z 907.3056.
5-O-(2,3,5-Tri-O-acetyl-β-d-ribofuranosyl)-1-N-[(S)-4-(benzyloxycarbonylamino)-2-hydroxybutanoyl]-6,3′,4′-tri-O-acetyl-3,2′,6′-triazidoneamine (27)
Please refer to the procedure for the preparation of compound 14. 1H NMR (400 MHz, CDCl3) δ 7.2 - 7.3 (m, 5H), 7.10 (d, J = 8.7 Hz, 1H), 5.90 (d, J = 3.7 Hz, 1H, H-1′), 5.45 (dd, J = 10.6, 9.3 Hz, 1H, H-3′), 5.44 (d, J = 5.2 Hz, 1H, H-1″), 5.2 (m, 1H), 5.21 (dd, J = 4.8, 4.8 Hz, 1H, H-3″), 5.0 - 5.1 (m, 3H), 5.0 (dd, J = 9.8, 9.6 Hz, 1H, H-4′), 4.89 (dd, J = 9.9, 9.8 Hz, 1H, H-6), 4.51 (ddd, J = 10.2, 5.2, 2.8 Hz, 1H, H-5′), 4.42 (dd, J = 12.1, 3.0 Hz, 1H, H-5″), 4.2 (m, 1H, H-4″), 4.15 (dd, J = 12.1, 3.8 Hz, 1H, H-5″), 4.0 - 4.1 (m, 2H), 3.95 (dd, J = 8.9, 8.8 Hz, 1H, H-5), 3.70 (dd, J = 9.7, 8.7 Hz, 1H, H-4), 3.58 (ddd, J = 12.0, 7.6, 4.4 Hz, 1H, H-3), 3.5 (m, 1H), 3.36 (dd, J = 13.5, 2.9 Hz, 1H, H-6′), 3.30 (dd, J = 13.5, 5.5 Hz, 1H, H-6′), 3.22 (dd, J = 10.7, 3.7 Hz, 1H, H-2′), 3.1 (m, 1H), 2.35 (ddd, J = 13.5, 4.5, 4.3 Hz, 1H, H-2eq), 2.1 (s, 6H), 2.08 (s, 3H), 2.07 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 2.0 (m, 2H), 1.56 (ddd, J = 12.7, 12.5, 12.3 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 173.7, 170.9, 170.7, 170.2, 169.9, 169.6 (2C), 158.3, 136.3, 128.7, 128.5, 128.2, 106.4, 96.6, 81.5, 79.5, 75.0, 74.5, 71.0, 70.2, 69.6, 69.3, 69.0, 67.4, 63.4, 61.1, 59.4, 51.2, 47.4, 37.2, 35.3, 32.5, 29.8, 21.0, 20.8 (2C), 20.7, 20.6, 20.5; HRESI Calcd for C41H54N11O20 ([M+H]+) m/z 1020.3546; measure m/z 1020.3545.
5-O-(β-d-ribofuranosyl)-1-N-[(S)-4-amino-2-hydroxybutanoyl]neamine (Butirosin B)
Please refer to the procedure for the preparation of neokacin. 1H NMR (400 MHz, D2O) δ 5.96 (d, J = 3.9 Hz, 1H), 5.33 (s, 1H), 4.29 (dd, J = 8.2, 4.0 Hz, 1H), 4.1 - 4.2 (m, 2H), 3.8 - 3.9 (m, 7H), 3.67 (dd, J = 12.6, 5.3 Hz, 1H), 3.59 (dd, J = 10.2, 8.8 Hz, 1H), 3.45 (dd, J = 9.8, 9.1 Hz, 1H), 3.3 - 3.4 (m, 3H), 3.2 (m, 1H), 3.1 (m, 2H), 2.1 (m, 2H), 1.9 (m, 1H), 1.68 (ddd, J = 12.6, 12.5, 12.3 Hz, 1H, H-2ax); 13C NMR (100 MHz, D2O) δ 175.8, 110.5, 95.8, 86.0, 82.4, 77.4, 75.6, 74.1, 70.9, 69.8, 69.3, 69.1, 68.6, 60.8, 53.9, 49.2, 49.1, 40.4, 36.8, 31.1 (2C); HRESI Calcd for C21H42N5O12 ([M+H]+) m/z 556.2829; measure m/z 556.2835.
6-O-(3-Azido-2,4,6-tri-O-benzyl-α-D-glucopyranosyl)-1-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-3,2′,6′-triazidoneamine (29a)
Please refer to the general procedure for glycosylation and hydrolysis. 1H NMR (400 MHz, CDCl3) δ 7.2 - 7.3 (m, 25H), 7.05 (d, J = 5.7 Hz, 1H, NH), 5.60 (d, J = 3.6 Hz, 1H), 5.29 (dd, J = 5.7, 5.5 Hz, 1H, NH), 5.1 (m, 2H), 4.99 (d, J = 12.0 Hz, 1H), 4.74 (d, J = 10.7 Hz, 1H), 4.68 (d, J = 13.5 Hz, 1H), 4.66 (d, J = 12.2 Hz, 1H), 4.57 (d, J = 12.3 Hz, 1H), 4.55 (d, J = 12.5 Hz, 1H), 4.5 (m, 1H), 4.45 (d, J = 12.2 Hz, 1H), 4.40 (d, J = 10.7 Hz, 1H), 4.2 (m, 1H, H-5′), 3.99 (dd, J = 9.5, 9.5 Hz, 1H), 3.93 (dd, J = 6.6, 4.7 Hz, 1H), 3.83 (dd, J = 10.1, 9.8 Hz, 1H), 3.8 (m, 1H), 3.7 (m, 1H), 3.1 - 3.6 (m, 14H), 2.6 (m, 1H, H-2eq), 2.1 (m, 1H), 1.9 (m, 1H), 1.20 (ddd, J = 13.2, 12.4, 12.3 Hz, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 173.9, 156.6, 137.5, 137.4, 137.2, 136.8, 136.7, 129.0, 128.9, 128.8, 128.6 (2C), 128.5, 128.3 (2C), 128.2, 128.1, 127.9, 127.5, 98.4 (2C), 85.4, 79.7, 78.3, 77.4, 76.3, 75.0, 73.6 (2C), 73.1, 71.8, 71.6 (2C), 71.3, 67.9, 66.9, 65.2, 63.0, 59.2, 51.6, 48.4, 37.4, 32.4, 32.2, 29.8; HRESI Calcd for C58H67N14O14 [M+H]+m/z 1183.4961; measure m/z 1183.4961.
6-O-(4-O-((R)-3-Azido-2-((R)-3-azido-2-O-hydroxylpropyl)propyl)-2,3-di-O-benzyl-6-deoxy-α-d-glucopyranosyl)-1-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)-butanoyl]-3,2′,6′-triazidoneamine (29b)
Please refer to the general procedure for glycosylation and hydrolysis. 1H NMR (400 MHz, CDCl3) δ 7.2 - 7.4 (m, 20H), 7.11 (d, J = 5.4 Hz, 1H, NH), 5.60 (d, J = 3.6 Hz, 1H), 5.3 (m, 1H, NH), 5.07 (d, J = 12.0 Hz, 1H), 4.96 (d, J = 12.1 Hz, 1H), 4.88 (d, J = 11.2 Hz, 1H), 4.70 (d, J = 11.3 Hz, 1H), 4.65 (d, J = 12.3 Hz, 1H), 4.55 (d, J = 12.3 Hz, 1H), 4.6 (m, 1H), 4.48 (s, 2H), 4.2 (m, 1H), 3.97 (dd, J = 9.7, 9.5 Hz, 1H), 3.93 (dd, J = 6.4, 5.1 Hz, 1H), 3.7 - 3.8 (m, 4H), 3.3 - 3.6 (m, 15H), 3.1 -3.3 (m, 4H), 3.14 (dd, J = 9.7, 9.0 Hz, 1H), 2.90 (dd, J = 9.2, 9.2 Hz, 1H), 2.56 (ddd, J = 12.8, 4.1, 3.5 Hz, 1H, H-2eq), 2.1 (m, 1H), 1.9 (m, 1H), 1.3 (m, 1H, H-2ax), 1.22 (d, J = 6.2 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 174.0, 156.6, 138.7, 138.6, 137.6, 136.6, 129.0, 128.9, 128.7 (2C), 128.6, 128.5 (2C), 128.4, 128.2, 127.9, 127.7, 127.3, 127.0, 98.8, 98.5, 85.0, 84.3, 81.2, 80.1, 79.5, 79.3, 78.2, 76.8, 75.3, 73.8, 73.5, 73.1, 71.9, 71.8, 71.6 (2C), 69.9, 68.4, 66.9, 63.2, 59.1, 53.3, 51.8, 51.6, 48.4, 37.5, 32.5, 29.8, 17.9; HRESI Calcd for C57H72N17O16 [M+H]+m/z 1250.5342; measure m/z 1250.5346.
6-O-(3-Azido-2-O-benzyl-α-d-xylopyranosyl)-1-N-[(S)-2-O-benzyl-4-(benzyloxycarbonylamino)butanoyl]-3,2′,6′-triazidoneamine (29c)
Please refer to the general procedure for glycosylation and hydrolysis. 1H NMR (400 MHz, CDCl3) δ 7.2 - 7.4 (m, 15H), 7.18 (d, J = 5.5 Hz, 1H), 5.50 (d, J = 2.9 Hz, 1H), 5.25 (dd, J = 5.4, 5.4 Hz, 1H), 5.10 (d, J = 12.2 Hz, 1H), 5.03 (d, J = 12.1 Hz, 1H), 4.7 (m, 1H), 4.65 (d, J = 12.1 Hz, 1H), 4.50 (d, J = 12.2 Hz, 1H), 4.46 (s, 2H), 4.3 (m, 1H), 4.1 - 4.2 (m, 1H), 3.8 - 4.0 (m, 2H), 3.5 - 3.7 (m, 5H), 3.4 - 3.5 (m, 4H), 3.2 - 3.3 (m, 5H), 3.07 (dd, J = 9.5, 9.3 Hz, 1H), 2.4 (m, 1H, H-2eq), 1.8 - 2.0 (m, 2H), 1.2 (m, 1H, H-2ax); 13C NMR (100 MHz, CDCl3) δ 174.1, 156.8, 137.2, 136.8, 136.5, 129.0 (2C), 128.9, 128.8, 128.7 (2C), 128.4, 98.8, 97.7, 83.6, 80.7, 78.4, 77.8, 77.4, 74.1, 73.4, 71.6, 68.9, 67.1, 65.2, 63.1, 59.1, 51.5, 48.1, 37.5, 32.4, 32.3, 29.9; HRESI Calcd for C43H53N14O13 [M+H]+m/z 973.3916; measure m/z 973.3900.
6-O-(3-Amino-α-d-glucopyranosyl)-1-N-[(S)-4-amino-2-hydroxybutanoyl]neamine (JLN007)
Please refer to the procedure for the preparation of neokacin. 1H NMR (400 MHz, D2O) δ 5.81 (d, J = 3.8Hz, 1H), 5.13 (d, J = 3.7 Hz, 1H), 4.24 (dd, J = 9.2, 3.7 Hz, 1H), 3.9 - 4.1 (m, 3H), 3.91 (dd, J = 10.7, 9.1 Hz, 1H), 3.7 - 3.9 (m, 6H), 3.64 (dd, J = 10.1, 10.1 Hz, 1H), 3.44 (dd, J = 9.6, 9.5 Hz, 1H), 3.3 - 3.4 (m, 4H), 3.24 (dd, J = 13.6, 7.0 Hz, 1H), 3.1 (m, 2H), 2.1 (m, 2H), 1.9 (m, 1H), 1.74 (ddd, J = 12.7, 12.6, 12.6 Hz, 1H, H-2ax); 13C NMR (100 MHz, D2O) δ 175.7, 98.2, 96.4, 80.5, 79.6, 74.9, 72.3, 71.0, 69.8, 69.3, 69.2, 68.2, 65.8, 60.0, 55.3, 53.9, 49.0, 48.8, 40.4, 37.2, 31.4, 31.0; HRESI Calcd for C22H45N6O12 [M+H]+m/z 585.3095; measure m/z 585.3068.
6-O-(4-O-((R)-3-Amino-2-((R)-3-amino-2-hydroxylpropyl)-propyl)-6-deoxy-α-d-glucopyranosyl)-1-N-[(S)-4-amino-2-hydroxybutanoyl]neamine (JLN033)
Please refer to the procedure for the preparation of neokacin. 1H NMR (400 MHz, D2O) δ 5.95 (d, J = 3.9 Hz, 1H), 5.01 (d, J = 4.0 Hz, 1H), 4.24 (dd, J = 9.2, 3.8 Hz, 1H), 3.9 - 4.1 (m, 5H), 3.7 - 3.8 (m, 5H), 3.69 (dd, J = 9.6, 9.5 Hz, 1H), 3.57 (dd, J = 10.1, 6.1 Hz, 1H), 3.52 (dd, J = 5.5, 4.2 Hz, 1H), 3.4 - 3.5 (m, 6H), 3.2 - 3.3 (m, 2H), 3.0 - 3.2 (m, 4H), 3.0 (m, 2H), 2.22 (ddd, J = 12.9, 4.3, 4.2 Hz, 1H, H-2eq), 2.1 (m, 1H), 1.9 (m, 1H), 1.82 (ddd, J = 12.8, 12.7, 12.6 Hz, 1H, H-2ax), 1.24 (d, J = 6.3 Hz, 3H, CH3); 13C NMR (100 MHz, D2O) δ 175.6, 99.2, 95.9, 84.1, 80.6, 78.2, 75.4, 74.9, 72.6, 71.9, 71.1, 70.8, 69.8, 69.4, 68.5, 67.5, 66.9, 53.7, 48.9, 48.8, 41.9, 40.9, 40.3, 37.2, 31.0, 30.5, 17.1; HRESI Calcd for C28H58N7O14 [M+H]+m/z 716.4041; measure m/z 716.4026.
6-O-(3-Amino-α-D-xylopyranosyl)-1-N-[(S)-4-amino-2-hydroxybutanoyl]neamine (JLN040)
Please refer to the procedure for the preparation of neokacin. 1H NMR (400 MHz, D2O) δ 5.81 (d, J = 3.8 Hz, 1H), 5.02 (d, J = 3.7 Hz, 1H), 4.16 (dd, J = 9.3, 3.6 Hz, 1H), 4.0 (m, 1H), 3.8 - 3.9 (m, 3H), 3.6 - 3.7 (m, 4H), 3.5 - 3.6 (m, 2H), 3.3 - 3.4 (m, 4H), 3.1 - 3.2 (m, 2H), 3.1 (m, 2H), 2.11 (ddd, J = 12.8, 4.2, 4.0 Hz, 1H, H-2eq), 2.0 (m, 1H), 1.8 (m, 1H), 1.70 (ddd, J = 12.7, 12.7, 12.7 Hz, 1H, H-2ax); 13C NMR (100 MHz, D2O) δ 175.5, 97.5, 96.1, 79.6, 78.5, 74.5, 70.8, 69.7, 69.2, 68.6, 68.0, 65.7, 61.9, 55.4, 53.7, 48.7, 48.6, 40.3, 37.1, 30.9, 30.8; HRESI Calcd for C21H43N6O11 [M+H]+m/z 555.2989; measure m/z 555.2965.
Supplementary Material
1H and 13C spectra of the synthesized compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
We acknowledge National Institutes of Health (AI053138) and DARPA for financial support. We thank Dr. Dobrowolski of Utah State University for assistance in NMR experiments. We thank Prof. Mobashery of Notre Dame University for providing the pTZ19U-3 and pSF815 plasmids. We also thank Prof. Vernon Parker and Prof. Alvan Hengge of Utah State University for providing valuable advice and Prof. Steve Scheiner of Utah State University for assistance in computational analysis.
References
- 1.Umezawa S, Tsuchiya T. In: Aminoglycoside Antibiotics. Umezawa H, Hooper IR, editors. Springer-Verlag; New York: 1982. pp. 37–110. [Google Scholar]
- 2.Hanessian S, Haskell TH. In: Antibiotics Containing Sugars. Pigmann H, Horton D, editors. Academic Press; New York/Lodon: 1970. pp. 139–211. [Google Scholar]
- 3.Haddad J, Kotra LP, Mobashery S. In: Glycochemistry Principles, Synthesis, and Applications. Wang PG, Bertozzi CR, editors. Marcel Dekker, Inc; New York/Basel: 2001. pp. 307–424. [Google Scholar]
- 4.Hanessian S, Kornienko A, Swayze EE. Tetrahedron. 2003;59:995–1007. [Google Scholar]
- 5.Tsuchiya T, Takagi Y, Umezawa S. Tetrahedron Lett. 1979;51:4951–4954. [Google Scholar]
- 6.Nagabhushan TL, Cooper AB, Turner WN, Tsai H, McCombie S, Mallams AK, Rane D, Wright JJ, Reichert P, Boxler DL, Weinstein J. J Am Chem Soc. 1978;100:5253–5254. [Google Scholar]
- 7.Roestamadjli J, Grapsas I, Mobashery S. J Am Chem Soc. 1995;117:11060–11069. [Google Scholar]
- 8.Umezawa H, Miyasaka T, Iwasawa H, Ikeda D, Kondo S. J Antibiot. 1981;34:1635–1640. doi: 10.7164/antibiotics.34.1635. [DOI] [PubMed] [Google Scholar]
- 9.Shitara T, Umemura E, Tsuchiya T, Matsuno T. Carbohydr Res. 1995;276:75–89. doi: 10.1016/0008-6215(95)00123-b. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Remers WA. J Org Chem. 1978;43:3327–3331. [Google Scholar]
- 11.Umezawa S, Tsuchiya T, Torii T. J Antibiot. 1982;35:58–61. doi: 10.7164/antibiotics.35.58. [DOI] [PubMed] [Google Scholar]
- 12.Sharma MN, Kumar V, Remers WA. J Antibiot. 1982;35:905–910. doi: 10.7164/antibiotics.35.905. [DOI] [PubMed] [Google Scholar]
- 13.Cappelletti LM, Spagnoli R, Spa P. J Antibiot. 1983;36:328–330. doi: 10.7164/antibiotics.36.328. [DOI] [PubMed] [Google Scholar]
- 14.Elchert B, Li J, Wang J, Hui Y, Rai R, Ptak R, Ward P, Takemoto JY, Bensaci M, Chang CWT. J Org Chem. 2004;69:1513–1523. doi: 10.1021/jo035290r. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Li J, Chen HN, Chang H, Tanifum CT, Liu HH, Czyryca PG, Chang CWT. J Med Chem. 2005;48:6271–6285. doi: 10.1021/jm050368c. [DOI] [PubMed] [Google Scholar]
- 16.Bayley H, Standring DN, Knowles JR. Tetrahedron Lett. 1978:3633–3634. [Google Scholar]
- 17.Li J, Chen HN, Chang H, Wang J, Chang CWT. Org Lett. 2005;7:3061–3064. doi: 10.1021/ol051045d. [DOI] [PubMed] [Google Scholar]
- 18.Nyffeler PT, Liang CH, Koeller KM, Wong CH. J Am Chem Soc. 2002;124:10773–10778. doi: 10.1021/ja0264605. [DOI] [PubMed] [Google Scholar]
- 19.Ariza X, Vrpi F, Viladomat C, Vilarrasa J. Tetrahedron Lett. 1998;39:9101–9102. [Google Scholar]
- 20.Moon MS, Jun SJ, Lee SH, Cheong CS, Kim KS, Lee BS. Bull Korean Chem Soc. 2003;24:163–164. [Google Scholar]
- 21.Wu BG, Yang J, He Y, Swayze EE. Org Lett. 2002;4:3455–3458. doi: 10.1021/ol026548n. [DOI] [PubMed] [Google Scholar]
- 22.(a) Knapp S, Shieh WC. Tetrahedron Lett. 1991;32:3627–3630. [Google Scholar]; (b) Antkowiak WZ, Krzyzosiak WJ. J of Chromatography. 1974;90:399–401. [Google Scholar]
- 23.The axial substituent is typically more shielded as compared to the axial one as indicated by the chemical shifts of H-2ax and H-2eq in this report. Similar results regarding the chemical shifts of methyl group on methylcyclohexane can be found in the literature as well ( Wiberg KB, Hammer JD, Castejon H, Bailey WF, DeLeon EL, Jarret RM. J Org Chem. 1999;64:2085. doi: 10.1021/jo990056f.). However, computational analysis of the electron density and electrostatic potential for the axial and equatorial azido groups on cyclohexane reveals only slight difference favoring equatorial azido group toward nucleophilic agent. Thus, we reason that a combination of stereoelectronic effect and steric hindrance should contribute to the lower reactivity of N-2‴.
- 24.Methods for Dilution Antimicrobial Susceptibility Testing for Bacteria that Grow Aerobically. Approved standard M7-A5, and Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved standard M2-A7, National Committee for Clinical Laboratory Standards, Wayne, PA.
- 25.Rasheed JK, Anderson GJ, Yigit H, Queenan AM, Domenech-Sanchez A, Swenson JM, Biddle JW, Ferraro MJ, Jacoby GA, Tenover FC. Antimicrob Agents Chemother. 2000;44:2382–2388. doi: 10.1128/aac.44.9.2382-2388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachiler H, Santanam P, Kayser FH. Antimicrob Agents Chemother. 1996;40:1254–1256. doi: 10.1128/aac.40.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ida T, Okamoto R, Shimauchi C, Okubo T, Kuga A, Inouqe M. J Clin Microb. 2001;39:3115–3121. doi: 10.1128/JCM.39.9.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright GD, Ladak P. Antimicrob Agents Chemother. 1997;41:956–960. doi: 10.1128/aac.41.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, J.; Wang, J.; Chang, C.-W. T. unpublished result.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H and 13C spectra of the synthesized compounds. This material is available free of charge via the internet at http://pubs.acs.org.