Abstract
Background
Comparative morphology identifies the digits of the wing of birds as 1,2 and 3, but they develop at embryological positions that become digits 2, 3 and 4 in other amniotes. A hypothesis to explain this is that a homeotic frame shift of digital identity occurred in the evolution of the bird wing, such that digits 1,2 and 3 are developing from embryological positions 2, 3 and 4. Digit 1 of the mouse is the only digit that shows no late expression of HoxD-11. This is also true for the anterior digit of the bird wing, suggesting this digit is actually a digit 1. If this is the case, we can expect closer relatives of birds to show no HoxD-11 expression only in digit 1. To test this prediction we investigate HoxD-11 expression in crocodilians, the closest living relatives of birds.
Methodology/Principal Findings
Using degenerate primers we cloned a 606 nucleotide fragment of exon 1 of the alligator HoxD-11 gene and used it for whole-mount in-situ detection in alligator embryos. We found that in the pentadactyl forelimbs of alligator, as in the mouse, late expression of HoxD-11 is absent only in digit 1.
Conclusions/Significance
The ancestral condition for amniotes is that late-phase HoxD-11 expression is absent only in digit 1. The biphalangeal morphology and lack of HoxD-11 expression of the anterior digit of the wing is like digit 1 of alligator and mouse, but its embryological position as digit 2 is derived. HoxD-11 expression in alligator is consistent with the hypothesis that both digit morphology as well as HoxD-11 expression are shifted towards posterior in the bird wing.
Introduction
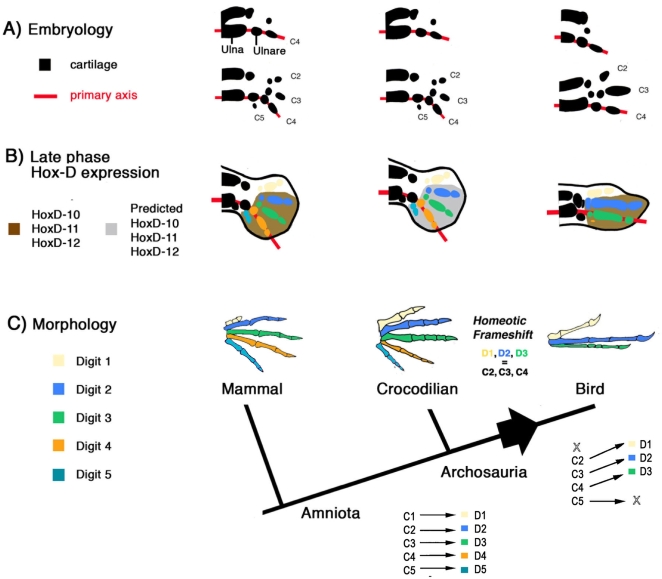
The identity of the digits of the bird wing is a classic problem of evolutionary biology, born out of apparently contradictory developmental and morphological evidence. If we follow the criterion of homology by embryological position of origin, we find that the wing digits develop from embryological positions corresponding to those of digits 2, 3 and 4 of crocodilians [1], [2]. Crocodilians are bird's closest living relatives [3] and thus the optimal reference point for developmental comparisons to the bird wing. In the alligator forelimb (as in mouse) the first cartilaginous digital condensation to form is spatially in line with the ulnare and ulna (Figure 1A, top row), and develops into digit 4 (Figure 1 A, bottom row). The spatial alignment of these elements is referred to as the “primary axis”, indicated by a red line in Figure 1. In the wing, the primary axis develops into the posterior digit, indicating the digits develop at positions 2, 3 and 4 [1], [2] (Figure 1A). However, the wing digits of early birds like Archaeopteryx are morphologically similar to digits 1, 2, and 3 of crocodilians, presenting 2, 3 and 4 phalanges, respectively (Figure 2). We arrive at the same conclusion if we compare Archaeopteryx to early dinosaurs, lizards, and even early branches of amniotes (Figure 2, See Captorhinus, Ophiacodon). Wing digits are labeled 1,2,3 in the fields of phylogenetic systematics and comparative anatomy [4], [5], [6], [7] As an explanation to this apparent contradiction with the embryological evidence, Wagner and Gauthier [8] suggested that a homeotic frame shift of digital identity had occurred in the evolution of the bird wing, such that in birds digits 1, 2 and 3 develop from embryological positions 2, 3 and 4 (Figure 1C).
Figure 1. Three levels to the avian digit homology problem: embryology, gene expression, and morphology.
A) Embryology: In pentadactyl amniotes like mammals and crocodilians, the primary axis of cartilage formation (red line) always develops into digit 4. Embryological condensations in this figure are labeled C1–C5 based on their spatial relation to the primary axis (C4). B) Gene expression (Late phase): In the mouse, expression of HoxD10-D12 is absent only in digit 1. In the chicken, expression is absent in the anterior digit, but it is one position closer to the primary axis, at the embryological position of C2. C) Comparative morphology: The wing digits are morphologically 1, 2, and 3. The hypothesis of a homeotic frame shift proposes that digits 1,2 and 3 have all shifted one embryological position towards posterior in the evolution of the bird line, such that digits D1, D2 and D3 (color coded: cream, blue and green) in the wing develop from the embryological positions C2, C3 and C4. If the frame shift hypothesis is correct, we expect to find that HoxD gene expression in crocodilians will be absent only at embryological position C1.
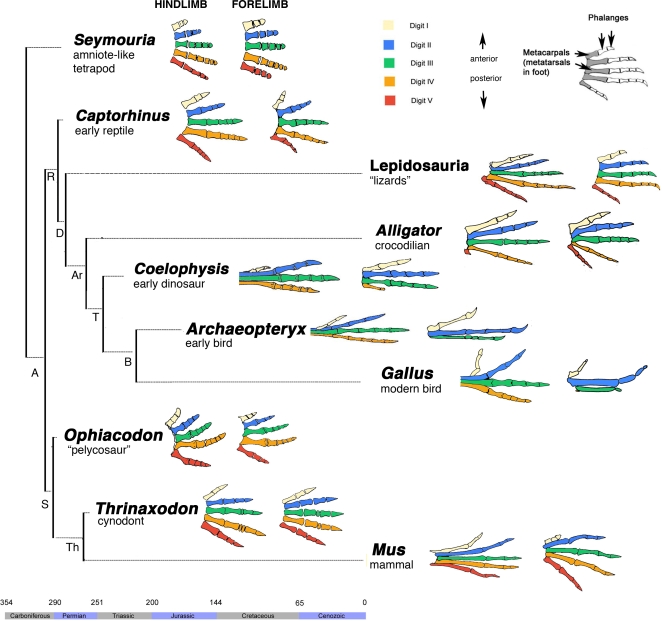
Figure 2. The evolution of digit morphology.
The forelimb and hind limbs of representative taxa illustrate the history of digit morphology in the lineages leading to the taxa compared in this study, the chicken (Gallus gallus), alligator (Alligator mississippiensis) and mouse (Mus musculus). The digits of early birds like Archaeopteryx, are specifically similar to digits 1, 2, and 3 of crocodilians, presenting 2,3 and 4 phalanges on each digit, respectively (node Ar). We arrive at the same conclusion if we compare Archaeopteryx to early dinosaurs, lizards, and early branches of amniotes (such as Captorhinus, Ophiacodon). No comparative morphological evidence has been presented for a 2,3,4 identification of wing digits. Molecular phylogenies confirm the relationships shown in this figure [3], [33]. Maximally parsimonious inference of morphological history is done following the method in [34]. The nodes of the tree are labeled for corresponding clades: A) Amniota R) Reptilia, D) Diapsida, Ar) Archosauria, T) Theropoda, B) Birds S) Synapsida Th) Therapsida. A geological time scale indicates the approximate time of lineage divergence.
Consistent with this hypothesis, the embryological position of HoxD gene expression appears to be shifted in the bird wing. The posterior HoxD genes (i.e. HoxD-10, HoxD-11, HoxD-12, and HoxD-13) are well known for their expression and function in developing digits [9], [10]. In the bird wing HoxD-10, -11 and -12 are absent only at the most anterior digit [11], [12] (embryological position 2, Figure 1B). Because the same is true only for digit 1 of the mouse [13], Vargas and Fallon [14] argued that HoxD gene expression in the wing suggests a digit 1 develops at the embryological position of digit 2. If the comparison of digit 1 of the mouse to the anterior wing digit is correct, we should expect closer relatives of birds to show no expression of these genes only in digit 1 (The predicted expression for alligator is shown in gray shading in Figure 1B). If we do not assume a frame shift, but rather that wing digits develop directly into digits 2,3 and 4, expression in crocodilian forelimbs could be absent in digit 2. To test these predictions, we investigate HoxD-11 expression in crocodilians (bird's closest living relatives). If expression in crocodilians is not uniquely absent in digit 1 (as in mouse), HoxD-11 would provide no support for the homeotic frame shift hypothesis. We cloned a fragment of exon 1 of HoxD-11 of the crocodilian Alligator mississippiensis and observed its transcription in developing digits. We found that, as in the mouse, in alligator forelimbs HoxD-11 mRNA is absent only at digit 1. We discuss the relevance of this result for the hypothesis of a homeotic frame shift in the bird wing.
Results
Cloning and sequence analysis
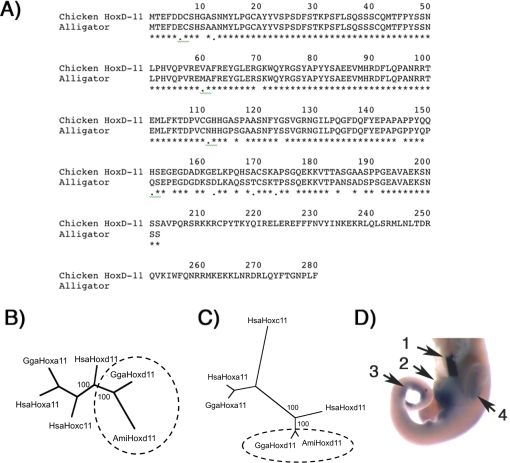
A genomic fragment was amplified by PCR with a primer pair targeting the conserved 5′ sequence of the HoxD-11 coding sequence and a part of the homeobox (see Material and Methods). These primers target a sequence that corresponds to nucleotides 22 to 690 of the chicken HoxD-11 coding sequence, but include the intron between exon 1 and 2. We obtained a PCR product of approximately 900 nucleotides and sequenced 819 nucleotides from the 5′ end of this sequence. This sequence contains the complete exon 1 of 606 nucleotides and the adjacent intron sequence with a putative 5′ splice site AG/GTAGGT (the G/G is the putative exon-intron boundary). The translated exon 1 sequence has 87% sequence conservation with the corresponding part of the chicken HoxD-11 gene (Figure 3A). A phylogenetic analysis of this and published paralog group 11 amino acid sequences reveals strong support for the hypothesis that the alligator sequence is a HoxD-11 ortholog. Our sequence forms a well supported clade with the chicken HoxD-11 sequence and together with the human HoxD-11 sequence is separated by a well supported node from HoxA-11 and HoxC-11 sequences (Figure 3 B, C). Furthermore, in situ hybridization revealed expression in all structures where HoxD-11 is known to be expressed in other amniotes, as can be observed in Figure 3D. The specimen is dissected to show the sharp anterior limit of hindgut expression (Figure 3D, 1) expression in the genital tubercle (Figure 3D, 2), distal tail (Figure 3D, 3) and limbs (Figure 3D 4, Figure 4). We thus conclude that we have isolated the exon 1 and 5′ part of the intron of alligator HoxD-11 gene (Genbank accession # EU597806).
Figure 3. Identification of the alligator HoxD-11 exon 1 sequence.
(EU597806): A) alignment of the deduced alligator amino acid sequence with the HoxD-11 sequence of chicken. In exon 1 the amino acid sequence conservation is 87%. B) Maximum parsimony tree of the aligned exon 1 amino acid sequences from HoxD-11, HoxA-11 and HoxC-11 sequences. C) Neighbor joining tree of the aligned exon 1 amino acid sequences from HoxD-11, HoxA-11 and HoxC-11 sequences. B+C) The numbers at the internal branches represent bootstrap support values. Note that the alligator HoxD-11 sequence, AmiHoxd11, forms a well-supported clade with the human, HsaHoxd11, and the chicken, GgaHoxd11, HoxD-11 sequences, confirming that the alligator sequence is a HoxD-11 ortholog. Furthermore, the alligator sequence is more closely related to the chicken sequence than to the mouse sequence, as expected based on the accepted species phylogeny. D) Expression of the alligator sequence in a stage 12 alligator embryo. The embryo is dissected to show the sharp anterior limit of hindgut expression (1). Expression is also present in genital tubercule (2), distal tail (3) and limb buds (4), all known expression domains of HoxD-11 in chicken and mouse that confirm the alligator sequence is a homolog of HoxD-11.
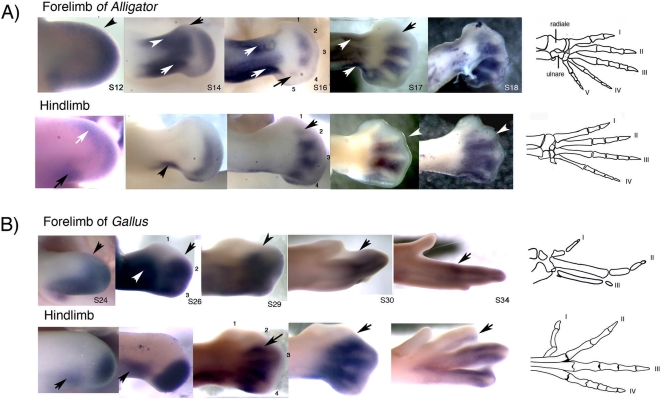
Figure 4. The expression of HoxD-11 in alligator and chicken limbs.
A) The expression of HoxD-11 in the developing forelimb and hind limb of the crocodilian Alligator mississippiensis (staging is according to Ferguson [29]) B) The expression of HoxD-11 in the chicken Gallus gallus (staging according to Hamburger-Hamilton [30]). Alligator forelimb: Early stages 12–14 show extension of HoxD-11 along the limb border, including anterior regions (black arrows). At stage 16 and onwards, there is no detectable expression in the anterior-most digit 1 region. At stage 16 posterior expression is temporarily down-regulated in the region of digits 4 and 5 (black arrow) but is re-expressed by stage 17. At stage 17, only very low expression is detectable in the interdigit between digit 1 and 2 (black arrow). Strong anterior and posterior expression in the wrist and forearm region at stage 14 continues up to stage 17 (white arrows). Alligator hind limb: Stage 12 shows some expression along the anterior margin (white arrow) but this is undetectable in the anterior-most digit 1 region from stage 14 onwards. At stage 16, only very low expression is detectable in the interdigit between digits 1 and 2 (black arrow). Expression in the foreleg is restricted to posterior (stages 12 and 14, black arrow) and absent in the ankle region. Stage 17 and stage 18 show a sharp anterior limit of HoxD-11 expression along the posterior margin of digit 2 (white arrows). Chicken wing: Stage 24 presents anterior expression (black arrow). Expression is undetectable in digit 1 in stage 26 and subsequent stages. No low expression is detectable between interdigits 1 and 2. In stages 26–34, expression of HoxD-11 extends to the anterior border of digit 2 (black arrows). Chicken hind limb: Early stage 24 shows no anterior expression. No expression is ever detected in digit 1 precursor cells or the interdigit between digit 1 and digit 2. In stages 29–34 HoxD-11 shows a sharp anterior limit along the posterior border of digit 2 (black arrows in stage 17–18 of alligator and stages 31–34 of chicken; stage 34 is shown in ventral view). Strong expression is found in the interdigit between digits 2 and 3 (stage 29, black arrow), as in forelimbs. HoxD-11 expression in alligator limbs is consistent with the notion that, as in chicken, normal digit 1 determination occurs at late stages with absent or very low HoxD-11 expression.
Expression of HoxD-11 in embryonic limbs of alligator
The expression pattern of HoxD-11 in the embryonic limbs of the alligator is presented in Figure 4A alongside with that of HoxD-11 in the chicken (Figure 4B) for comparison. In the early alligator forelimb bud (stage 12, n = 1), the expression of HoxD-11 extends along most of the margin of the limb bud, including anterior regions indicated by the black arrow. Early mouse forelimbs show similar anterior extension of HoxD-11 transcripts (see stage 10.5 photographs in [15], [16]). Anterior expression in alligator forelimbs persists into stage 14 (n = 2), presumably including cell precursors of digit 1 (black arrow). However, as development proceeds and digital rays become apparent, anterior expression of HoxD-11 in alligator forelimb is down-regulated and becomes undetectable in digit 1, as observed in stage 16 (n = 3), stage 17 (n = 3) and stage 18 (n = 1). In the forelimbs of the chicken, and in forelimbs and hind limbs of the mouse [11], strong expression extends up to the anterior border of digit two, as indicated by black arrows in stages 26 to 34 of the chicken wing (Figure 4B). In the foot of both chicken and alligator, late HoxD-11 expression is slightly more shifted towards posterior: The anterior limit of expression is found along the posterior border of digit 2 (white arrows in hind limb stages 17–18 of the alligator, black arrows for stages 30 and 34 of the chicken hind limb).
A phenomenon particular to the alligator forelimb is a transient down-regulation of HoxD-11 expression in the posterior region of digits 4 and 5 at stage 16, as indicated by the black arrow. Expression is recovered by stage 17. In contrast, the posterior expression of HoxD-11 is uninterrupted in the hind limb of the alligator, as well as both forelimbs and hind limbs of chicken and mouse. Digits 4 and 5 of the adult alligator forelimb are special in that they are unusually slender, have no claws, and are proportionally reduced compared to reptilian outgroups such as lizards (specially digit 4; see Lepidosauria, Figure 1 node D). Assuming that HoxD-11 expression affects growth rates [17], we can hypothesize that transient down-regulation of HoxD-11 may relate to the partial reduction of these digits.
In all forelimbs examined. i.e. alligator, chicken, and mouse, there is early expression of HoxD-11 in the mesopodial (“wrist”) and zeugopodial (“forearm”) regions [11], [13] (Figure 4A). In alligator, strong anterior and posterior expression in the mesopodial-zeugodial region of the forelimb persists into late stages (white arrows in Stages 14, 16 and 17). In the chicken and alligator hind limb, mesopodial expression is absent, and zeugopodial expression is found in a posteriorly restricted domain (black arrow in alligator stage 14 and chicken stage 26 hind limbs). In the mouse hind limb, mesopodial expression is present, but weaker than in forelimbs [13]. This expression in the wrist of alligator may relate to the unusual lengthening of the radiale and ulnare wrist-bones, a derived trait of crocodilians (Figure 4A, adult skeleton). This difference is reminiscent of the stronger HoxA-11 expression in the frog hind limb compared to the forelimb, where the frog hind limb also has a pair of elongated mesopodial bones [18].
Discussion
Expression of HoxD-11 in alligator digits is consistent with its function in other amniotes
When cartilaginous digital rays are formed they are at first undetermined. Digital identity becomes fixed at fairly late stages under the influence of the surrounding tissues (HH26–30 in the chicken foot) [19], [20], [21]. Here we will be concerned with late-phase gene expression in the mesenchyme surrounding digital rays during digit determination. At these late stages, the digital ray corresponds mostly to the future metacarpal (or metatarsal); only the distal most tip gives rise to phalanges, at the PFR (Phalanx Forming Region) [21]. The mesenchyme immediately posterior to the PFR is crucial to phalangeal number and morphology. Small heterotopic grafts of interdigital mesenchyme pinned into this region are sufficient to cause homeotic transformations of digits in the chicken foot [21]. In mouse and chicken limbs, late phase expression of HoxD-10, HoxD-11 and HoxD-12 is absent only in digital ray 1 and the interdigital mesenchyme between digit 1 and digit 2, but these are strongly expressed elsewhere. Forced expression of HoxD-12 in the entire mouse limb frequently leads to the transformation of digit 1 into a triphalangeal digit 2 [22]. In the chicken, forced expression of HoxD-11 in the entire hind limb often leads to the transformation of digit 1 into digit 2 [23]
Late phase-expression of HoxD-11 in the alligator forelimb is consistent with a similar role for HoxD-11 as in other amniotes. The transient expression in precursor cells of digit 1 in alligator forelimbs appears too early to participate in digit determination. Transient anterior expression is also apparent in early chicken and mouse forelimbs. In the chicken foot, digit 1 is the last digit to be determined, at stage 30 [21] (See Figure 4B). HoxD-11 is absent in digit 1 of alligator forelimbs at a comparable late stage (Figure 4A, stage 17). Our in situs also reveal some weak expression in the interdigital mesenchyme between digit 1 and digit 2 of alligator limbs (indicated by the arrow in stage 17 alligator forelimb and stage 16 of the hind limb, Figure 4). Very low expression levels of HoxD-11 in interdigit 1 may not be mechanistically relevant, specially in the case of HoxD genes, who show functional overlap and quantitative, additive effects [9], [10]. Importantly, there is no expression in the mesenchyme immediately posterior to the distal phalanx-forming region (PFR) of digit 1 in alligator, unlike strong expression for digit 2. Expression in the alligator suggests that, as in mouse and chicken limbs, digit 1 determination normally occurs in absence of HoxD-11 expression.
The asymmetric late HoxD-11 expression related to “thumbness” is conserved in alligator
Late phase expression of HoxD-11 is asymmetric in the pectoral fins of the basal bony fish Polyodon spathula, with no expression present in the anterior most region of developing fin radials [24]. The autopod (digit ray region) of amniotes shows similar late asymmetric expression related to “thumbness” [25], [26], with no anterior expression in digit 1. Hence an anterior autopodial domain of no HoxD-11 expression is an ancient marker of positional identity along the anterior-posterior axis of paired appendages. As expected, in alligator lack of expression of HoxD-11 was found only at the biphalangeal digit 1 of both the forelimb and hind limb. Adding crocodilians to the comparison of mouse and chicken allows the inference of ancestral and derived expression patterns. The ancestral condition for amniotes is observed in the mouse and the alligator: The anterior limit of late-phase HoxD-11 expression does not extend into the biphalangeal digit 1, and is separated by two digital positions from the primary axis. In the wing of birds, the development of a biphalangeal digit in absence of HoxD-11 expression is conserved, but the embryological position of HoxD-11 expression is derived, a full digital position closer to the primary axis (Figure 1C). The shifted morphology and HoxD-11 expression suggests both were effected by a mechanism upstream of HoxD-11 and other HoxD genes. Our analysis is consistent with that of other authors that have argued the primary axis in the bird wing exceptionally develops into digit 3, rather than digit 4 [6], [28], [29]. To further understand the role of HoxD-11 expression in the evolution of digits, we encourage the study of more taxa across a broad taxonomic sample, including other species where morphology also seems to have shifted embryological position, for instance the three-toed Italian skink, Chalcides chalcides [2], [29]
Materials and Methods
Cloning and sequencing of an exon 1 fragment from the alligator HoxD-11 gene
DNA extractions were performed on alligator embryo samples preserved in 95% ethanol using the DNAEasy Tissue kit (Qiagen inc) according to the manufacturer's protocol. Degenerate primers for HoxD-11 were designed targeting the conserved 5′ region of exon one and part of the homeo box.
1FD11: ATGAMCGASTTTGACGAKTGC
1RD11: CKTTTCTCTTTGTTTATGTABAC
PCR was performed with a range of annealing temperatures between 46 and 48°C. The resulting PCR product was about 900 bp long and cloned. Several clones were sequenced in order to evaluate PCR errors. To confirm its identity as HoxD-11 the sequence was analyzed using Neighbor Joining and Likelihood analysis as implemented in Phylip (http://evolution.genetics.washington.edu/phylip.html). To prepare an in situ probe, we cloned a fragment of exon 1 into a bacterial plasmid vector that was used to transcribe a labeled anti-sense mRNA probe.
Egg collection
The Alligator mississippiensis eggs were collected from the Rockefeller Wildlife Refuge in southwestern Louisiana in June of 2006. Under the direction of refuge biologist Ruth Elsey, J. V. collected recently deposited eggs. To reveal the eggs, the top layer of vegetation was removed and set aside. At the nest, all of the eggs were removed one by one and carefully marked with a black pencil to indicate the side of the egg that was oriented toward the top of the nest. This is necessary because if the eggs are turned over, the embryos contained inside run a high risk of drowning in the albumen or becoming detached from the top membrane of the egg, which shortly leads to death of the embryo. The eggs were then transported to Yale University, in wire mesh cages containing vegetation collected directly from the nest.
Incubation
The eggs were incubated in Plexiglas aquaria (30″×12″×12″) covered with machined Plexiglas lids with holes cut for ventilation. The incubators were filled with tap water to a depth of five inches. RenaCal™ Basic 100 Watt aquarium heaters were inserted under the water and set to a temperature of ∼31.5°C to regulate the internal temperature of the incubators as well as create a high humidity atmosphere within the incubator. To create a platform for the eggs, two 6″ high×12″ long drying racks that together covered most of the length of the incubators while leaving gaps for water to evaporate through were placed on the bottom of the aquaria. This setup left a space of 1″ between the top of the water and the top of the drying racks. A layer of nesting material that was brought back from the alligator nests was placed on top of the drying racks. The eggs were transferred from the cages and rested on top of this layer of vegetation. A second layer of nesting materials was then used to cover the eggs. The natural nesting material is ideal for insulation of the eggs and allowing them to stay moist, but not wet. The relative humidity level was regulated to approach 100%. The incubators were kept in a temperature and humidity controlled animal care room, in which the temperature was set at 31°C and the relative humidity at 60%. Within the incubators, the temperature was held constant at 31.5°C and the relative humidity was held at near 100%. Because of approximately 1–2 weeks of uncertainty regarding the date when eggs were laid, staging was done according to the embryological series and stages described in [30]
Chicken fertilized eggs were obtained from Charles Rivers Laboratories and incubated in polysterene egg incubators (Hova-bator) at 37°C with a water tray for humidity. Embryos were collected at 12-hour intervals, staged (according to [31]) and fixed in fixed overnight in 4% paraformaldehyde, rinsed in PBS, dehydrated in a sequence of methanol concentrations and preserved in methanol 100% at −20°C.
In situ hybridization
Alligator embryos were collected at 1-day intervals and fixed, dehydrated and stored as with chicken embryos above. Antisense probes for chicken and alligator HoxD-11 labeled with digoxigenin were prepared to visualize the transcripts of these genes in the developing limbs. In situ hybridization was carried out following standard procedures described in [32]. Chicken HoxD-11 plasmid was obtained from C. Tabin's laboratory. No special modifications of the standard protocol were required to successfully perform in situ hybridization on alligator embryos.
Acknowledgments
We thank Cliff Tabin for a clone of the chicken HoxD-11 gene and Ruth Elsey from the Rockefeller Wildlife Refuge in southwestern Louisiana for her help in alligator egg collection. Thanks to “Nucleo Decenio” for inspiration.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support came from NSF grant IOB 002488. Alexander Vargas is a fellow of PEW Latin American Program of fellowships in Biomedical sciences.
References
- 1.Müller GB, Alberch P. Ontogeny of the limb skeleton in Alligator mississippiensis: Developmental invariance and change in the evolution of archosaur limbs. Journal of Morphology. 1990;203:151–164. doi: 10.1002/jmor.1052030204. [DOI] [PubMed] [Google Scholar]
- 2.Burke AC, Feduccia A. Developmental patterns and the identification of homologies in the avian hand. Science. 1997;278:666–668. [Google Scholar]
- 3.Lee M, Reeder T, Slowinski J, Lawson R. Resolving reptile relationships. In: Cracraft J, Donoghue MJ, editors. Assembling the tree of life. Oxford university press; 2004. pp. 461–467. [Google Scholar]
- 4.Romer AS. The Vertebrate Body. 1st edition. WB Saunders company; 1950. [Google Scholar]
- 5.Gauthier JA. Saurischian monophyly and the origin of birds. Mem California Acad Sci. 1986;8:1–55. [Google Scholar]
- 6.Padian K, Chiappe L. The origin and early evolution of birds. Biological Reviews. 1998;73:1–42. [Google Scholar]
- 7.Shapiro MD, Shubin NH, Downs JP. Limb diversity and digit reduction in reptilian evolution. In: Hall BK, editor. Fins into limbs. The University of Chicago Press; 2007. pp. 225–244. [Google Scholar]
- 8.Wagner GP, Gauthier JA. 1,2,3 = 2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proc Natl Acad Sci U S A. 1999;96:5111–5116. doi: 10.1073/pnas.96.9.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis AP, Capecchi MR. A mutational analysis of the 5′ HoxD genes: dissection of genetic interactions during limb development in the mouse. Development. 1996;122:1175–1185. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- 10.Zákány J, Fromental-Ramain C, Warot X, Duboule D. Regulation of number and size of digits by posterior hox genes: A dose-dependent mechanism with potential evolutionary implications. Proc Natl Acad Sci USA. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, et al. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- 12.Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- 13.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Developmental Biology. 2001;236:421–35. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 14.Vargas AO, Fallon JF. Birds have dinosaur wings: The molecular evidence. J Exp Zool B Mol Dev Evol. 2005;304:86–90. doi: 10.1002/jez.b.21023. [DOI] [PubMed] [Google Scholar]
- 15.te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–30. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 16.Panman L, Galli A, Lagarde N, Michos O, Soete G, et al. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133:3419–28. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- 17.Reno PL, McCollum MA, Cohn MJ, Meindl RS, Hamrick M, et al. Patterns of correlation and covariation of anthropoid distal forelimb segments correspond to Hoxd expression territories. J Exp Zool B Mol Dev Evol. 2007;310:240–258. doi: 10.1002/jez.b.21207. [DOI] [PubMed] [Google Scholar]
- 18.Blanco MJ, Misof BY, Wagner GP. Heterochronic differences of HoxA-11 expression in Xenopus fore- and hind limb development: Evidence for a lower limb identity of the anuran ankle bones. Dev Gen & Evol. 1998;208:175–187. doi: 10.1007/s004270050172. [DOI] [PubMed] [Google Scholar]
- 19.Dahn RD, Fallon JF. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science. 2000;289:438–441. doi: 10.1126/science.289.5478.438. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Ogura T. Tbx Genes Specify Posterior Digit Identity through Shh and BMP Signaling. Dev Cell. 2004;6(1):43–53. doi: 10.1016/s1534-5807(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci USA. 2008;105:4185–4190. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knezevic V, De Santo R, Schughart K, Huffstadt U, Chiang C, et al. HoxD-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- 23.Morgan BA, Izpisúa-Belmonte JC, Duboule D, Tabin CJ. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature. 1992;358:236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- 24.Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–6. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- 25.Montavon T, Le Garrec JF, Kerszberg M, Duboule D. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 2008;22:346–59. doi: 10.1101/gad.1631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner GP, Vargas AO. On the nature of thumbs. Genome Biology. 2008;9:213–214. doi: 10.1186/gb-2008-9-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shubin NH. The phylogeny of development and the origin of homology. In: Grande L, Rieppel O, editors. Interpreting the hierarchy of nature. San Diego, CA: 1994. pp. 201–225. [Google Scholar]
- 28.Chatterjee S. Counting the fingers of birds and dinosaurs. Science. 1998;280:355. [Google Scholar]
- 29.Wagner GP. The developmental evolution of avian digit homology: an update. Theory Biosci. 2005;124:165–83. doi: 10.1007/BF02814482. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson MWJ. The reproductive biology and embryology of crocodilians. In: Gans C, Billett F, Maderson PFA, editors. Biology of the Reptilia. Vol. 14 (Development B) New York: John Wiley & Son; 1985. pp. 329–491. [Google Scholar]
- 31.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Developmental Dynamics. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 32.Nieto MA, Patel K, Wilkinson DG. In situ analysis of chick embryos in whole mount and tissue sections. In: Bronner-Fraser M, editor. Methods in Cell Biology Vol. 51. New York: Academic Press; 1996. pp. 219–235. [DOI] [PubMed] [Google Scholar]
- 33.Meyer A, Zardoya R. Recent advances in the (molecular) phylogeny of vertebrates. Annu Rev Ecol Evol Syst. 2003;34:311–38. [Google Scholar]
- 34.Donoghue MJ. Phylogenies and the analysis of evolutionary sequences, with examples from seed plants. Evolution. 1989;43:1137–1156. doi: 10.1111/j.1558-5646.1989.tb02565.x. [DOI] [PubMed] [Google Scholar]