Abstract
We previously reported that mitochondrial function, intracellular ATP levels, and complex I activity are decreased in renal proximal tubular cells (RPTC) after oxidant (tert-butyl hydroperoxide; TBHP)-induced injury. This study examined the hypothesis that succinate supplementation decreases mitochondrial dysfunction, ameliorates energy deficits, and increases viability in TBHP-injured RPTC. Basal and uncoupled respirations in injured RPTC decreased 33 and 35%, respectively, but remained unchanged in injured RPTC supplemented with 10 mM succinate (electron donor to respiratory complex II). State 3 respiration supported by electron donors to complex I decreased 40% in injured RPTC but improved significantly by succinate supplements. The activity of mitochondrial complex I in TBHP-injured RPTC decreased 48%, whereas complex II activity remained unchanged. Succinate supplementation prevented decreases in complex I activity. ATP levels decreased 43% in injured RPTC but were maintained in injured cells supplemented with succinate. Lipid peroxidation increased 19-fold in injured RPTC but only 9-fold in injured cells supplemented with succinate. Exposure of primary cultures of RPTC to TBHP produced 24% cell injury and lysis but no apoptosis. In contrast, no cell lysis was found in RPTC supplemented with succinate. We conclude that mitochondrial dysfunction and energy deficits in oxidant-injured RPTC are ameliorated by succinate, and we propose that succinate supplementation may prove therapeutically valuable. Succinate 1) uses an alternate pathway of mitochondrial energy metabolism, 2) improves activity of complex I and oxidation of substrates through complex I, and 3) decreases oxidative stress and cell lysis in oxidant-injured RPTC.
The major source of energy for metabolic processes and active transport in renal proximal tubular cells (RPTC) is oxidative metabolism, and ATP levels in RPTC are maintained almost entirely by oxidative phosphorylation. Therefore, decreases in ATP levels are the initiating factor in the development of both lethal and sublethal RPTC injury, and the degree of ATP decline determines the severity of injury (Bonventre and Weinberg, 2003). RPTC are the major target of ischemic and nephrotoxic injury within the kidney due to their dependence on oxidative phosphorylation and their limited capacity for generating ATP in glycolysis (Bagnasco et al., 1985; Bonventre and Weinberg, 2003). Ischemia and toxicants impair oxidative phosphorylation in RPTC both in vivo and in vitro and result in ATP depletion, leading to the loss of ion homeostasis, opening of the glycine-sensitive plasma membrane death channels/pores, and necrotic cell death (Miller et al., 1994; Dong et al., 1998; Chen et al., 2001; Bonventre and Weinberg, 2003).
Structural and functional alterations of mitochondria are important pathogenic factors underlying injury induced by ischemia, hypoxia, and toxicants in many cell types, including RPTC (Rouslin, 1983; Lash and Anders, 1987; Schnellmann et al., 1987, 1988; Aleo et al., 1991; Siegel et al., 1994; Gonzalez-Flecha and Boveris, 1995; Di Lisa and Bernardi, 1998; Nowak et al., 1998; Weinberg et al., 2000a; Jo et al., 2001). Some nephrotoxicants act as mitochondrial uncouplers and dissipate mitochondrial proton gradient, whereas the others inhibit respiratory chain, both resulting in decreases in the proton-motive force and ATP synthesis (Boveris and Chance, 1973; Turrens and Boveris, 1980; Schnellmann et al., 1987; Aleo et al., 1991; Kushnareva et al., 2002; Feldkamp et al., 2005). Furthermore, under some pathological conditions associated with the collapse of the proton-motive force, mitochondria not only cease to be the ATP producers but they also can become major ATP consumers due to the inverse operation of the F0F1-ATPase in the attempt to restore the proton gradient (Rouslin et al., 1997; Di Lisa and Bernardi, 1998). In many cases, ischemia and toxicants target respiratory complexes or dehydrogenases of the citric acid cycle, decreasing flow of electrons through the respiratory chain and the proton gradient across the inner mitochondrial membrane (Rouslin, 1983). Decreased activity of complex I, the major complex targeted by ischemia and nephrotoxicants, contributes to mitochondrial dysfunction and cell injury by reducing the electron transport rate and the proton-motive force and by producing superoxide and initiating oxidative stress (Rouslin, 1983; Gonzalez-Flecha and Boveris, 1995; Rouslin et al., 1997; Nowak et al., 2004). Mitochondrial functional deficits limit both structural and functional recovery of RPTC after toxicant-induced injury (Siegel et al., 1994; Nowak et al., 1999). Reducing ATP depletion or promoting ATP repletion diminishes the extent of injury or accelerates the process of proximal tubule repair (Nowak et al., 1999, 2000).
In addition to reducing ATP synthesis, inhibition of the respiratory chain results in generation of reactive oxygen species (ROS). Mitochondrial ROS are a major source of oxidative stress in the cell. The NADH dehydrogenase subunit of complex I is a critical site of mitochondrial superoxide production, which markedly increases during conditions associated with the dysfunction of complex I (Kushnareva et al., 2002). In particular, the iron-sulfur clusters, the flavin mononucleotide binding site, and the ubiquinone-binding sites of NADH dehydrogenase have been implicated in superoxide generation by complex I (Kushnareva et al., 2002; Lambert and Brand, 2004; Chen et al., 2005a). In a previous study, we have shown that RPTC develop energy deficits due to the inhibition of the respiratory chain after exposure to the oxidant tert-butyl hydroperoxide (TBHP) and that complex I is a major target of this oxidant within the respiratory chain (Nowak et al., 1998, 2004, 2006).
Previous studies documented that energy deficits caused by hypoxia in renal proximal tubules in vitro can be ameliorated by α-ketoglutarate and aspartate supplements (Weinberg et al., 2000a). Supplementation with succinate is protective in renal proximal tubules when provided during reoxygenation but not during hypoxia (Weinberg et al., 2000b). However, the exact mechanism of protection offered by succinate is not clear, and it is not known whether succinate ameliorates energy deficits induced by other insults, including toxicant or oxidant injury. We hypothesized that providing substrates, the oxidation of which bypasses impaired complex I, restores electron flow through the respiratory chain and ameliorates ATP deficits in oxidant-injured RPTC. Therefore, the goal of this study was to determine whether supplementation with succinate prevents mitochondrial dysfunction, restores ATP levels, and improves viability in oxidant-injured RPTC. Another aim of this study was to begin to determine the mechanism of succinate-mediated protection of mitochondrial function in oxidant-injured RPTC.
Materials and Methods
Isolation and Culture of Renal Proximal Tubular Cells
Female New Zealand White rabbits (2.0–2.5 kg) were purchased from Myrtle’s Rabbitry (Thompson Station, TN). The excision of kidneys was performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Renal proximal tubules were isolated by the iron oxide perfusion method and cultured in 35-mm culture dishes in improved conditions as described previously (Nowak and Schnellmann, 1996). The culture medium was a 50:50 mixture of Dulbecco’s modified Eagle’s essential medium and Ham’s F-12 nutrient mix without phenol red, pyruvate, and glucose, supplemented with 15 mM NaHCO3, 15 mM HEPES, and 6 mM lactate, pH 7.4 (295 mosmol/kg). Human transferrin (5 μg/ml), selenium (5 ng/ml), hydrocortisone (50 nM), bovine insulin (10 nM), and L-ascorbic acid-2-phosphate (50 μM) were added to the media immediately before daily media change (2 ml/dish).
TBHP Treatment of RPTC Monolayer
RPTC monolayers became 100% confluent 1 day before the oxidant treatment. The mono-layers were treated with tert-butyl hydroperoxide (C4H10O2; 350 μM) (TBHP) for 45 min. Our previous studies have shown that this concentration results in approximately 25 to 30% cell death and loss of the monolayer and a significant sublethal injury to the remaining cells (Nowak et al., 1998). However, the cell loss is not due to apoptosis at this concentration of TBHP (Nowak et al., 2006). TBHP exposure was terminated by aspirating medium containing TBHP, and cells were cultured for additional 2 days. Controls were treated with the diluent, 0.1% dimethyl sulfoxide. Potassium succinate (10 mM, final concentration; pH 7.4) was added to culture media 0.5 h before TBHP exposure and with every media change after TBHP exposure.
Assessment of RPTC Viability
Release of lactate dehydrogenase (LDH) into the culture medium was used as a marker of RPTC cell lysis. An aliquot of cell culture medium was collected from each dish and used to determine the activity of LDH released from the cells to the medium. Then, the cells were suspended in the remaining medium, sonicated, and spun down at 1000g for 2 min to pellet down the cellular debris. The supernatant resulting from this centrifugation (cell lysate) was used to assess total LDH activity. LDH activity was determined spectrophotometrically at 340 nm by measuring NADH (0.3 mM) oxidation in the presence of 1.8 mM pyruvate as a substrate as described by Moran and Schnellmann (1996). Annexin V/propidium iodide binding assay was used to determine apoptosis as we have described previously (Nowak et al., 2003). Propidium iodide and annexin V-fluorescein-5-isothiocyanate (FITC) fluorescence was quantified by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA) using excitation at 488 nm and emission at 590 and 530 nm for propidium iodide and annexin V-FITC, respectively. For each sample, 10,000 events were counted. Cells positive for annexin V and negative for propidium iodide were considered apoptotic.
Oxygen Consumption
Oxygen consumption (QO2) was measured polarographically using a Clark type electrode as described previously (Nowak and Schnellmann, 1996; Nowak et al., 2004). Basal and uncoupled QO2s were used as markers of overall mitochondrial function and electron transfer rate, respectively. Uncoupled QO2 was measured in the presence of carbonyl cyanide p-(trifluoro-methoxy)phenylhydrazone (2 μM).
State 3 Respiration
State 3 respiration (the maximal rate of mitochondrial respiration coupled to ATP synthesis) supported by electron donors to the respiratory complex I or complex II was measured in whole RPTC in 2 ml of warm (37°C) solution resembling an intracellular electrolyte milieu (120 mM KCl, 5 mM KH2PO4, 10 mM HEPES, 1 mM MgSO4, and 2 mM EGTA, pH 7.4) containing digitonin (0.1 mg/ml), 5 mM pyruvate + 5 mM malate (electron donors to complex I), or 10 mM succinate (electron donor to complex II) + 0.1 μM rotenone as described previously (Nowak et al., 2006). State 3 respiration was initiated by addition of 0.4 mM ADP. State 4 respiration was measured after addition of oligomycin (0.5 μg/ml) to mitochondria respiring at state 3. Respiratory control ratio (RCR) was calculated by dividing state 3 respiration by state 4 respiration.
Intracellular ATP Content
Intracellular ATP content in RPTC lysates was measured by the luciferase method using an ATP Bioluminescence Assay Kit HS II (Roche Diagnostic, Indianapolis, IN) and the manufacturer’s protocol as described previously (Borkan et al., 1993; Nowak et al., 2004, 2006).
Isolation of RPTC Mitochondria
RPTC were homogenized in the ice-cold isolation buffer containing 225 mM mannitol, 10 mM HEPES, pH 7.4, 75 mM sucrose, 0.1 mM EGTA, and 0.1% bovine serum albumin (BSA; fatty acid free) and centrifuged at 1000g for 5 min at 4°C. Supernatant was recentrifuged at 15,000g for 15 min at 4°C. The pellet was washed twice in the washing buffer (395 mM sucrose, 10 mM HEPES, 0.1 mM EGTA, pH 7.4) and spun down again at 15,000g for 15 min at 4°C. The final mitochondrial pellet was resuspended in the hypotonic assay buffer (25 mM potassium phosphate buffer containing 5 mM MgCl2, pH 7.2) for direct assessment of the activities of the respiratory complexes.
Activities of Respiratory Complexes
Activities of respiratory complexes I and II were measured in isolated mitochondria according to the method of Birch-Machin and Turnbull (2001). Immediately after isolation, mitochondria were suspended in the assay buffer and freeze-thawed in liquid nitrogen. Complex I (NADH/ubiquinone oxidoreductase) activity was assayed spectrophotometrically by following the oxidation of NADH (0.25 mM) at 340 nm at 30°C in the presence of 62.5 μM ubiquinone, 0.25% BSA, antimycin A (2 μg/ml), and mitochondria. The decrease in absorbance was recorded for 3 min, rotenone (10 μg/ml) was added, and the absorbance was recorded for another 2 min. Complex I activity was calculated as the rotenone-sensitive NADH/ubiquinone oxidoreductase activity. Complex II (succinate/ubiquinone oxidoreductase) activity was assayed spectrophotometrically at 590 nm by following the reduction of dichlorophenolindophenol (0.25 mM) at 30°C in the presence of succinate (20 mM), antimycin A (2 μg/ml), rotenone (10 μg/ml), 0.25% BSA, and ubiquinone (62.5 μM). The results were corrected for changes in the absorbance in blank samples (containing no mitochondria) in all assays.
Lipid Peroxidation
Malondialdehyde (MDA) concentration was used as a marker of lipid peroxidation and was measured fluoro-metrically according to the method of Casini at al. (1982). RPTC were scraped off culture dishes, suspended in the culture medium, precipitated using trichloroacetic acid (5% final concentration), and spun down. The supernatant was incubated with 2-thiobarbituric acid (0.3% final concentration) at 100°C for 15 min, and the product of the reaction was extracted using 1-butanol. The fluorescence of the extract was measured at 485 nm/535 nm (excitation/emission).
Protein Assay
Protein concentration was determined using the bicinchoninic acid assay with bovine serum albumin as the standard.
Statistical Analysis
Data are presented as the mean ± S.E. and were analyzed for significance by analysis of variance. Multiple means were compared using the Student-Newman-Keuls test with the level of significance of P < 0.05. Renal proximal tubular cells isolated from an individual rabbit represented one experiment (n = 1) consisting of data obtained from two to three culture plates.
Results
RPTC Viability
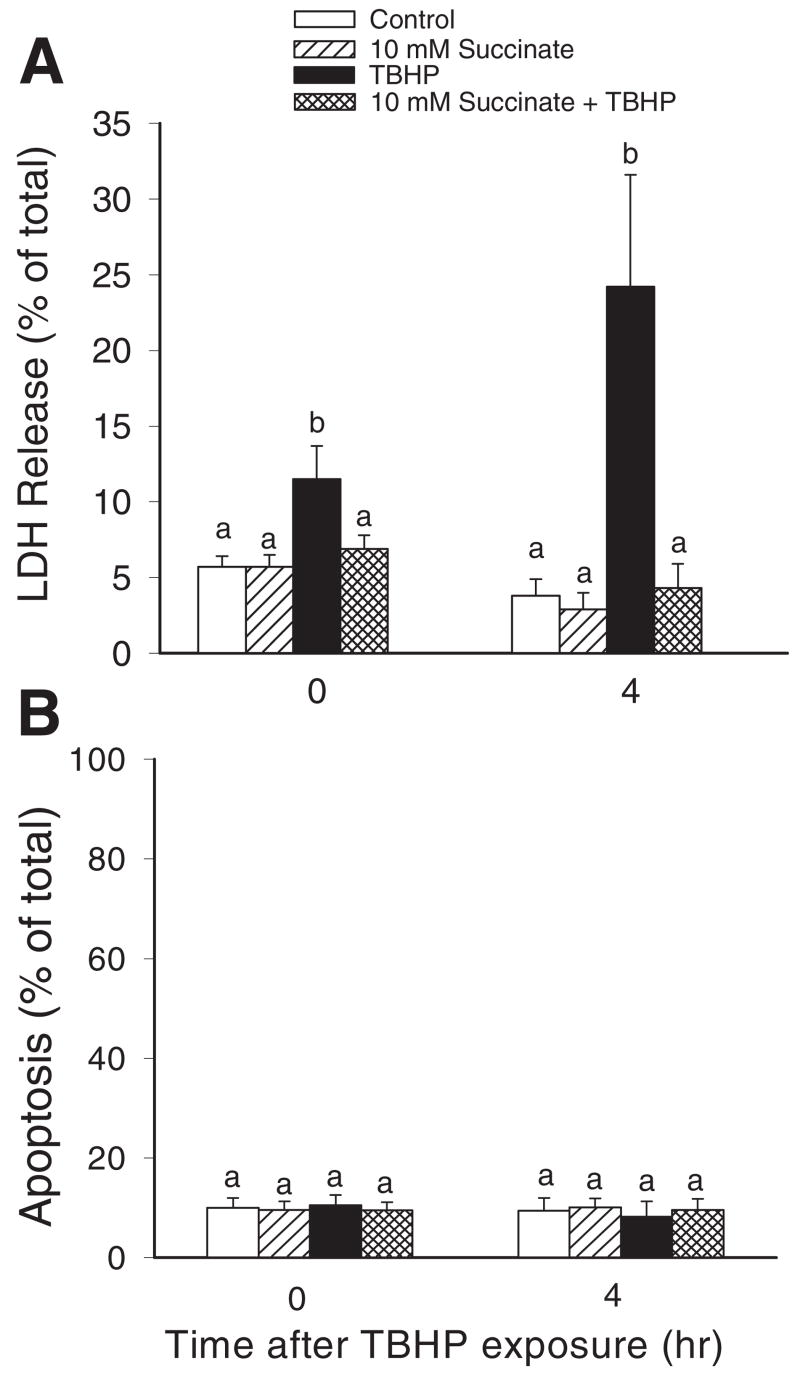
Treatment of RPTC with TBHP produced 12% cell lysis at the end of the exposure (45 min), which was a 2-fold increase in comparison with controls (Fig. 1A). At 4 h after termination of TBHP exposure, RPTC lysis increased to 24%, which constituted a 6-fold increase in comparison with controls at the same time point (Fig. 1A). TBHP-induced injury did not produce apoptosis (Fig. 1B). Supplementation of culture media with succinate prevented TBHP-induced RPTC lysis at both time points examined (Fig. 1A).
Fig. 1.
The effects of TBHP (350 μM)-induced injury and supplementation with succinate (10 mM) on RPTC viability. Succinate was present 0.5 h before, during, and after TBHP exposure. A, lactate dehydrogenase release from RPTC as a marker of necrotic cell death. B, the percentage of annexin V-FITC-positive/propidium iodide-negative (apoptotic) RPTC. Results are the average ± S.E. of three to four independent experiments (RPTC isolations). Values with dissimilar superscripts are significantly different (P < 0.05) from each other.
Oxygen Consumption
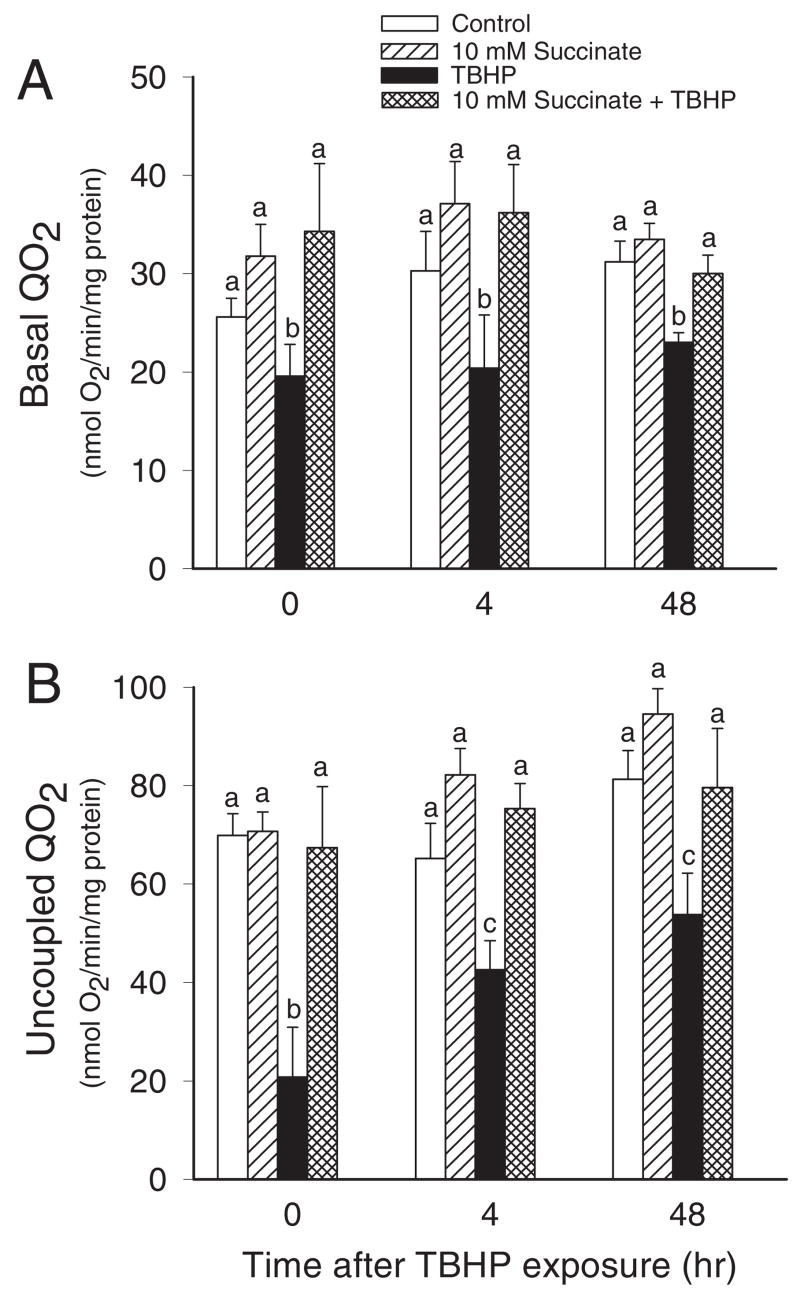
Basal QO2 was used as a marker of the overall respiration in RPTC. Basal QO2 decreased to 67% of control levels at 4 h and remained at this level at 48 h after TBHP exposure (Fig. 2A). Supplementation of the culture medium with 10 mM succinate did not change basal QO2 in noninjured cells but prevented decreases in basal respiration in TBHP-injured RPTC after injury. Beneficial effects of succinate on RPTC respiration were still present at 48 h after TBHP injury (Fig. 2A). TBHP-induced decreases in basal QO2 were also prevented when succinate was absent during TBHP exposure and added after the termination of the exposure (data not shown).
Fig. 2.
The effects of supplementation of RPTC with succinate (10 mM) on M)-induced basal (A) and uncoupled (B) QO2 in RPTC after TBHP (350 μ injury. Succinate was present 0.5 h before, during, and after TBHP exposure. Results are the average ± S.E.M. of four independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
Uncoupled QO2 was used as a marker of the mitochondrial electron transfer rate and the integrity of respiratory chain. Uncoupled QO2 was decreased to 30 and 65% of control levels at the end (0 h) and 4 h, respectively, after TBHP exposure in RPTC and did not recover at 48 h after injury (Fig. 2B). Supplementation of culture media with 10 mM succinate during and after TBHP exposure maintained uncoupled QO2 in injured RPTC at control levels (Fig. 2B). TBHP-induced decreases in uncoupled QO2 were also prevented when culture media were supplemented with 10 mM succinate after TBHP exposure only (data not shown).
These data show that supplementation of RPTC with succinate as an oxidative substrate prevents TBHP-induced decreases in RPTC respiration and maintains function of the respiratory chain.
State 3 Respiration
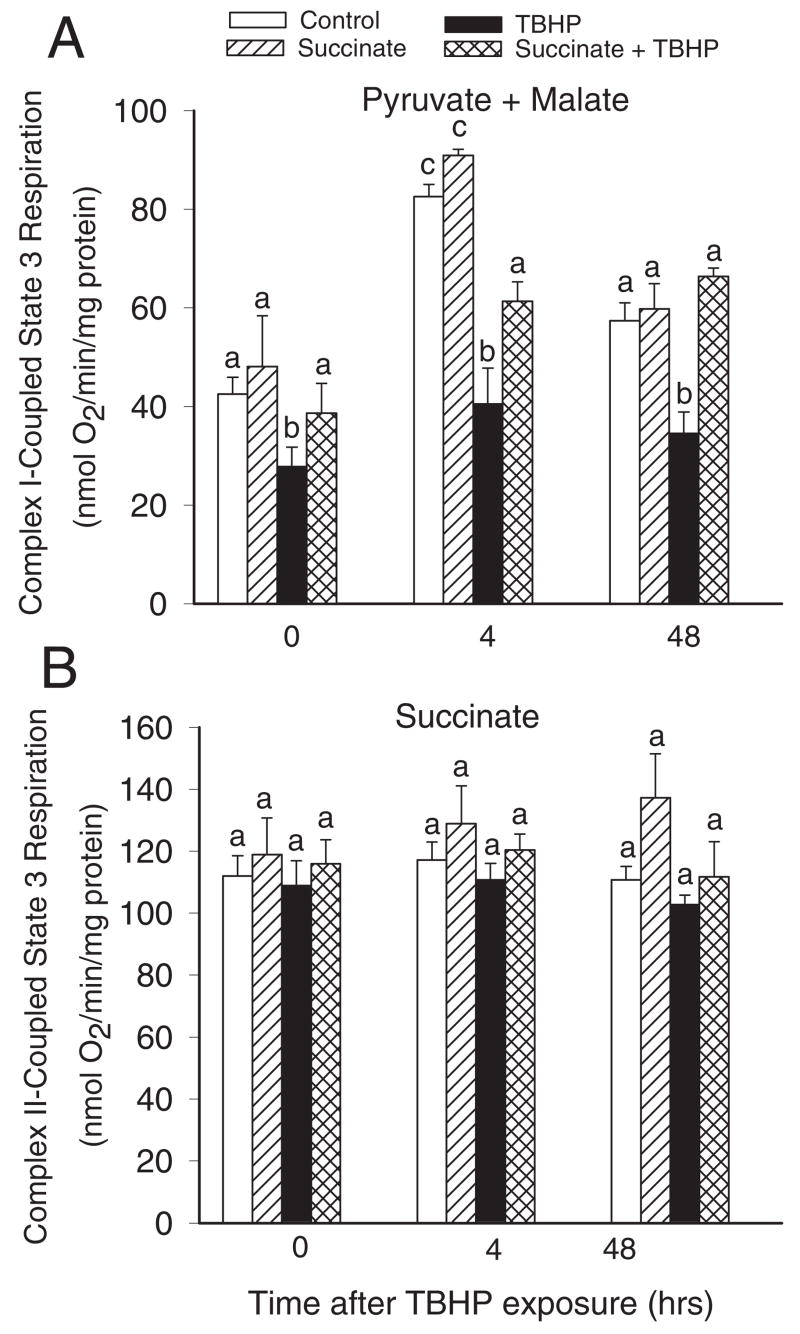
Because our data showed that TBHP targets the respiratory chain, we examined the effect of succinate supplementation on electron flow coupled to respiratory complexes I and II. Different electron donors were used to differentiate between substrate oxidation and electron flow coupled to complex I (pyruvate + malate) or complex II (succinate + rotenone). Complex I-coupled state 3 respiration using pyruvate and malate as electron donors decreased 35 and 43% at the end and at 4 h after TBHP exposure, respectively, and remained reduced at 48 h after the treatment (Fig. 3A). Supplementation of injured RPTC with 10 mM succinate during and after TBHP exposure significantly reduced decreases in state 3 respiration coupled to complex I (Fig. 3A). The protective effect of succinate was still present at 48 h after TBHP exposure (Fig. 3A). State 3 respiration using electron donors to complex II (succinate) was not affected by the exposure of RPTC to TBHP and the addition of succinate to culture media (Fig. 3B).
Fig. 3.
State 3 respiration in noninjured and TBHP-injured RPTC supplemented with succinate (10 mM). Succinate was present 0.5 h before, during, and after TBHP exposure. A, complex I-coupled state 3 respiration (state 3 respiration measured using electron donors to complex I of the respiratory chain: 5 mM pyruvate + 5 mM malate). B, complex II-coupled state 3 respiration (state 3 respiration measured using an electron donor to complex II: 10 mM succinate + 0.1 μM rotenone). Results are the average ± S.E.M. of four independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
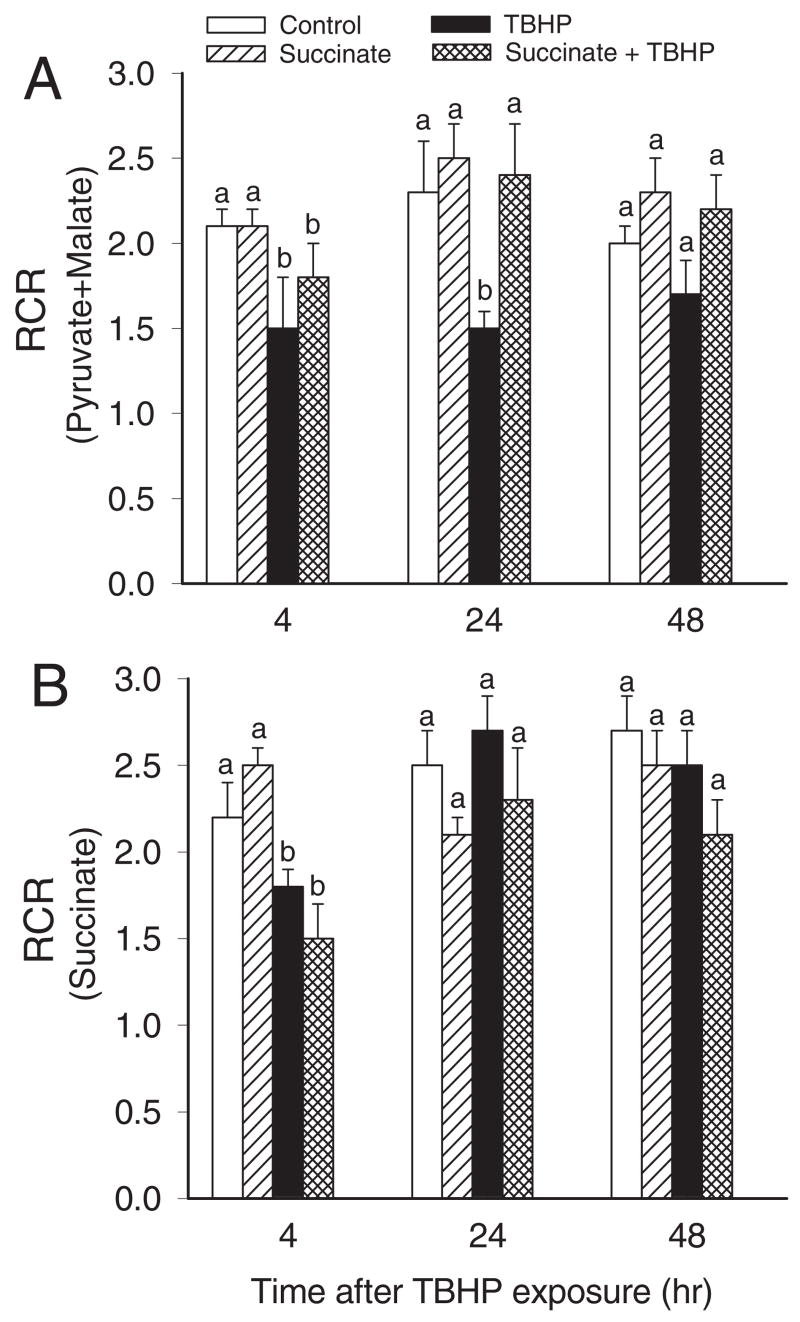
RCR in the presence of pyruvate and malate was 3.3 ± 0.2 and decreased to 1.5 ± 0.3 in TBHP-injured RPTC (Fig. 4A). The decrease in RCR was consistent with the decrease in state 3 respiration coupled to complex I and persisted at 48 h after TBHP exposure (Figs. 3A and 4A). TBHP did not produce any increases in state 4 respiration coupled to complex I at any time point examined (data not shown). Supplementation of RPTC cultures with succinate did not have any effect on RCR at the end of the exposure or at 4 h after TBHP exposure, but it promoted the recovery of RCR. At 24 h, RCR in injured RPTC supplemented with succinate was equal to RCR in controls (Fig. 4A). RCR coupled to substrate oxidation through complex II was decreased 4 h after TBHP injury regardless of the presence or absence of succinate in the media (Fig. 4B). This decrease was due to an increase in state 4 respiration in TBHP-treated RPTC, which suggests a decrease in mitochondrial coupling at complex II in oxidant-injured RPTC (Fig. 4B). RCR coupled to complex II recovered within 24 h after injury (Fig. 4B).
Fig. 4.
RCR in noninjured and TBHP-injured RPTC supplemented with succinate (10 mM). Succinate was present 0.5 h before, during, and after TBHP exposure. A, RCR measured using electron donors to complex I: 5 mM pyruvate + 5 mM malate. B, RCR measured using an electron donor to complex II: 10 mM succinate + 0.1 μM rotenone. Results are the average ± S.E.M. of three independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
These data demonstrate the following: 1) the decrease in RPTC respiration in oxidant-injured RPTC is due to a decrease in electron flow through complex I but not complex II; 2) providing succinate as an alternate oxidative substrate maintains the mitochondrial electron transport rate in TBHP-injured RPTC; 3) succinate supplementation accelerates the repair of complex I-coupled substrate oxidation and electron flow; 4) oxidant exposure uncouples electron flow through complex II but not complex I in RPTC mitochondria, and the coupling recovers at 24 h of the recovery period; and 5) supplementation of RPTC with succinate does not prevent uncoupling at complex II.
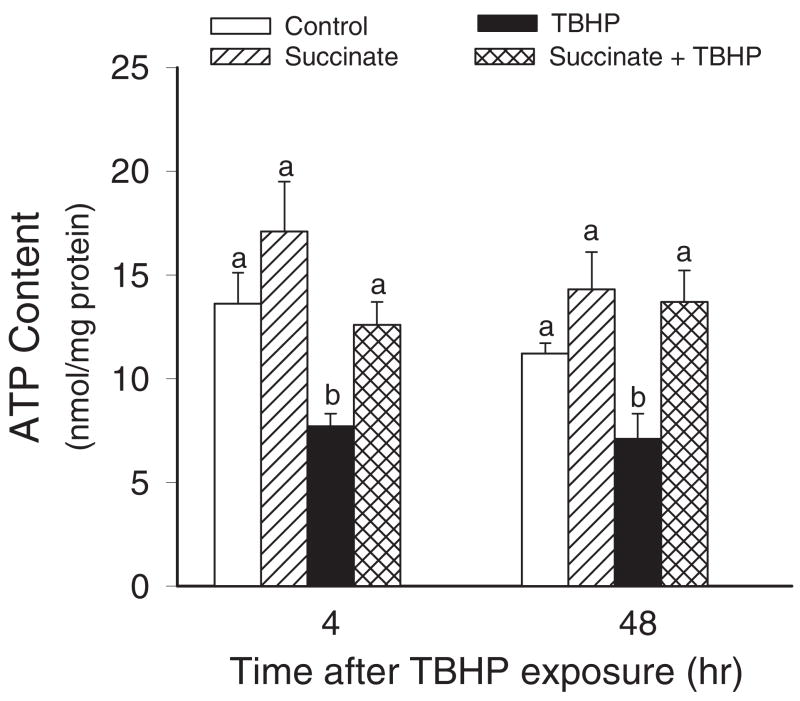
ATP Content
In the next series of experiments, we tested whether improved respiration in TBHP-injured RPTC supplemented with succinate results in improved energy status. Therefore, intracellular ATP content was measured in TBHP-injured RPTC cultured in the presence and absence of succinate during and after TBHP exposure. Figure 5 demonstrates that the injury was associated with a 50% decrease in ATP levels at 4 h after TBHP exposure and that ATP content did not recover at 48 h of the recovery period. The support of mitochondrial respiration by succinate was associated with improved intracellular ATP levels in TBHP-injured RPTC. Supplementation with succinate maintained ATP levels in injured RPTC at all of the time points examined in this study (Fig. 5). Thus, our data demonstrate that energy deficits caused by oxidant exposure can be alleviated by supplementing RPTC with succinate.
Fig. 5.
Intracellular ATP content in noninjured and TBHP-injured RPTC supplemented with succinate (10 mM). Succinate was present 0.5 h before, during, and after TBHP exposure. Results are the average ± S.E.M. of four independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
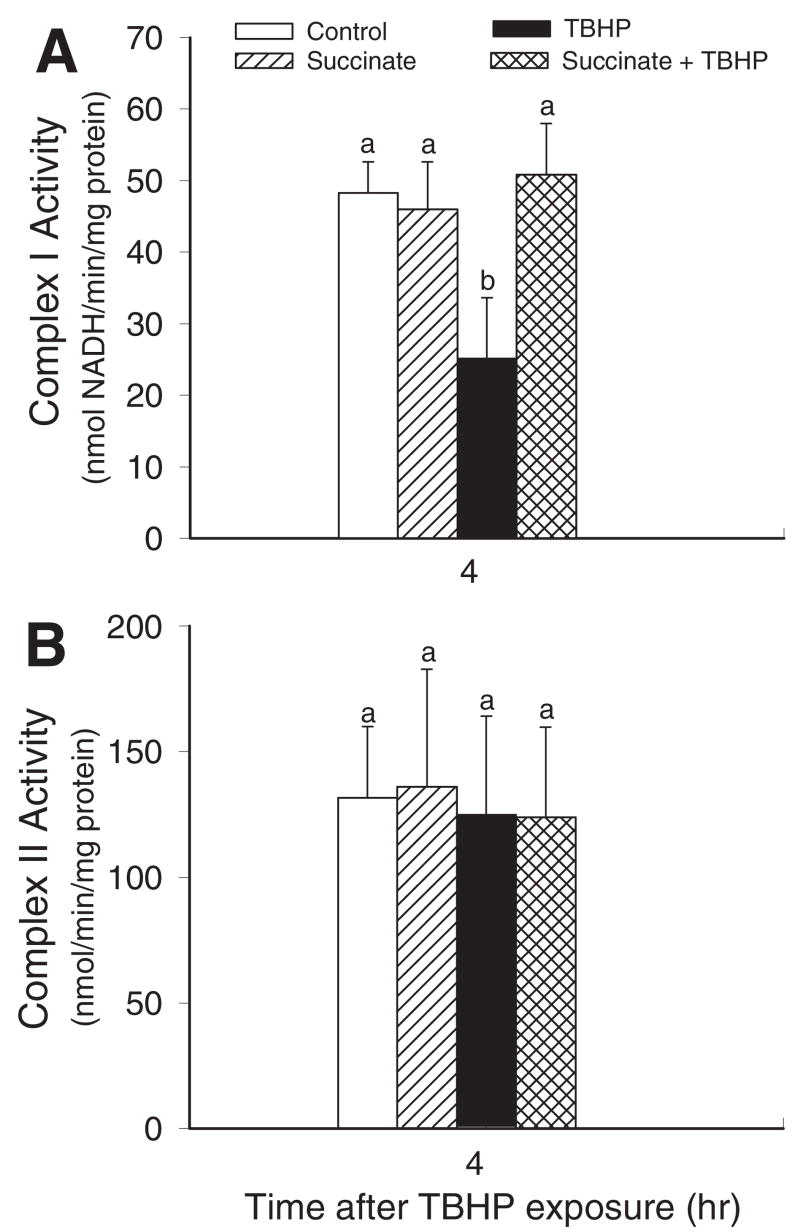
Respiratory Complexes
The activity of complex I (NADH/ubiquinone oxidoreductase) in mitochondria isolated from non-injured and TBHP-injured RPTC was examined to determine whether decreases in complex I-coupled respiration are due to disrupted function of complex I. Figure 6A shows that the activity of complex I decreased 48% at 4 h after TBHP exposure. In contrast, the activity of complex II was not changed by oxidant injury (Fig. 6B). Supplementation of TBHP-injured RPTC with succinate resulted in maintaining complex I activity in isolated mitochondria and had no effect on complex II activity (Fig. 6). These data demonstrate that succinate supplementation protects against dysfunction of complex I in oxidant-injured RPTC.
Fig. 6.
The effects of supplementation of RPTC with succinate (10 mM) on complex I (NADH/ubiquinone oxidoreductase) activity (A) and complex II (succinate/ubiquinone oxidoreductase) (B) in mitochondria isolated from RPTC at 4 h after TBHP (350 μM) exposure. Succinate was present 0.5 h before, during, and after TBHP exposure. Results are the average ± S.E.M. of six independent experiments (RPTC isolations). Values with dissimilar superscripts are significantly different (P < 0.05) from each other.
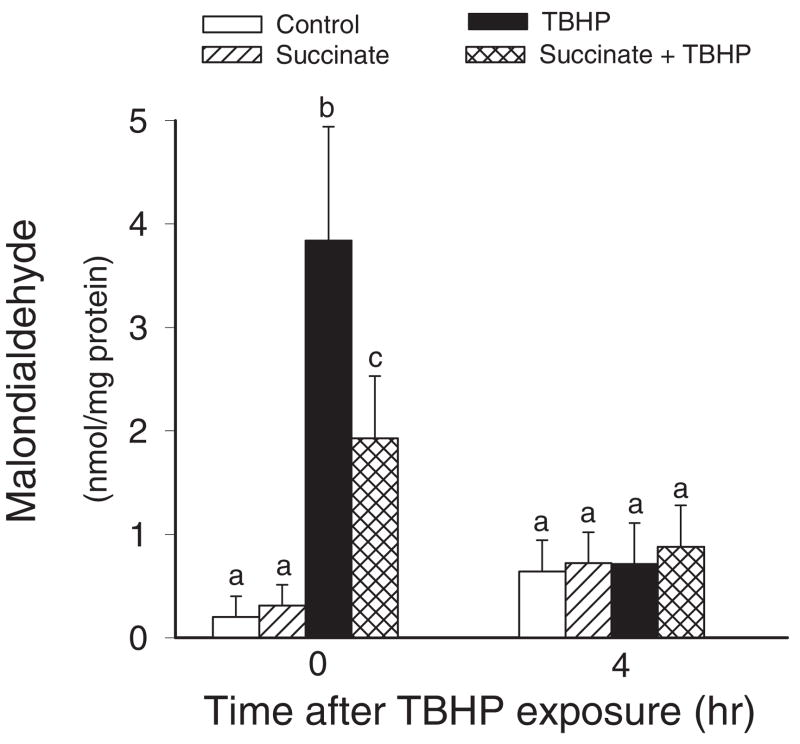
Oxidative Stress
Experiments were also carried out to test whether supplementation of RPTC with succinate has an effect on lipid peroxidation and oxidative stress induced by TBHP in RPTC. Exposure of RPTC to TBHP resulted in a 19-fold increase in MDA levels (Fig. 7). Supplementation of RPTC with succinate decreased generation of MDA in TBHP-injured RPTC by 50% (Fig. 7). These results demonstrate that succinate decreases oxidant-induced lipid peroxidation in RPTC and suggest that supplementation of RPTC with succinate decreases oxidative stress induced by TBHP. MDA generation was transient, and at 4 h of the recovery period, lipid peroxidation in injured RPTC was not significantly different from controls.
Fig. 7.
The effects of supplementation of RPTC with succinate (10 mM) on malondialdehyde content in RPTC after TBHP (350 μM)-induced injury. Succinate was present 0.5 h before, during, and after TBHP exposure. Results are the average ± S.E.M. of six independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
Discussion
Alterations in mitochondrial structure and function are important pathogenic factors that determine the extent of ischemic and toxic injury in renal cortical cells. Mitochondrial dysfunction results in decreases in oxidative phosphorylation and ATP levels, and the resulting energy deficits are especially pronounced in cell types that require high-energy input and generate the majority of their ATP in oxidative phosphorylation. Because oxidative stress is a common mechanism of ischemia/reperfusion and nephrotoxicant-induced injury in renal proximal tubules, we used oxidant injury as an experimental model to test whether supplementation with succinate alleviates mitochondrial dysfunction, energy deficits, and cellular injury in RPTC. Our previous studies have shown that oxidant (TBHP) injury results in decreases in the function and activity of complex I in injured RPTC. In contrast, substrate oxidation coupled to complexes II and IV is not affected by oxidant injury (Nowak et al., 2004, 2006). Therefore, we hypothesized that energy deficits in oxidant-injured RPTC could be reduced by providing a substrate that bypasses complex I and is oxidized by remaining complexes. Supplementation of RPTC with succinate during and after oxidant exposure maintained overall cellular respiration and mitochondrial electron transport rate. As a result, succinate supplementation maintained intracellular ATP levels and prevented cell lysis in oxidant-injured RPTC.
At first, we assumed that the mechanism of protective actions of succinate was through serving as an alternative oxidative substrate that bypasses the injury of complex I and maintains oxidative phosphorylation and intracellular ATP levels. It has been shown previously that energy deficits caused by hypoxia/reoxygenation injury in renal proximal tubules can be ameliorated by other metabolites of the citric acid cycle such as α-ketoglutarate and aspartate (Weinberg et al., 2000a, 2000b). It was postulated that the mechanism of this protection is through anaerobic metabolism of α-ketoglutarate and aspartate to succinate and ATP generation through substrate level phosphorylation (Weinberg et al., 2000a). Although it is likely that, as long as NAD+ is available, ATP levels can be increased by anaerobic metabolism of α-ketoglutarate to succinate at the step of succinyl-CoA conversion to succinate, this reaction provides only one molecule of high-energy compound (GTP) for each molecule of α-ketoglutarate metabolized. All substrates with protective properties were metabolized to succinate, whereas the substrates that did not protect were not metabolized to succinate (Weinberg et al., 2000b). Succinate, the product of the proposed anaerobic metabolism of α-ketoglutarate, was not protective during hypoxia but protected during reoxygenation after hypoxia, suggesting that oxygen availability is a crucial factor in the mechanism of succinate-mediated protection against hypoxia/reoxygenation-induced injury (Weinberg et al., 2000b).
In our experiments, beneficial effects of succinate were not limited to bypassing complex I and supporting oxidative phosphorylation through maintaining the respiratory chain active (complexes II, III, and IV). Our data show that state 3 respiration using electron donors to complex I was significantly improved by succinate supplementation in injured RPTC. These results were unexpected because succinate is not oxidized via complex I and donates electrons to the respiratory chain through FADH2 at the level of complex II, thus bypassing complex I. In a previous study, we reported that state 3 respiration coupled to electron donors to complex I requires more than 4 days to recover in TBHP-injured RPTC (Nowak et al., 2006). Our present data show that complex I-coupled state 3 respiration in injured RPTC supplemented with succinate is significantly improved at 4 h and is equal to state 3 respiration in controls at 24 h after TBHP-induced injury. The mechanism of protective effects of succinate was not through increased coupling of injured mitochondria. Succinate did not improve coupling in mitochondria respiring in the presence of electron donors to complex I. In contrast, RCR and state 4 respiration data suggest that succinate produced a mild and transient uncoupling of mitochondria respiring in the presence of electron donor to complex II. The most unexpected results showed that succinate improved the activity of complex I. The measurements of complex I activity in isolated mitochondria demonstrated that supplementation of RPTC with succinate during and after injury protected against oxidant-induced dysfunction of complex I. Because succinate was absent in the media used to isolate mitochondria and measure complex I activity, succinate must have exerted its actions during TBHP exposure and/or early recovery period (4 h) after injury and before mitochondrial isolation. However, the effect of succinate was still present after mitochondrial isolation.
Because TBHP is a strong oxidant, we tested whether succinate exerted its protective effects through decreasing oxidative stress. Succinate supplementation reduced lipid peroxidation in RPTC at the end of TBHP exposure, suggesting that succinate decreased oxidative stress in injured RPTC. In a previous study, succinate has been shown to decrease lipid peroxidation in γ-irradiated animals and increase survival when administered in vivo before irradiation (Ronai et al., 1987). Lipid peroxidation in mitochondria, specifically in the inner mitochondrial membrane, can also be prevented by succinate, but the exact mechanism of this effect is not clear (Szabados et al., 1987). Inhibition of lipid peroxidation by succinate prevents modification of mitochondrial proteins, inactivation of several mitochondrial enzymes, including succinate dehydrogenase (SDH) and isocitrate dehydrogenase, and loss of the inner mitochondrial membrane integrity (Tretter et al., 1987). In previous studies, it has been shown in the brain that antioxidant properties of several intermediates of Krebs cycle, including succinate, are abolished by cyanide and heat (Puntel et al., 2005). This suggests that the protective effect of succinate is dependent on an enzyme activity and active respiratory chain. The effects of succinate have been attributed to maintaining SDH activity (complex II) (Ronai et al., 1987). However, the protective effects of succinate in our model were not through maintaining SDH activity because SDH was not affected by TBHP. Our study shows a novel observation that succinate maintains the activity of complex I, which, in addition to its role in NADH oxidation, is a major mitochondrial site of superoxide generation and subsequent oxidative stress. A significant increase in ROS formation requires approximately 70% inhibition of complex III activity but only 20% inactivation of complex I (Sipos et al., 2003). The activity of complex I decreased 48% in our model of RPTC injury. This decrease was blocked by succinate and was associated with a 50% reduction in lipid peroxidation, suggesting that succinate plays a major role in decreasing ROS generation in TBHP-injured RPTC. It has been postulated that the two major sources of superoxide and ROS in renal proximal tubules are NADPH oxidase and mitochondrial electron transport chain, specifically complexes I and III (Geiszt et al., 2000; Chen et al., 2005b). Because succinate decreases mitochondrial injury in RPTC, our data support a hypothesis that the reduction in lipid peroxidation observed in our study was due to a decrease in the mitochondrial pathway of ROS generation rather than the NADPH oxidase pathway.
In conclusion, this study demonstrates that mitochondrial dysfunction and energy deficits in oxidant-injured RPTC are ameliorated by succinate supplements. Succinate 1) uses alternate pathways of mitochondrial energy metabolism, 2) maintains activity of complex I and oxidation of substrates through complex I, 3) decreases lipid peroxidation induced by an oxidant, and 4) protects against cell lysis in injured RPTC. Because oxidative stress and mitochondrial dysfunction are common mechanisms of ischemic and nephrotoxic acute kidney injury, supplementation with succinate may advance therapies to treat renal injury by improving metabolic functions and survival of renal proximal tubules.
Acknowledgments
We thank Malinda Godwin for excellent technical assistance.
This work was supported by the NIDDK, National Institutes of Health Grant R01DK59558.
ABBREVIATIONS
- RPTC
renal proximal tubular cells
- ROS
reactive oxygen species
- TBHP
tert-butyl hydroperoxide
- LDH
lactate dehydrogenase
- FITC
fluorescein-5-isothiocyanate
- QO2
oxygen consumption
- RCR
respiratory control ratio
- BSA
bovine serum albumin
- MDA
malondialdehyde
- SDH
succinate dehydrogenase
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
References
- Aleo MD, Wyatt RD, Schnellmann RG. Mitochondrial dysfunction is an early event in ochratoxin A but not oosporein toxicity to rat renal proximal tubules. Toxicol Appl Pharmacol. 1991;107:73–80. doi: 10.1016/0041-008x(91)90332-9. [DOI] [PubMed] [Google Scholar]
- Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Physiol. 1985;248:F522–F526. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- Borkan SC, Emami A, Schwartz JH. Heat stress protein-associated cytoprotection of inner medullary collecting duct cells from rat kidney. Am J Physiol Renal Physiol. 1993;265:F333–F341. doi: 10.1152/ajprenal.1993.265.3.F333. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini A, Giorli M, Hyland RJ, Serroni A, Gilfor D, Farber JL. Mechanisms of cell injury in the killing of cultured hepatocytes by bromobenzene. J Biol Chem. 1982;257:6721–6728. [PubMed] [Google Scholar]
- Chen J, Liu X, Mandel LJ, Schnellmann RG. Progressive disruption of the plasma membrane during renal proximal tubule cellular injury. Toxicol Appl Pharmacol. 2001;171:1–11. doi: 10.1006/taap.2000.9105. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gill PS, Welch WJ. Oxygen availability limits renal NADPH-dependent superoxide production. Am J Physiol Renal Physiol. 2005a;289:F749–F753. doi: 10.1152/ajprenal.00115.2005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen C, Zhang L, Green-Church KB, Zweier JL. Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. J Biol Chem. 2005b;280:37339–37348. doi: 10.1074/jbc.M503936200. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem. 1998;184:379–391. [PubMed] [Google Scholar]
- Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA. Development of porous defects in plasma membranes of adenosine triphosphate-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Lab Invest. 1998;78:657–668. [PubMed] [Google Scholar]
- Feldkamp T, Kribben A, Weinberg JM. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2005;288:F1092–F1102. doi: 10.1152/ajprenal.00443.2004. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flecha B, Boveris A. Mitochondrial sites of hydrogen peroxide production in reperfused rat kidney cortex. Biochim Biophys Acta. 1995;1243:361–366. doi: 10.1016/0304-4165(94)00160-y. [DOI] [PubMed] [Google Scholar]
- Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- Lash LH, Anders MW. Mechanism of S-(1,2-dichlorovinyl)-L-cysteine- and S-(1,2-dichlorovinyl)-L-homocysteine-induced renal mitochondrial toxicity. Mol Pharmacol. 1987;32:549–556. [PubMed] [Google Scholar]
- Miller GW, Lock EA, Schnellmann RG. Strychnine and glycine protect renal proximal tubules from various nephrotoxicants and act in the late phase of necrotic cell injury. Toxicol Appl Pharmacol. 1994;125:192–197. doi: 10.1006/taap.1994.1064. [DOI] [PubMed] [Google Scholar]
- Moran JH, Schnellmann RG. A rapid β-NADH-linked fluorescence assay for lactate dehydrogenase in cellular death. J Pharmacol Toxicol Methods. 1996;36:41–44. doi: 10.1016/1056-8719(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Nowak G, Aleo MD, Morgan JA, Schnellmann RG. Recovery of cellular functions following oxidant injury. Am J Physiol Renal Physiol. 1998;274:F509–F515. doi: 10.1152/ajprenal.1998.274.3.F509. [DOI] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Clifton GL. Protein kinase C-ε modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol. 2004;286:F307–F316. doi: 10.1152/ajprenal.00275.2003. [DOI] [PubMed] [Google Scholar]
- Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–F855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Price PM, Schnellmann RG. Lack of a functional p21WAF1/CIP1 gene accelerates caspase-independent apoptosis induced by cisplatin in renal cells. Am J Physiol Renal Physiol. 2003;285:F440–F450. doi: 10.1152/ajprenal.00233.2002. [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. L-Ascorbic acid regulates growth and metabolism of renal cells: Improvements in cell culture. Am J Physiol Cell Physiol. 1996;271:C2072–C2080. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- Puntel RL, Nogueira CW, Rocha JB. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30:225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- Ronai E, Tretter L, Szabados G, Horvath I. The inhibitory effect of succinate on radiation-enhanced mitochondrial lipid peroxidation. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51:611–617. doi: 10.1080/09553008414552141. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol Heart Circ Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- Rouslin W, Long I, Richard B, Broge CW. Why are ATP depletion rates in situ in ischemic myocardium so much lower than one might predict from the activity of the mitochondrial ATPase in sonicated heart mitochondria? J Mol Cell Cardiol. 1997;29:1505–1510. doi: 10.1006/jmcc.1997.0417. [DOI] [PubMed] [Google Scholar]
- Schnellmann RG. Mechanisms of t-butyl hydroperoxide-induced toxicity to rabbit renal proximal tubules. Am J Physiol Cell Physiol. 1988;255:C28–C33. doi: 10.1152/ajpcell.1988.255.1.C28. [DOI] [PubMed] [Google Scholar]
- Schnellmann RG, Ewell FP, Sgambati M, Mandel LJ. Mitochondrial toxicity of 2-bromohydroquinone in rabbit renal proximal tubules. Toxicol Appl Pharmacol. 1987;90:420–426. doi: 10.1016/0041-008x(87)90134-7. [DOI] [PubMed] [Google Scholar]
- Siegel NJ, Devarajan P, Van Why S. Renal cell injury: Metabolic and structural alterations. Pediatr Res. 1994;36:129–136. doi: 10.1203/00006450-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Sipos I, Tretter L, Adam-Vizi V. The production of reactive oxygen species in intact isolated nerve terminals is independent of the mitochondrial membrane potential. Neurochem Res. 2003;28:1575–1581. doi: 10.1023/a:1025634728227. [DOI] [PubMed] [Google Scholar]
- Szabados G, Ando A, Tretter L, Horvath I. Effect of succinate on mitochondrial lipid peroxidation. 1. Comparative studies on ferrous ion and ADP: Fe/NADPH-induced peroxidation. J Bioenerg Biomembr. 1987;19:21–30. doi: 10.1007/BF00769729. [DOI] [PubMed] [Google Scholar]
- Tretter L, Szabados G, Ando A, Horvath I. Effect of succinate on mitochondrial lipid peroxidation. 2. The protective effect of succinate against functional and structural changes induced by lipid peroxidation. J Bioenerg Biomembr. 1987;19:31–44. doi: 10.1007/BF00769730. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000a;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000b;279:F927–F943. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]