Abstract
During Toxoplasma gondii infection, macrophages, dendritic cells, and neutrophils are important sources of pro-inflammatory cytokines from the host. To counteract the pro-inflammatory activities, T. gondii is known to have several mechanisms inducing down-regulation of the host immunity. In the present study, we analyzed the production of pro- and anti-inflammatory cytokines from a human myelomonocytic cell line, THP-1 cells, in response to treatment with T. gondii lysate or lipopolysaccharide (LPS). Treatment of THP-1 cells with LPS induced production of IL-12, TNF-α, IL-8, and IL-10. Co-treatment of THP-1 cells with T. gondii lysate inhibited the LPS-induced IL-12, IL-8 and TNF-α expression, but increased the level of IL-10 synergistically. IL-12 and IL-10 production was down-regulated by anti-human toll-like receptor (TLR)-2 and TLR4 antibodies. T. gondii lysate triggered nuclear factor (NF)-κB-dependent IL-8 expression in HEK293 cells transfected with TLR2. It is suggested that immunosuppression induced by T. gondii lysate treatment might occur via TLR2-mediated NF-κB activation.
Keywords: Toxoplasma gondii, LPS, macrophage, cytokine, toll-like receptor, NF-κB
INTRODUCTION
Toxoplasma gondii is an intracellular protozoan parasite and infection of this parasite is widely distributed in the world. In T. gondii infection, activated macrophages play an important role in the host defense and secrete molecules that regulate the inflammatory responses [1]. Macrophages activated by various pathogens activate innate as well as initial immune responses. When stimulated with T. gondii tachyzoite extracts or peptides, macrophages, neutrophils, and dendritic cells can produce interleukin-12 (IL-12) [2]. Lipopolysaccharide (LPS) is a potent activator of macrophages and stimulates secretion of various cytokines. LPSs from gram-negative bacteria induce an intense inflammatory response as well as massive production of pro-inflammatory cytokines including IL-12 and tumor necrosis factor-α (TNF-α).
On the other hand, T. gondii has been shown to be a suppressor of LPS-induced signaling [3-5]. During Toxoplasma infection, activated macrophages are a major source of TNF-α secretion [6]. TNF-α plays a significant role in the host resistance and acts synergistically with IFN-γ. The function of TNF-α is known to activate macrophages and mononuclear leukocytes in the infection sites and stimulate the microbicidal activities of these cells. IL-8 produced by THP-1 cells, a human macrophage cell line, treated with T. gondii lysate, has a role during the activation and migration of neutrophils [7]. Macrophages are also an important source of IL-10, which is a cytokine that acts to down-regulate IL-12 synthesis [3]. IL-10 is an inhibitor of activated macrophages and controls the innate immune responses. T. gondii induces high levels of pro-inflammatory cytokines, IL-12 and TNF-α, and also triggers anti-inflammatory cytokines, like transforming growth factor-β (TGF-β) and IL-10 [8,9]. Following a parasite infection, the balance between induction and suppression of the immune responses through pro-inflammatory and anti-inflammatory cytokines is important for the survival of the host [10].
Toll-like receptors (TLRs) are important transmembrane molecules that function during the recognition and signaling in the immune system. TLRs are capable of recognizing a wide range of organisms including bacteria, virus, fungi, and protozoa. Engagement of TLRs leads to the production of a variety of pro-inflammatory and immunoregulatory cytokines, chemokines, and costimulatory molecules [11]. TLRs play a major role in LPS signaling in macrophages. However, little is known about how TLRs mediate the innate immunity responses to T. gondii. Pro-inflammatory cytokine synthesis is initiated with TLR signaling followed by activation of nuclear factor-kappa B (NF-κB). Activation of cells, such as macrophages, results in activation of NF-κB and production of cytokines as well as other inflammatory molecules. In the present study, we tested the secretion or expression of IL-12, TNF-α, IL-8, and IL-10 in THP-1 cells treated with T. gondii lysate and/or LPS, using ELISA or reverse transcriptase (RT)-PCR. TLRs were examined whether involved in NF-κB activation and cytokine production when induced with T. gondii lysate.
MATERIALS AND METHODS
Lysate of T. gondii tachyzoites
Tachyzoites of T. gondii (RH) were maintained via intraperitoneal passages in ICR mice (Daehan Biolink Co., Eumseoung, Korea), and tachyzoites were harvested from infected mice after 3-4 days. Mice were peritoneally injected with RPMI 1640 (Gibco, Carlsbad, California, USA), and peritoneal fluid was centrifuged with low and high speeds for removal of peritoneal cells. The tachyzoites were washed with phosphate-buffered saline (PBS) and kept at -70℃ until use. To prepare the T. gondii lysate, tachyzoites were frozen and thawed 3 times and ultrasonicated at 100% amplitude, 0.75 cycles, 17-20 times, in the presence of 1 ml of protease inhibitor cocktails (Boehringer Ingelheim, Darmstadt, Germany). The total homogenation of tachyzoites was checked for T. gondii lysate under a light microscope. The sonicated material was dialyzed against PBS and centrifuged at 20,000 g for 1 hr, and then the supernatant was collected and filtered through a membrane with pore size 0.2 µm (Millipore, Billerica, Massachusetts, USA). The protein concentration of the T. gondii lysate was determined using the Bradford assay (Bio-Rad, Hercules, California, USA).
Culture of cells
A human myelomonocytic cell line, THP-1 (ATCC, American Type Cellular Collection, Manassas, Virginia, USA), was maintained in RPMI 1640 culture medium supplemented with 10% fetal bovine serum (FBS) (Gibco), penicillin (100 IU/ml), and streptomycin (50 µg/ml). For cell differentiation, THP-1 cells were treated with 10 nM phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, Missouri, USA) and incubated at 37℃ in 5% CO2 for 48 hr. THP-1 cells were cultured in 96-well (2 × 105 cells/well) or 12-well (1 × 106 cells/well) culture plates (Becton Dikinson, Meylan Cedex, France) for ELISA or RT-PCR, respectively. THP-1 mononuclear cells were stimulated by 10 µg/ml of LPS (Escherichia coli strain, 026; B6, Sigma-Aldrich) or 50 µg/ml T. gondii lysate for 24 hr and 48 hr. For transient transfection, HEK293 cells (human embryonic kidney 293 cells) (ATCC) were cultured in a 250 ml culture flask with RPMI 1640 and 10% FBS at 37℃ in 5% CO2 and subcultured every 2-3 days. The cells were detached with 0.05% trypsin-EDTA (WelGene Co., Daegu, Korea) and 1 × 106 HEK293 cells were cultured in 12-well plates using Dulbecco's modified Eagle's medium (DMEM) with 10% FBS for 18-24 hr.
RT-PCR
In a 12-well culture plate, THP-1 cells (1 × 106/well) were stimulated with 10 µg/ml of LPS and 50 µg/ml of T. gondii lysate for 1, 18, or 24 hr, and the mRNA expression of TNF-α was measured by RT-PCR. The total RNA was extracted from cells using 1 ml of TRIzol Reagent (Invitrogen, Carlsbad, California, USA), and 200 µl of chloroform (Sigma-Aldrich). The mixture was incubated at room temperature for 3 min and centrifuged at 12,000 g for 15 min. The cDNA was synthesized from 2 µg of the total RNA using Superscript III (Invitrogen). PCR was performed with the cDNA template, 1 µl of PCR premix (Bioneer Co., Daejeon, Korea), 1 µl of bovine serum albumin (BSA) (2 mg/ml) (Sigma-Aldrich), and 20 ρmole/µl of sense and anti-sense primers. PCR was performed in a thermocycler (MJ Research, INC, Watertown, Massachusetts, USA). RT-PCR was used for identifying TLR transfection of HEK 293 cells using TLR4 or TLR2 specific primers. The PCR products were analyzed on 2% agarose gels and stained with ethidium bromide. RT-PCR was repeated 3 times and showed similar banding patterns. The samples were analyzed by RT-PCR using TNF-α, TLR2, TLR4, and β-actin. Primer sequences and PCR conditions used for amplification of β-actin, TNF-α, TLR2, and TLR 4 were as follows: β-actin (sense) 5'-CCAGAGCAAGAGAGGTATCC-3', (antisense) 5'-CTGTGGTGGTGAAGCTGTAG-3'; 32 cycles with 45 sec at 95℃ of denaturation, 45 sec at 60℃ for annealing, 1 min 30 sec at 72℃ for extension. TNF-α (sense) 5'-ACTCTTCTGCCTGCTGCACTTTGG-3', (antisense) 5'-GTTGACCTTTGTCTGGTAGGAGACGG-3'; 30 cycles, 30 sec at 94℃ for denaturation, 30 sec at 55℃ for annealing, 1 min at 72℃ for extension. TLR2 (sense) 5'-GATGCCTACTGGGTGGAGAA-3', (antisense) 5'-CGCAGCTCTCAGATTTACCC-3'; 30 cycles, 1 min at 94℃ for denaturation, 1 min at 55℃ for annealing, 1 min at 72℃ for extension. TLR4 (sense) 5'-CAACAAAGGTGGGAATGCTT-3', (antisense) 5'-TGCCATTGAAAGCAACTCTG-3'; 30 cycles, 1 min at 94℃ for denaturation, 1 min at 55℃ for annealing, 1 min at 72℃ for extension.
ELISA
The levels of IL-12, IL-8, and IL-10 in the supernatants of THP-1 cells grown under different culture conditions were measured by ELISA. In 96-well culture plates (Falcon Becton Dickinson), THP-1 cells (1 × 105 cells/well) were co-cultured with LPS (10 µg/ml) and/or T. gondii lysate (50 µg/ml) for 24 hr or 48 hr. The supernatant was collected and measured for cytokine levels with anti-human IL-12, anti-human IL-8, and anti-human IL-10 (1 : 250) (BD, PharMingen, Sandiego, California, USA) primary antibodies and streptoavidin-horseradish peroxidase conjugate (1 : 250) (BD, PharMingen) secondary antibody. Secretion of IL-12 and IL-10 from THP-1 cells treated with LPS and/or T. gondii lysate was measured after treatment with 10 µg/ml of anti-human TLR2 or anti-human TLR4 antibodies (Serotec, Oxford, UK) for 24 hr. All results were presented as the mean of triplicate wells and are representative of at least 3 independent experiments.
Transient transfection
The plasmids, pGL3-minIL6P, pcDNA3.1(+), pFLAG-CMV-1, and pCH110 were purchased from Amersham Pharmacia Biotech, Invitrogen, or Sigma. HEK 293 cells (1 × 106) were cultured with DMEM with 10% FBS in 12-well culture plates (Becton Dickinson, Bergen County, New Jersey, USA). HEK293 cells were transiently transfected with 1 µg of plasmid DNA containing NF-κB + luciferase reporter genes, MyD88, or pCH110 plasmid DNA expressing human TLR2 or TLR4 gene mixed with 1 µg of lipofectamine 2000 (Invitrogen). After 24 hr, the transfected cells were cultured in presence of 10 µg/ml LPS and/or 50 µg/ml T. gondii lysate for another 24 hr. The plasmid DNA was transformed into DH5a competent cells in LB medium with penicillin, and purified with a plasmid miniprep kit (Qiagen, Valencia, California, USA) after cultivation.
Luciferase assay
After treatment of HEK293 cells with LPS and/or T. gondii lysate for 24 hr, a NF-κB-dependent luciferase reporter assay was performed using a dual luciferase kit (Promega, Madison, Wisconsin, USA). The HEK293 cells were lysed with cell lysis reporter buffer and chemiluminescence was determined with luciferase substrate (Berthord Detectio system, Bad Wildbad, Germany). Activities were determined and normalized to the activity of the co-transfected plasmid or β-galactosidase.
Statistical analysis
Data obtained were analyzed statistically using the Mann-Whitney U-test, where P < 0.05 indicates significance.
RESULTS
Inflammatory cytokine production by THP-1 cells stimulated with T. gondii lysate
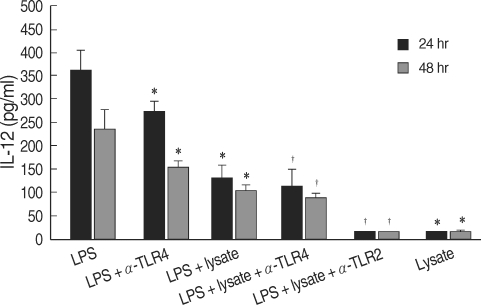
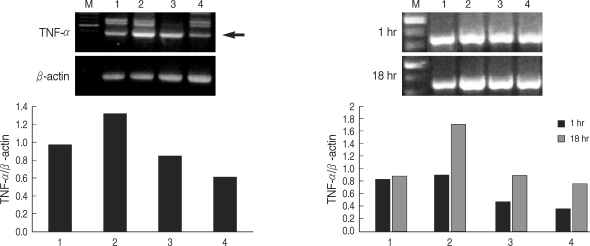
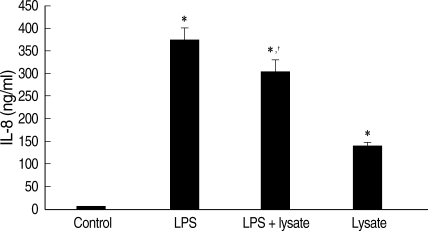
T. gondii lysate did not induce a significantly high IL-12 secretion by THP-1 cells, but suppressed LPS-stimulated IL-12 secretion (Fig. 1). A treatment with anti-human TLR2 antibody showed that TLR2 played a critical role in IL-12 secretion (P < 0.05, Fig. 1). THP-1 cells stimulated with LPS and/or T. gondii lysate for 1-24 hr were assayed for TNF-α by RT-PCR. The expression of TNF-α was increased following LPS treatment and suppressed by T. gondii lysate (Fig. 2). IL-8 in culture supernatant of THP-1 cells treated with T. gondii lysate or LPS was 140.8 ± 0.01 ng/ml and 373.8 ± 0.03 ng/ml, respectively. T. gondii lysate suppressed the secretion of IL-8 (304.6 ± 0.02 ng/ml) in cells stimulated with LPS (P < 0.05, Fig. 3).
Fig. 1.
Production of IL-12 by human myelomonocytic THP-1 cells stimulated with LPS or T. gondii lysate. LPS, lipopolysaccharide of Escherichia coli; lysate, T. gondii lysate; α-TLR2 & 4, anti-TLR2 & 4 antibodies.
Fig. 2.
Expression of TNF-α by THP-1 cells treated with LPS or T. gondii lysate. M, 100 bp marker; lane 1, cell only; lane 2, LPS; lane 3, LPS and T. gondii lysate; lane 4, T. gondii lysate. Arrow indicates TNF-α.
Fig. 3.
IL-8 levels in culture supernatant of THP-1 cells treated with LPS and/or T. gondii lysate for 24 hr. Control, cell only; Lysate, T. gondii lysate. *P < 0.05 (versus control); †P < 0.05 (versus LPS).
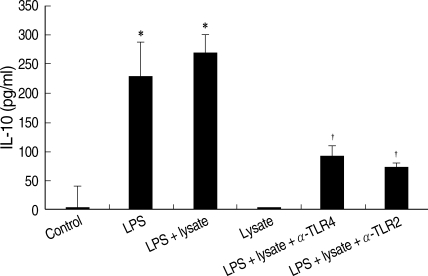
THP-1cells treated only with T. gondii lysate did not secrete IL-10. The IL-10 production of THP-1 cells treated with LPS or LPS plus T. gondii lysate was measured at 231.0 ± 0.06 ρg/ml and 271.0 ± 0.03 ρg/ml, respectively. Interestingly, a combination of T. gondii lysate and LPS showed a synergistic effect on IL-10 production by THP-1 cells (Fig. 4). Anti-human TLR2 and TLR4 antibodies suppressed IL-10 production of THP-1 cells stimulated with LPS and T. gondii lysate.
Fig. 4.
IL-10 of THP-1 cells treated with LPS and/or T. gondii lysate, or in combination with anti-human TLR2 or TLR4 antibodies. Control, cell only; Lysate, T. gondii lysate. *P < 0.05 (versus control); †P < 0.05 (versus LPS + lysate).
NF-κB activation by TLR2 or TLR4 in THP-1 cells treated with T. gondii lysate
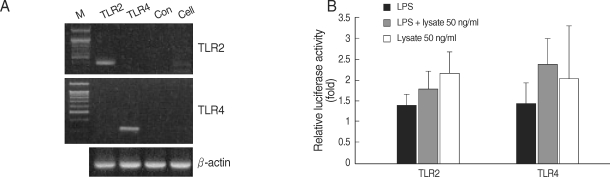
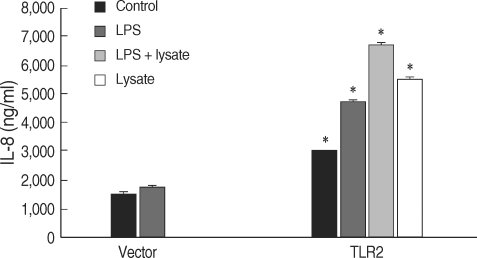
HEK293 cells were transiently transfected with a NF-κB/luciferase report plasmid, and the luciferase expression was determined. These cells were co-transfected with plasmid DNA and TLR2 or TLR4. Gene transcripts of TLR2 and TLR4 in HEK293 cells were confirmed by RT-PCR (Fig. 5A). When challenged with LPS and/or T. gondii lysate for 24 hr, the TLR-transfected HEK293 cells showed increased luciferase levels (Fig. 5B). The secretion of IL-8 from the TLR2-transfected HEK293 cells was increased by LPS and/or T. gondii lysate (Fig. 6).
Fig. 5.
RT-PCR of HEK293 cells without transfection (A), and NF-κB activity in HEK 293 cells treated with LPS and/or T. gondii lysate (B). M, marker; TLR2, transfection with TLR2 expression plasmid; TLR4, transfection with TLR4 expression plasmid; con, transfection with control plasmid; cell, HEK293 cells without transfection.
Fig. 6.
IL-8 secreted from TLR2-transfected HEK293 cells.*P < 0.05.
DISCUSSION
Macrophages infected by T. gondii are capable of microbicidal activity through production of inflammatory mediators, i.e., IL-12 and TNF-α, which are critical to the protective immune response during T. gondii infection [2,8,10,12]. On the other hand, reports have shown that T. gondii-infected macrophages are blocked for production of IL-12 and TNF-α that are induced by LPS [3,5,13,14]. T. gondii soluble antigen or crude lysates have been shown incapable of inducing IL-12 or TNF-α production in human monocytes [14,15]. LPS is a potent activator of macrophages and induces production of several pro-inflammatory cytokines such as IL-12 and TNF-α. In the present study, increased IL-12 secretion and TNF-α mRNA expression were observed in THP-1 cells after LPS stimulation and expression of these pro-inflammatory cytokines was blocked by co-treatment with T. gondii lysate. However, cells treated with T. gondii lysate alone did not significantly induce IL-12 or TNF-α expression.
T. gondii induced activation of signal transducer and activator of transcription 3 (STAT3) plays an important role in suppression of LPS induced expression of TNF-α and IL-12 [5,16]. However, the activation of STAT3 was not induced by soluble T. gondii extract or heat-killed tachyzoite [3]. In previous studies, IL-8 expression was increased in cells infected by T. gondii or by Trichomonas vaginalis [17,18]. In the present study, IL-8 secretion of THP-1 cells was induced not only by LPS treatment but by T. gondii lysate. Futhermore, higher IL-8 levels were observed following LPS treatment and this was also suppressed by co-treatment with T. gondii lysate. The anti-inflammatory cytokine, IL-10, is produced by a variety of cells including macrophages. The IL-10 signaling cascade is a major pathway involved in the control of pro-inflammatory mediators such as IL-12 and TNF-α [3,13]. Interestingly, we observed that IL-10 was synergistically increased by co-treatment with LPS and T. gondii lysate in THP-1 cells. However, no secretion of IL-10 was detected in the cells treated only with T. gondii lysate. The balance between pro-inflammatory and anti-inflammatory cytokines is essential to the control of Toxoplasma infection [10]. The suppression of macrophage function may be a strategy that prevents hyper-inflammation responses [5].
TLRs are important transmembrane proteins capable of recognizing a wide range of microorganisms. Bacterial pathogen-associated molecular patterns (PAMPs) activate cells via TLRs, triggering anti-microbial responses and cytokine production [19]. The TLR family consists of 13 members found in mammalian cells with each TLR having its own intrinsic signaling pathway [20]. TLR2 is a mediator of cellular responses to a wide variety of infectious pathogens and a product of a gram-positive bacterium, Mycobacterium, and TLR4 ligand is combined with LPS of gram-negative bacteria [21,22]. TLR4 is the receptor linking LPS and cluster of differentiation 14 (CD14) interactions to NF-κB translocation and induction of pro-inflammatory cytokines. However, little is known about how TLRs mediate innate immunity against protozoa. Involvement of TLR2, but not TLR4, is essential during the induction of IL-12, TNF-α, and nitric oxide (NO) by murine macrophages activated by Trypanosoma cruzi glycosylphosphatidyl inositol (GPI) [23,24]. TLR4 is involved in the protective mechanisms of T. gondii, and low levels of cytokines are produced in peritoneal exudate cells of TLR2- and myeloid differentiation factor 88 (MyD88)-deficient mice [25,26]. T. gondii activates dendritic cells through TLR11 and induces IL-12 production [27]. Recognition by TLRs initiates signal pathways through the common adaptor molecule MyD88, leading to activation of the mitogen-activated protein kinase (MAPK) family and NF-κB transcription factors [28].
Entamoeba histolytica lipopeptidophosphoglycan (LPPG), a cell surface molecule, induces IL-12, TNF-α, and IL-8 production and also activates NF-κB through TLR2 and TLR4 [22,29]. The NF-κB pathway is inhibited by infection with viable T. gondii [16]. In the present study, we investigated NF-κB activation via TLR2 and TLR4 expressions. NF-κB activation was induced in HEK 293 cells stimulated with T. gondii lysate and/or LPS. The production of IL-8 by HEK 293 cells stimulated with T. gondii lysate and/or LPS was observed in cells expressing TLR2. The treatment with T. gondii lysate suppressed production of IL-12 and TNF-α expression in human macrophages stimulated by LPS. Through a transfection assay and the use of blocking monoclonal anti-TLR2 and anti-TLR4 antibodies, it was found that T. gondii lysate suppressed IL-12, TNF-α, IL-10, and IL-8 production with NF-κB activation via TLR2 or TLR4.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant (KRF-2005-015-E00101) funded by the Korean Government (MOEHRD).
References
- 1.Langermans JA, Nibbering PH, Van Vuren-Van Der Halst ME, Van Furth R. Transforming growth factor-beta suppresses interferon-gamma-induced toxoplasmastatic activity in murine macrophages by inhibition of tumor necrosis factor- alpha production. Parasite Immunol. 2001;23:169–175. doi: 10.1046/j.1365-3024.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. Cutting edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-α in host macrophages. J Immunol. 2005;174:3148–3152. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 4.Buzoni-Gatel D, Werts C. Toxoplasma gondii and subversion of the immune system. Trends Parasitol. 2006;22:448–452. doi: 10.1016/j.pt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Bennouna S, Denkers EY. Screening for Toxoplasma gondii-regulated transcriptional responses in lipopolysaccharide-activated macrophages. Infect Immun. 2006;74:1916–1923. doi: 10.1128/IAI.74.3.1916-1923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belloni A, Villenu I, Gomez JE, Polloux H, Bonhomme A, Guenounoh M, Pinon JM, Aubert D. Regulation of tumor necrosis factor alpha and its specific receptors during Toxoplasma gondii infection in human monocytic cells. Parasitol Res. 2003;89:207–213. doi: 10.1007/s00436-002-0735-z. [DOI] [PubMed] [Google Scholar]
- 7.Friedland JS, Shattock RJ, Johnson JD, Remick DG, Holliman RE, Griffin GE. Differential cytokine gene expression and secretion after phagocytosis by a human monocytic cell line of Toxoplasma gondii compared with Mycobacterium tuberculosis. Clin Exp Immunol. 1993;91:282–286. doi: 10.1111/j.1365-2249.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J Immunol. 2001;167:2193–2201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- 9.Lang C, Gross U, Luder CG. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol Res. 2007;100:191–203. doi: 10.1007/s00436-006-0306-9. [DOI] [PubMed] [Google Scholar]
- 10.Aldebert D, Durand F, Mercier C, Brenier-Pinchart M, Cesbron-Delauw M, Pellowx H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the THP-1 human monocyte cell line. Cytokine. 2007:206–211. doi: 10.1016/j.cyto.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–44. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Manson N, Aliberti J, Caamano JC, Liou H, Hunter CA. Identification of c-Rel-dependent and -independent pathways of IL-12 production during infections and inflammatory stimuli. J Immunol. 2002;168:2590–2594. doi: 10.4049/jimmunol.168.6.2590. [DOI] [PubMed] [Google Scholar]
- 13.Deckert-Schluter M, Buck C, Weiner D, Kaefer N, Rang A, Hof H, Wiestler OD, Schluter D. Interleukin-10 down regulates the intracerebral immune response in chronic Toxoplasma encephalitis. J Neuroimmunol. 1997;76:167–176. doi: 10.1016/s0165-5728(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 14.Belloni A, Aubert D, Gomez-Marin JE, Naour RL, Bonhomme A, Guenounou M, Pinon JM. Involvement of tumor necrosis factor-α during infection of human monocytic cells by Toxoplasma gondii. Parasitol Res. 2000;86:406–412. doi: 10.1007/s004360050685. [DOI] [PubMed] [Google Scholar]
- 15.Buzoni-Gatel D, Werts C. Toxoplasma gondii and subversion of the immune system. Trends Parasitol. 2006;22:448–452. doi: 10.1016/j.pt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-γ signaling. J Immunol. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Oh YK, Kim YJ, Cho SJ, Ahn MH, Cho YJ. Nuclear factor-kappa B plays a major role in the regulation of chemokine expression of HeLa cells in response to Toxoplasma gondii infection. Parasitol Res. 2001;87:758–763. doi: 10.1007/s004360100447. [DOI] [PubMed] [Google Scholar]
- 18.Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of Interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–1332. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 20.Urematsu S, Akira S. Toll-like receptor and innate immunity. J Mol Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 21.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2004;165:5780–5787. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 22.Yarovinsky F, Sher A. Toll-like receptor recognition of Toxoplasma gondii. Int J Parasitol. 2006;36:255–259. doi: 10.1016/j.ijpara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procópio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 24.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 25.Mun HS, Aosai F, Norose K, Chen M, Piao L, Takeuchi O, Akira S, Ishikura H, Yano A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 26.Furuta T, Kikuchi T, Akira S, Watanabe N, Yoshikawa Y. Roles of the small intestine for induction of toll-like receptor 4-mediated innate resistance in naturally acquired murine toxoplasmosis. Int Immunol. 2006;18:1655–1662. doi: 10.1093/intimm/dxl099. [DOI] [PubMed] [Google Scholar]
- 27.Yarovinsky F, Zhang D, Ansersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hiehy S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 28.Del Rio L, Butcher BA, Bennouna S, Hieny S, Sher A, Denkers EY. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J Immunol. 2004;172:6954–6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado-Bernal C, Kirschning C, Rosenstein Y, Rocha LM, Rios-Sarabia N, Espinosa-Cantellno M, Becker I, Estrada I, Salazar-Gonzalesz RM, Lopez-Macias C, Wanga H, Sanchez J, Isibasi A. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 2005;27:127–137. doi: 10.1111/j.1365-3024.2005.00754.x. [DOI] [PubMed] [Google Scholar]