Abstract
Background
We recently demonstrated that a 2-year subantimicrobial-dose doxycycline (SDD) regimen (double-masked, placebo-controlled clinical trial) in postmenopausal (PM) women exhibiting mild systemic bone loss (osteopenia) and local bone loss (periodontitis) reduced the progression of periodontal attachment loss (intent-to-treat analysis) and the severity of gingival inflammation and alveolar bone loss (subgroups) without producing antibiotic side effects. We now describe SDD effects on biomarkers of collagen degradation and bone resorption in the gingival crevicular fluid (GCF) of the same vulnerable subjects.
Methods
GCF was collected from SDD- and placebo-treated PM subjects (n = 64 each) at the baseline and 1- and 2-year appointments; the volume was determined; and the samples were analyzed for collagenase activity (using a synthetic peptide as substrate), relative levels of three genetically distinct collagenases (Western blot), a type-1 collagen breakdown product/bone resorption marker (a carboxyterminal telopeptide cross-link fragment of type I collagen [ICTP]; radioimmunoassay), and interleukin-1β (enzyme-linked immunosorbent assay). Statistical analyses were performed using generalized estimating equations; primary analyses were intent-to-treat.
Results
Collagenase activity was significantly reduced by SDD treatment relative to placebo based on intent-to-treat (P = 0.01). ICTP showed a similar pattern of change during SDD treatment, and GCF collagenase activity and ICTP were positively correlated at all time periods (P < 0.001). Matrix metalloproteinase (MMP)-8 accounted for ~80% of total collagenase in GCF, with much less MMP-1 and -13, and SDD reduced the odds of elevated MMP-8 by 60% compared to placebo (P = 0.006).
Conclusion
These observations support the therapeutic potential of long-term SDD therapy to reduce periodontal collagen breakdown and alveolar bone resorption in PM women; effects on serum biomarkers of systemic bone loss in these subjects are being analyzed.
Keywords: Clinical trial, collagenases, gingival crevicular fluid, osteopenia, periodontitis, postmenopause
More than 2 decades ago, Golub et al.1 discovered, and other groups2–4 confirmed, that tetracyclines (TCs), such as doxycycline and minocycline, can inhibit host-derived matrix metalloproteinases (MMPs), such as collagenases and gelatinases, and by a mechanism unrelated to the antibiotic activity of these drugs. The first mechanism identified was the ability of TCs to directly inhibit already activated MMPs by binding the metal ions, calcium and zinc, in the catalytic domain of the enzymes.1–3,5 Additional pleiotropic mechanisms soon became apparent, such as the ability of these drugs to downregulate the expression of inactive precursor pro-MMPs and to block the activation of these zymogens. Two strategies were pursued to translate this previously unrecognized, non-antibiotic property of TCs into new therapies to inhibit pathologically excessive connective tissue destruction, including bone resorption. One strategy was to chemically modify the TC molecule to eliminate its antibiotic properties (i.e., bacteriostatic) but to retain (even enhance) its MMP-inhibitory properties, i.e., chemically-modified tetracyclines (CMTs) 1 through 10.2,3 The second strategy was to titrate downward the oral dose of doxycycline to produce blood levels of the drug too low to produce antibiotic activity (and, thus, eliminate side effects of antibiotic administration) but which still produced MMP-inhibitory effects and clinical improvement in patients with periodontitis.2–4 Early in these studies, TCs and CMTs were found to inhibit bone resorption in organ culture6,7 and in animal models of bone-deficiency diseases, including the estrogen-deficient (ovariectomized) osteoporotic aged female rat8 and the diabetes-induced osteopenic rat.9 These effects were associated, in part, with the MMP-inhibitory properties of these drugs. These drugs also “normalized” pathologic bone turnover by inhibiting osteoclast activity and bone resorption and by enhancing osteoblast activity, type I collagen synthesis, and bone formation.10–12
Because estrogen deficiency in postmenopausal (PM) women is the most common cause of osteoporosis, involves accelerated bone resorption overpowering the rate of bone formation, and has been associated with increased tooth loss and oral bone loss,13–15 we hypothesized that subantimicrobial-dose doxycycline (SDD), by a non-antimicrobial mechanism, can reduce bone loss and improve clinical measures of periodontitis in these vulnerable subjects. As a result, we recently completed a double-masked, placebo-controlled clinical trial on PM women who exhibited mild systemic bone loss (osteopenia) and periodontitis and who were administered a 2-year regimen of SDD or placebo adjunctive to periodontal maintenance therapy and calcium and vitamin D supplements. Our data demonstrated that SDD significantly reduced the progression of periodontal attachment loss (intent-to-treat analysis) and reduced the severity of gingival inflammation and alveolar bone loss (in subgroups of these subjects), without producing side effects associated with antibiotic therapy.16–18 We now present our findings, in the same clinical trial, describing the effect of SDD on biochemical “markers” of collagen degradation and bone resorption in the gingival crevicular fluid (GCF) from this vulnerable population. To the best of our knowledge, this study is the first to show that 1) SDD can reduce collagenase levels and activity over a prolonged period of time (previous studies2–4 described effects on collagenases over several weeks to 3 months, which did not preclude the subsequent potential loss of drug effect), 2) effects on collagenase are positively correlated with a biomarker of bone resorption in the same GCF samples over a long period of time, and 3) long-term SDD therapy can produce these effects in PM women exhibiting bone loss locally and systemically, whereas previous studies2,3,19,20 on this topic of host-modulation therapy did not target subjects with this important systemic factor, estrogen deficiency associated with the menopause.
MATERIALS AND METHODS
The details of this clinical trial, as well as the methods used for clinical, radiographic, and microbiologic measurements, were described in our earlier articles.16–18
In brief, the study was a two-center, double-masked, placebo-controlled clinical trial with each of 128 PM subjects randomly assigned to take placebo (n = 64 subjects) or SDD (doxycycline hyclate, 20 mg; n = 64) tablets twice daily for 2 years. Subjects were recruited and randomized between June 2002 and October 2003. The last subject completed the clinical trial in October 2005. All subjects received calcium (600 mg) and vitamin D (200 IU) supplements twice daily with instructions for use and were scheduled to receive periodontal maintenance therapy every 3 to 4 months, all of which was provided at no cost to the participants during the 2-year protocol. Enrolled subjects were 45 to 70 years of age, PM for ≥6 months, diagnosed as osteopenic (not osteoporotic, because this disease would have required treatment with a United States Food and Drug Administration–approved medication, e.g., a bisphosphonate) based on dual-energy x-ray absorptiometry (DEXA; i.e., T scores of −1.0 to −2.5 inclusive) of the lumbar spine or femoral neck, had moderate to advanced periodontitis, and were undergoing periodontal maintenance therapy. Additional enrollment criteria were described by us previously.16 However, once enrolled, subjects were not removed from the trial if they did not adhere to the protocol (e.g., started bisphosphonate therapy or chronic non-steroidal anti-inflammatory drug therapy) based on an intent-to-treat paradigm. These occurrences of non-adherence to the protocol were recorded and addressed during data analysis (see Statistical Analysis). All subjects provided written informed consent to participate in the study. The study protocol was reviewed and approved by the Stony Brook Institutional Review Board and the University of Nebraska Medical Center Institutional Review Board.
Computer-assisted densitometric image analysis of oral posterior bite-wing radiographs and DEXA scans of the lumbar spine and femoral neck to assess local and systemic bone loss, respectively, as well as clinical measurements of periodontal disease and subgingival plaque samples for microbiologic analysis, were taken at regular intervals over 2 years; these data were described previously.16–18
Collection of GCF Samples
At each of three appointments (baseline and 1 and 2 years), GCF samples were collected from two pocket sites (5 to 9 mm in depth) per subject identified at a previous screening appointment. The GCF collection technique and measurement of GCF volume were described by us previously.19,20 In brief, the identified pocket sites were isolated with cotton rolls and gently air dried. Supragingival plaque was carefully removed using periodontal curets, then precut presterilized filter paper strips# were inserted into each isolated periodontal pocket until slight resistance was felt. The filter strips were left in place for 10 seconds, and the volume absorbed onto the paper strip was immediately determined in a calibrated GCF flow meter.** GCF samples visually contaminated with blood were discarded. Immediately after measurement, the GCF samples were placed into a microfuge tube on ice at chairside and stored frozen at −80°C within 10 minutes of collection. GCF collection preceded any clinical measurements.
Assay Methods for GCF Biomarkers
The frozen GCF samples (one pooled sample per subject/appointment) were thawed (4°C) for 15 minutes. Then, 400 μl 50 mM Tris/0.2 M NaCl/5 mM CaC12 buffer (pH 7.6) containing a proteinase-inhibitor cocktail (which blocked serine, cysteine, and thiol proteinases, but not MMPs), consisting of antipain (1 mg/l), aprotinin (1 mg/l), N-ethylmaleimide (125 mg/l), leupeptin (1 mg/l), and 50 mg/l detergent,†† were added to the pooled GCF samples. The two strips (pooled) containing the GCF were exhaustively mixed and extracted (1 hour, 4°C), and aliquots were taken for analysis of the following: collagenase (MMP) activity, the only type of proteinase that can degrade the triple-helical collagen molecule under physiologic conditions;3,4 a carboxyterminal telopeptide cross-link fragment of type I collagen [ICTP], a degradation fragment of type I collagen and a bone resorption marker;20 relative protein levels of the three different collagenases (MMP-1, -8, and -13) in GCF;20 and interleukin (IL)-1β, a proinflammatory cytokine that can induce osteoclastic activity and bone resorption.21 If one of the two teeth selected for GCF sampling was extracted before the 2-year protocol ended, the GCF collected on a filter strip from the remaining tooth was eluted in 200 μl instead of 400 μl buffer, and the aliquots for each of the assays below were reduced by half. These assays were carried out as follows.
Total Collagenase Activity
The details for measuring GCF collagenase activity were described by us previously.19 Seventy microliters of GCF extract were transferred to a microfuge tube containing a synthetic, collagenase-susceptible octapeptide (dinitrophenol [DNP]-Pro-Gln-Gly-Ile-Ala-Gly-Gln-dArg)‡‡ that served as the enzyme substrate. Following incubation at 37°C, the reaction mixture was quenched with l, 10-phenanthroline (a zinc chelator that binds this cation in the collagenase molecule), the tripeptide breakdown product was separated by high-performance liquid chromatography§§ using a reverse-phase C18 column (4.6 × 75 mm, 3.5-μm macroporous spherical support), and the eluate was monitored at 375 nm for quantifying the DNP-labeled peptides. The collagenase activity measured by this assay was further characterized as a host-derived collagenase based on its response in vitro to several different proteinase inhibitors and activators19,20 and was scored on a scale of 0% to 100% hydrolysis of the synthetic octapeptide.
ICTP and IL-1β Analyses
As described previously, l00-μl aliquots were taken and analyzed by radioimmunoassay for ICTP20 using a commercial kit,|||| and duplicate 50-μl aliquots were analyzed for IL-1β using an enzyme immunoassay¶¶ based on a double-antibody sandwich technique.21
Western Blot Analysis of MMP-1, -8, and -13
In brief, lyophilized GCF extracts (100 μl containing 10 to 20 μg protein) were treated with Laemmli buffer (pH 7.0) containing 5 mM dithiothreitol and heated for 5 minutes at 100°C. High- and low-range prestained sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis standard proteins were used as molecular weight markers. The samples were electrophoresed on 7.5% SDS-polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes, and Western blot analysis was carried out as described by us previously.20,22
Specific immunoreactivity was visualized as dark bands against a clear background, and the membranes were scanned with an imaging densitometer## using a program*** that corrects for background values. The densitometric units were measured in the linear range of immunoreactivity for each of the three MMPs; purified human MMP-1, -8, and -13 were used as positive controls.
Statistical Analysis
Statistical analytical procedures were described by us in detail.16–18 The method of generalized estimating equations, with a working exchangeable correlation structure, was used.23 For the collagenase, ICTP, and IL-1β measures, a linear regression model was fit, for which the outcome was the natural log-transformed follow-up measure and the baseline biochemical value, a time effect (12- or 24-month), and a study drug effect; randomization stratification factors (study center and baseline smoking status) were independent variables. The models were adjusted for a batch effect (assays were run in three different batches, and all samples were analyzed in the same batch for a given subject; batches were well-balanced by treatment group), along with all two- and three-way interaction terms among treatment, batch, andtime. Non-significant interaction terms among time, batch, and treatment were dropped, and the model was refit. Among all subjects in the intent-to-treat analyses, interactions involving treatment and the time or batch terms were not significant; therefore, the treatment effects are summarized and reported across time periods and batches. The influence of extreme data points, defined as falling more than three standard deviations away from the mean, was investigated by refitting regression models without such points. Because MMP distributions were highly skewed, the measures were coded into two or three categories based on the median value or tertiles and were analyzed using a similar modeling approach as described above with a binomial (logistic link) or multinomial (cumulative logit link) regression model, respectively. A Pearson correlation coefficient was calculated to summarize the association between collagenase and ICTP measures.
The primary analysis was intent-to-treat; data were analyzed from all randomized subjects, regardless of protocol adherence. As a secondary analysis, only measurements from subjects up to the time when a lack of protocol adherence occurred (e.g., initiation of significant concomitant medications, such as bisphosphonates, or subject adherence to study medications or calcium/vitamin D below an 80% threshold) were analyzed (per-protocol analysis). Reasons for exclusion from per-protocol analysis were described previously;16,17 overall, the SDD group had a slightly larger per-protocol subset (n = 32) than the placebo group (n = 27). Placebo and SDD groups exhibited similar characteristics, including age, ethnicity, race, years following estimated onset of menopause, smoking, number of teeth, and probing depths at baseline.16 The effect of SDD compared to placebo also was investigated for subgroups defined by smoking status, time since onset of menopause, adherence to study medications, and significant concomitant medication use, using tests of interactions in the regression models as described in detail previously.16,17
RESULTS
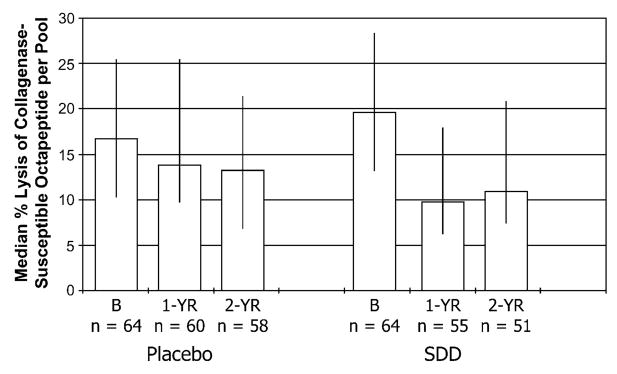
The data in Figure 1 show the effect of SDD therapy on collagenase activity expressed per pool of GCF from two pockets per subject. Using techniques we published previously,19,20 the SDD-treated PM women showed ~50% reduction in GCF collagenase activity over the 2-years compared to their own baseline values. In contrast, the placebo values appeared to decrease only slightly. Moreover, based on linear regression analysis, the SDD-treated group showed a statistically significant 22% reduction in median GCF collagenase activity compared to placebo-treated subjects over the study period, based on intent-to-treat analysis (95% confidence interval [CI]: 37% lower to 5% lower; P = 0.01), and a 29% reduction in median GCF collagenase activity compared to placebo subjects based on the per-protocol analysis (95% CI: 48% lower to 4% lower; P = 0.02) after adjusting for baseline values. When the GCF collagenase data were expressed as enzyme activity per microliter GCF, the greater reduction over time for SDD compared to placebo was not statistically significant based on intent-to-treat (P = 0.2), but it was significant based on the per-protocol analysis (P = 0.05; data not shown). For subgroup analyses, the effect of SDD seemed to depend on smoking status (P = 0.05), and there was a significant interaction between time and treatment for non-smokers (P = 0.02). At 1 year, median levels of collagenase activity per pool of GCF were 40% lower for SDD subjects compared to placebo subjects in the non-smoking group, which was statistically significant (95% CI: 53% lower to 22% lower; P < 0.0001). However, the 17% reduction for SDD-treated subjects compared to placebo in the non-smokers at 2 years was not statistically significant (P = 0.2). The smoking group did not show significant reductions with SDD compared to placebo (P = 0.3), and no other subgroup effects were significant.
Figure 1.
GCF collagenase activity in PM women with chronic periodontitis: effect of placebo and SDD administration. The median % lysis of a collagenase-susceptible octapeptide per pool of two GCF samples per subject at baseline (B), 1 year (1-YR), and 2 years (2-YR) is represented by the bar height; whiskers are drawn between the 25th and 75th percentiles. The estimated effect on median collagenase activity levels was a 22% reduction (95% CI: 37% lower to 5% lower; P = 0.01), comparing combined 1- and 2-year values between SDD and placebo after adjustment for baseline levels.
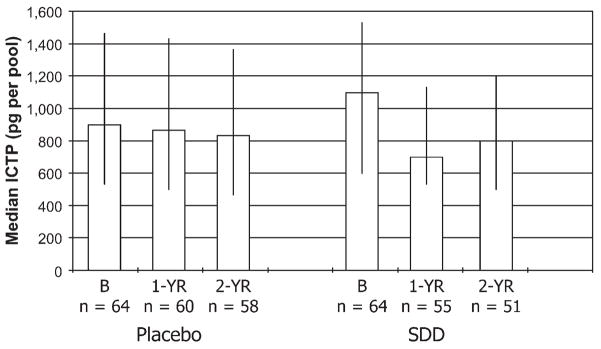
A similar pattern of change over time was seen for ICTP expressed per pool of GCF (Fig. 2) collected from the placebo- and SDD-treated PM subjects. As described by us previously,20 ICTP is a breakdown product of type I collagen, and this collagen makes up >90% of the organic matrix of bone. Thus, ICTP measurements in GCF, blood, and urine have been considered a diagnostic biomarker of bone resorption24 and are believed to reflect (at least in part) collagenase-mediated breakdown of the triple-helical collagen molecule. Once again, placebo treatment had no effect. In contrast, SDD therapy over the study period seemed to reduce the median ICTP levels per pool of GCF by ~30% compared to this group’s own baseline values. Using linear regression analysis, the SDD-treated group showed a 16% reduction in median GCF ICTP levels compared to placebo-treated subjects, after adjusting for baseline values (P = 0.08). However, when three extreme baseline values were excluded (two values in the placebo group and one in the SDD group), the SDD effect was statistically significant, with a median follow-up measure for SDD subjects that was 19% lower than for placebo subjects (95% CI: 33% lower to 2% lower; P = 0.03). Among the per-protocol subset, SDD was associated with a 16% reduction in median ICTP levels compared to placebo, which was not significant (P = 0.2). With regard to subgroup analyses, no significant effects were seen between SDD- and placebo-treated subjects for smoking status, years PM, adherence to study medications, or use of concomitant medications based on regression modeling.
Figure 2.
GCF ICTP levels in PM women with chronic periodontitis: effect of placebo and SDD administration. The median ICTP per subject (expressed as picograms ICTP per pool of two GCF samples) at baseline (B), 1 year (1-YR), and 2 years (2-YR) is represented by the bar height; whiskers are drawn between the 25th and 75th percentiles. The estimated effect on median ICTP levels was a 16% reduction (95% CI: 31% lower to 2% higher; P = 0.08, comparing combined 1- and 2-year values between SDD and placebo after adjustment for baseline levels.
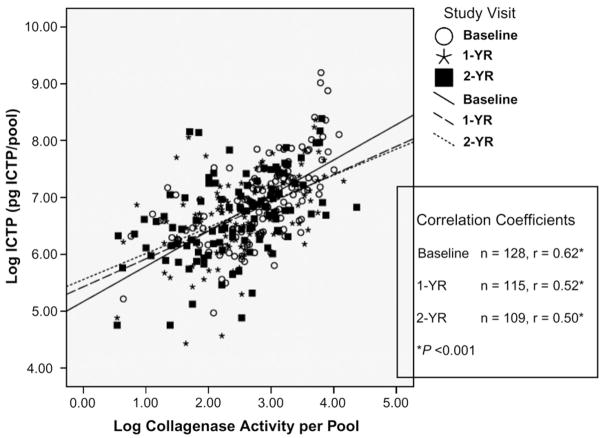
Because the initiation of the degradation of the native triple-helical collagen molecule is mediated by collagenases under physiologic conditions2–4 and collagen degradation is a key event in bone resorption, the correlation between the values for collagenase activity and ICTP in the GCF of these subjects, at all time periods, was determined and summarized across placebo and SDD groups (Fig. 3). The data for GCF collagenase activity and GCF ICTP levels were converted to a log value that demonstrated that the collagenase activity and the ICTP in the GCF were linearly related with positive correlation coefficients (r) of 0.62, 0.52, and 0.50 for baseline, 1 year, and 2 years, respectively; all three r values were highly statistically significant (P < 0.001). In general, the higher the values for collagenase activity per pool of GCF, the greater was the level of bone collagen breakdown products (ICTP).
Figure 3.
Correlation between natural log–transformed collagenase activity (percentage lysis of collagenase-susceptible substrate) and natural log–transformed ICTP in GCF of PM women with chronic periodontitis over the 2-year clinical protocol for SDD and placebo subjects combined. Linear regression lines are drawn for each time point. 1-YR = 1 year; 2-YR = 2 years.
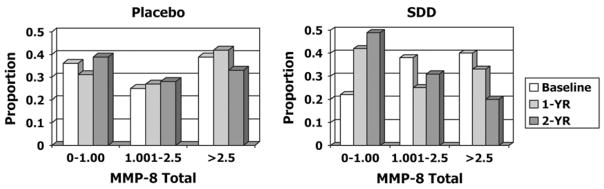
In addition to measuring total collagenase activity in the GCF (Figs. 1 and 3) of these PM women, the relative protein levels of the three genetically distinct collagenases, previously identified in human GCF,20,22 were also assessed (Table 1). Using the Western blot technique, MMP-1 (collagenase-1), -8 (collagenase-2), and -13 (collagenase-3) were detected in the GCF samples. Then, after densitometrically scanning the electrophoretic gels for the different molecular forms of each type of collagenase, the data were expressed as a percentage of the total collagenase protein. Regardless of whether the data were expressed as a mean or median value, MMP-8 (which included 65- to 75-kDa leukocyte and 45- to 55-kDa mesenchymal isoforms of this enzyme22) was the predominant collagenase type in the GCF, accounting for ~80% of the total. This was followed by MMP-13 at 0% to 18% (expressed as 25th to 75th percentiles) and MMP-1, which was detected at only very low levels (0% to 9%). Focusing on changes in the dominant type of collagenase, MMP-8, in the GCF of these PM women (Fig. 4), and based on intent-to-treat analysis, SDD therapy reduced the odds of elevated MMP-8 values (across the ordered categories of 0 to 1.00, 1.001 to 2.5, and >2.5 units) by 60% compared to placebo during the 2-year study period. This treatment effect was highly statistically significant (odds ratio [OR] = 0.40; 95% CI: 0.21 to 0.77; P = 0.006). Consistent with this pattern, SDD therapy increased the odds of lower values (among the ordered categories of 0 to 1.00, 1.00l to 2.5, and >2.5 units) for this type of collagenase, compared to placebo therapy, over the study period. Based on per-protocol analysis, this effect was even more dramatic because the odds of higher values for MMP-8 in SDD-treated subjects were 78% lower than in those receiving placebo tablets (OR = 0.22; 95% CI: 0.07 to 0.66; P = 0.007). Moreover, 1) the reduction of MMP-8 in SDD-treated subjects likely was driven by differences in the higher molecular weight polymorphonuclear leukocyte (PMN)-type MMP-8 (65 to 75 kDa), with a 38% reduction in the odds of higher PMN values for SDD subjects compared to placebo subjects (P = 0.1), and a much smaller effect on the mesenchymal-type MMP-8 (45 to 55 kDa) where the odds of non-zero values were 12% lower for SDD subjects compared to placebo subjects (P = 0.8), and 2) subgroup analysis demonstrated that the dramatic reduction in MMP-8 levels due to SDD therapy reflected an 83% lower odds of high values for this collagenase in subjects who did not use concomitant medications (P = 0.0002), whereas this effect was not significant in subjects who used concomitant medications (P = 0.7). Reductions in GCF collagenase activity and MMP-8 immunoreactive levels due to SDD therapy were not complete (i.e., residual collagenase activity and MMP-8 protein levels could be detected at 1 and 2 years; the therapeutic advantage of this less-than-complete reduction is addressed in the Discussion). No other significant associations between treatment and MMP levels were observed.
Table 1.
Distribution of MMP-1 (collagenase-1), MMP-8 (collagenase-2), and MMP-13 (collagenase-3)
| Type of Collagenase | Samples (N) | Mean | Median (25th to 75th percentile) |
|---|---|---|---|
| MMP-1 | 349 | 6.7 | 1.1 (0 to 8.6) |
| MMP-8 | 349 | 77.9 | 87.5 (39.2 to 98.5) |
| MMP-13 | 349 | 14.8 | 3.5 (0 to 17.6) |
Data reported as the percentage of total collagenase protein in GCF.
Figure 4.
The effect of SDD (versus placebo) administration on the risk of low, medium, or high levels of MMP-8 (leukocyte-type collagenase) in the GCF of PM women with chronic periodontitis over the 2-year clinical protocol: 0 to 1.00, 1.001 to 2.5, and >2.5 represent low, medium, and high levels of MMP-8, respectively. Data were available for 64, 59, and 57 placebo subjects and for 63, 55, and 51 SDD subjects at the baseline, 1-year (1-YR), and 2-year (2-YR) visits, respectively. The odds of higher MMP-8 values were reduced by 60% in SDD subjects compared to placebo subjects after adjustment for baseline levels (OR = 0.40; 95% CI: 0.21 to 0.77; P = 0.006).
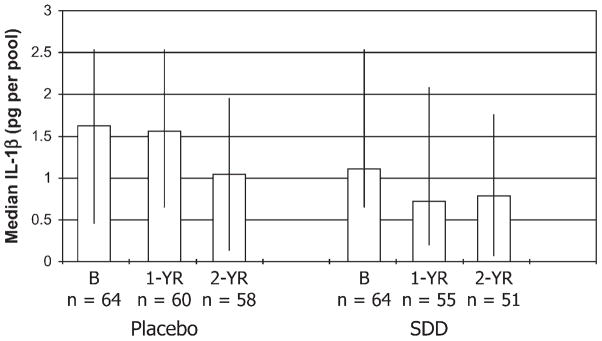
Regarding the levels of IL-1β in the GCF samples from placebo- and SDD-treated subjects over the 2-year time period, the pattern of change was similar to that seen for collagenase and ICTP, although the reduction in IL-1β levels was not significant except for a subgroup of subjects (see below). In general, based on intent-to-treat and on per-protocol analyses, the SDD subjects exhibited ~20% and 33% lower median values, respectively, for IL-1β over the study time period compared to placebo subjects, after adjusting for different baseline values, but these data were not statistically significant (P >0.2 for each; Fig. 5). Regarding subgroup analyses, the data for IL-1β were not statistically significant for subjects within 5 years of menopause (P = 0.1). However, for those subjects beyond 5 years of menopause, subjects administered SDD showed a statistically significant 51% lower median value for IL-1β per pool of GCF than placebo-treated subjects (OR = 0.49; 95% CI: 76% lower to 1% lower; P = 0.05). When the data were expressed per microliter of GCF, a similar pattern of change was seen, and results from the intent-to-treat and per-protocol analyses were similar, but these data were not statistically significant (data not shown).
Figure 5.
GCF IL-1β levels in PM women with chronic periodontitis: effect of placebo and SDD administration. The median GCF IL-1β per subject (expressed as picogram GCF IL-1β per pool of two GCF samples) at baseline (B), 1 year (1-YR), and 2 years (2-YR) is represented by the bar height; whiskers are drawn between the 25th and 75th percentiles. The estimated effect on median IL-1β levels was a 20% reduction (95% CI: 54% lower to 39% higher; P = 0.4), comparing combined 1- and 2-year values between SDD and placebo after adjustment for baseline levels.
DISCUSSION
The rationale for the current interventional (i.e., long-term administration of SDD) human clinical trial on PM women exhibiting local (periodontitis) and mild systemic (osteopenia) bone loss was two-fold. First, organ and cell culture, in vivo animal, and human studies1–3,8–11,16,20,25–29 over the past 25 years demonstrated beneficial, non-antibiotic effects of TC compounds (e.g., SDD and CMTs) on pathologic local and systemic bone loss. Second, it is increasingly being recognized that patients and experimental animals with systemic bone-deficiency disease, particularly PM osteoporosis (but also other disorders such as diabetes-induced osteopenia), can exhibit accelerated local (alveolar) bone loss beyond that induced by subgingival periodontopathogens, and all of these diseases might also benefit from treatment with TC compounds.2,3,30
Regarding these rationales, soon after the MMP-inhibitory activity of TCs was discovered, the relevance of this non-antimicrobial property of TCs to bone resorption was explored. Using standard aseptic organ culture systems, traditional and chemically modified TCs were found to inhibit bone resorption, regardless of whether the loss of the mineral and organic matrix constituents of bone was induced by parathyroid hormone, prostaglandin E2, or endotoxin.6,7 Non-TC antibiotics were ineffective in this system. Mechanisms included the ability of TCs to inhibit MMPs expressed by osteoblasts and osteoclasts (for reviews, see Golub et al.2,3). More recently, TCs were found to enhance bone formation and inhibit bone resorption.10,25–28 As examples, using ultracytochemistry, autoradiography, and dynamic histomorphometry on the osteoporotic bones of diabetic and ovariectomized (surgically induced menopausal) rats, TCs were found to enhance osteoblast activity, type I collagen synthesis, and bone formation. In the estrogen-deficient osteoporotic rat, the increased production of new bone as a result of TC treatment increased the connectivity of the resorbed discontinuous trabeculae in long bones,28 and, in an arthritic rat model, these drugs increased bone biomechanical strength and resistance to experimental fracture.29 Of interest, both alveolar bone loss and systemic (skeletal) bone loss benefited from these therapeutic effects of TCs in an animal model of PM osteoporosis.8
Two earlier clinical studies also provided a rationale for the current 2-year clinical trial. In a small pilot study, Payne et al.31 found that PM women diagnosed with periodontitis and systemic bone loss (osteopenia or osteoporosis) and treated with a 1-year cyclical SDD regimen showed less alveolar bone height loss and alveolar bone density loss (based on computer-assisted densitometric image analysis) than placebo-treated subjects. In an earlier study, Golub et al.20 monitored biomarkers of collagen and bone destruction, as well as bone formation and turnover, in GCF of male and female subjects with chronic periodontitis (who were not diagnosed with osteopenia or osteoporosis); a 2-month regimen of SDD (adjunctive to scaling) significantly reduced collagenase activity and ICTP, with no change in osteocalcin. Osteocalcin was originally viewed as a biomarker of bone formation because this Gla protein (rich in gamma-carboxy glutamic acid) is expressed only by osteoblasts, and, once secreted, small quantities are released into the bloodstream where they can be measured in serum samples. However, more recently, osteocalcin has been considered a biomarker of bone turnover (not just bone formation) because, after secretion by osteoblasts, this highly anionic matrix protein binds to Ca++ in mineralized bone; during bone resorption, the calcium-bound osteocalcin is released into the circulation.32 Because the earlier study20 on subjects with chronic periodontitis found that SDD decreased the biomarker of bone resorption (ICTP) but did not affect the levels of the bone turnover biomarker (osteocalcin), and the rate of bone turnover reflects a combination of bone formation plus bone resorption, these findings suggested that this TC treatment suppressed bone resorption and may have enhanced bone formation, with the net effect being no change in the bone turnover marker osteocalcin (placebo treatment had no effect on either GCF biomarker: ICTP or osteocalcin). These clinical results are consistent with earlier studies10,25–28 using cell culture and animal models of bone-deficiency disease in which TCs, such as doxycycline and minocycline, and the chemically modified TC derivative (CMT-1) increased bone formation and inhibited bone resorption. Although it has long been assumed that elevated levels of collagenase activity in humans likely reflects pathologic/ongoing collagen breakdown, based on well-established biologic concepts (i.e., the ability of only collagenase[s], but not other neutral proteinases, to degrade the undenatured triple-helical collagen molecule under physiologic conditions of pH and temperature), to the best of our knowledge, this was the first study20 to directly link these two biochemical events in subjects in situ.
The current, more definitive clinical trial confirmed this link by demonstrating, in the same pooled GCF samples, a strong statistically significant linear relationship between the level of collagenase activity and the ICTP degradation products of type I collagen, presumably released during resorption of alveolar bone at the same pocket site (based on the bone-specific pyridinoline content of the collagen telopeptide cross-link fragments) in these PM women with local and systemic bone loss. In fact, this positive correlation was maintained for placebo- and SDD-treated subjects over the 2-year protocol. The beneficial effects of SDD on these biomarkers of connective tissue and bone destruction during this long-term clinical trial likely contributed to improved clinical and radiologic measures of periodontal disease severity (at least in subgroups of these subjects) described by us previously.16,17 Moreover, abnormally elevated levels of these GCF biomarkers might signal an increased susceptibility of these subjects to more severe periodontal (including alveolar bone) breakdown if their commitment to regular periodontal maintenance therapy (provided to these subjects at no cost to them during the 2-year protocol to enhance compliance) should ever falter.
Perhaps the most dramatic effect of SDD therapy was the strong, long-term reduction in collagenase activity in the periodontal pockets of PM women, a finding supported by an equally dramatic reduction in protein levels of the most predominant type of collagenase, MMP-8, in the GCF (MMP-8 accounted for ~80% of the total collagenase protein, with much smaller relative amounts of MMP-13 [0% to 18%] and MMP-1 [0% to 9%], which is very similar to the pattern described for these same interstitial collagenases in an earlier study20 on GCF from adult females and males with chronic periodontitis). The highly significant reduction in MMP-8 during the 2-year regimen of SDD reflected decreased levels of 65- to 75-kDa leukocyte-type collagenase, because the smaller molecular weight (45 to 55 kDa) mesenchymal forms of this proteinase did not seem to be affected. The ~50% reduction of collagenase activity (compared to its own baseline) was measured by a functional assay using, as a substrate, an octapeptide with the mammalian collagenase–susceptible Gly–Ile peptide bond. The similar (less than complete) reduction of MMP-8 (collagenase-2) protein levels was measured using a polyclonal antibody to this genetically distinct type of collagenase. As we described previously,4,33 complete inhibition of MMPs (in contrast to a reduction of just the pathologically excessive levels of MMPs and their activity) may not be desirable because these neutral proteinases have physiologic functions such as processing of anti-inflammatory cytokines and chemokines, which are needed for host defense. In this regard, the current clinical trial also demonstrated that SDD (relative to placebo) significantly reduced GCF IL-1β (a proinflammatory and bone-resorbing cytokine) and alveolar bone height loss16 in the subjects who were PM for >5 years. The amplitude and duration (2 years) of these effects of SDD therapy in the absence of significant adverse events (AEs)16–18 provided further evidence of the therapeutic potential of SDD in subjects with periodontitis characterized by alveolar bone loss and, perhaps, in subjects with systemic bone loss.30 Regarding the latter condition, serum biomarkers of bone remodeling (note that SDD produced a significant reduction in biomarkers of systemic bone resorption in serum, at least in subgroups of these PM subjects34) as well as systemic inflammation are being analyzed for the current clinical trial and, recently, markers of systemic inflammation, such as C-reactive protein, were found to reflect susceptibility to skeletal bone–deficiency disease (PM osteoporosis).35 This acute-phase protein in blood samples also was reduced by SDD administration in subjects with severe cardiovascular disease.36 The data on biomarkers of systemic inflammation (e.g., C-reactive protein) in serum samples of these PM osteopenic women with periodontitis are being analyzed and will be reported elsewhere.
As described previously,16 and of clinical importance particularly considering that all subjects received the study medications daily over a prolonged period (2 years), AEs (such as gastrointestinal upset, infection, and aches/pains) were similar for the placebo-and SDD-treated groups. However, significantly fewer SDD subjects experienced a dermatologic AE, such as rash, acne, rosacea, and hives, during the clinical trial (2% for the SDD group versus 17% for the placebo group; P = 0.002). These data are consistent with previous studies showing evidence of the safety and efficacy of SDD in adults with the inflammatory skin diseases acne and rosacea37–39 and in subjects with the inflammatory joint disease, rheumatoid arthritis.40
Acknowledgments
The authors acknowledge the following individuals for their dedication to this clinical trial: E. Boilesen, programmer/analyst II, College of Public Health Office of the Dean, A. Lahners, research coordinator, Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, M. Morris, research nurse coordinator, Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, J. Layton, research coordinator, Department of Surgical Specialties, College of Dentistry, University of Nebraska Medical Center, T. Meinberg, dental hygenist, Department of Surgical Specialties, University of Nebraska Medical Center, T. Powell, office associate 1, Dental administration, College of Dentistry, University of Nebraska Medical Center, M. Schmid, research technologist II, Dental Administration, College of Dentistry, University of Nebraska Medical Center, and Ruth Tenzler, research nurse, Stony Brook University School of Dental Medicine. We thank the Nebraska Periodontitis Referral Network and several Long Island clinicians and Stony Brook University faculty for referring subjects to this clinical trial. The authors thank CollaGenex Pharmaceuticals, Newtown, Pennsylvania, for providing SDD and matched placebo tablets. We also thank Rene Martin, Stony Brook University School of Dental Medicine, for typing assistance. The project was supported by grant R01DE012872 from the National Institute of Dental and Craniofacial Research (NIDCR) (JBP, principal investigator [PI], and LMG, co-PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR or the National Institutes of Health. Additional support was provided by a grant to TS from the Academy of Finland, Helsinki, Finland, and Helsinki University Central Hospital research funds. Dr. Golub is listed as an inventor on several patents for the drug mentioned in this publication, and these patents have been fully assigned to his institution, State University of New York at Stony Brook. Drs. Golub and Ryan were consultants for CollaGenex Pharmaceuticals. Dr. Sorsa is listed as an inventor on four oral fluid biomarker/diagnostic patents. Drs. Lee, Stoner, Reinhardt, Wolff, Nummikoski, and Payne report no conflicts of interest related to this study.
Footnotes
Periopaper, Proflow, Amityville, NY.
Periotron 6000, Proflow.
Zwittergent, Calbiochem-Novabiochem, La Jolla, CA.
Bachem, King of Prussia, PA.
Waters Alliance 2695 System, Waters Alliance, Milford, MA.
Immunodiagnostic Systems, Fountain Hills, AZ.
Biosource, Camarillo, CA.
Bio-Rad Model GS-700, Bio-Rad, Hercules, CA.
Analyst, Bio-Rad, Hercules, CA.
References
- 1.Golub LM, Lee HM, Lehrer G, et al. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 2.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: New therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 3.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 4.Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 5.Ryan ME, Usman A, Ramamurthy NS, Golub LM, Greenwald RA. Excessive matrix metalloproteinase activity in diabetes: Inhibition by tetracycline analogues with zinc reactivity. Curr Med Chem. 2001;8:305–316. doi: 10.2174/0929867013373598. [DOI] [PubMed] [Google Scholar]
- 6.Gomes BC, Golub LM, Ramamurthy NS. Tetracyclines inhibit parathyroid hormone-induced bone resorption in organ culture. Experientia. 1984;40:1273–1275. doi: 10.1007/BF01946671. [DOI] [PubMed] [Google Scholar]
- 7.Golub LM, Ramamurthy N, McNamara TF, et al. Tetracyclines inhibit tissue collagenase activity. A new mechanism in the treatment of periodontal disease. J Periodontal Res. 1984;19:651–655. doi: 10.1111/j.1600-0765.1984.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 8.Golub LM, Ramamurthy NS, Llavaneras A, et al. A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Ann N Y Acad Sci. 1999;878:290–310. doi: 10.1111/j.1749-6632.1999.tb07691.x. [DOI] [PubMed] [Google Scholar]
- 9.Golub LM, Ramamurthy NS, Kaneko H, Sasaki T, Rifkin B, McNamara TF. Tetracycline administration prevents diabetes-induced osteopenia in the rat: Initial observations. Res Commun Chem Pathol Pharmacol. 1990;68:27–40. [PubMed] [Google Scholar]
- 10.Sasaki T, Ramamurthy NS, Golub LM. Tetracycline administration increases collagen synthesis in osteoblasts of streptozotocin-induced diabetic rats: A quantitative autoradiographic study. Calcif Tissue Int. 1992;50:411–419. doi: 10.1007/BF00296771. [DOI] [PubMed] [Google Scholar]
- 11.Rifkin BR, Vernillo AT, Golub LM, Ramamurthy NS. Modulation of bone resorption by tetracyclines. Ann N Y Acad Sci. 1994;732:165–180. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- 12.Craig RG, Yu Z, Xu L, et al. A chemically modified tetracycline inhibits streptozotocin-induced diabetic depression of skin collagen synthesis and steady-state type I procollagen mRNA. Biochim Biophys Acta. 1998;1402:250–260. doi: 10.1016/s0167-4889(98)00008-1. [DOI] [PubMed] [Google Scholar]
- 13.Tezal M, Wactawski-Wende J, Grossi SG, Dmochowski J, Genco RJ. Periodontal disease and the incidence of tooth loss in postmenopausal women. J Periodontol. 2005;76:1123–1128. doi: 10.1902/jop.2005.76.7.1123. [DOI] [PubMed] [Google Scholar]
- 14.Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol. 1997;68:24–31. doi: 10.1902/jop.1997.68.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 16.Payne JB, Stoner JA, Nummikoski PV, et al. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34:776–787. doi: 10.1111/j.1600-051X.2007.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhardt RA, Stoner JA, Golub LM, et al. Efficacy of subantimicrobial dose doxycycline in post-menopausal women: Clinical outcomes. J Clin Periodontol. 2007;34:768–775. doi: 10.1111/j.1600-051X.2007.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker C, Puumala S, Golub LM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: Microbiologic results. J Periodontol. 2007;78:1590–1601. doi: 10.1902/jop.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golub LM, McNamara TF, Ryan ME, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: Effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146–156. doi: 10.1034/j.1600-051x.2001.028002146.x. [DOI] [PubMed] [Google Scholar]
- 20.Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 21.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–567. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 22.Kiili M, Cox SW, Chen HY, et al. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: Molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol. 2002;29:224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 23.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.Herr AE, Hatch AV, Giannobile WV, et al. Integrated microfluidic platform for oral diagnostics. Ann N Y Acad Sci. 2007;1098:362–374. doi: 10.1196/annals.1384.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polson AM, Bouwsma OJ, McNamara TF, Golub LM. Enhancement of alveolar bone formation by tetracy-cline administration in squirrel monkeys. J Appl Res Clin Dentist. 2005;2:32–42. [Google Scholar]
- 26.Williams S, Barnes J, Wakisaka A, Ogasa H, Liang CT. Treatment of osteoporosis with MMP inhibitors. Ann N Y Acad Sci. 1999;878:191–200. doi: 10.1111/j.1749-6632.1999.tb07684.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams S, Wakisaka A, Zeng QQ, et al. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–644. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]
- 28.Aoyagi M, Sasaki T, Ramamurthy NS, Golub LM. Tetracycline/flurbiprofen combination therapy modulates bone remodeling in ovariectomized rats: Preliminary observations. Bone. 1996;19:629–635. doi: 10.1016/s8756-3282(96)00280-3. [DOI] [PubMed] [Google Scholar]
- 29.Zernicke RF, Wohl GR, Greenwald RA, Moak SA, Leng W, Golub LM. Administration of systemic matrix metalloproteinase inhibitors maintains bone mechanical integrity in adjuvant arthritis. J Rheumatol. 1997;24:1324–1331. [PubMed] [Google Scholar]
- 30.Payne JB, Reinhardt RA. Potential application of low-dose doxycycline to treat periodontitis in post-menopausal women. Adv Dent Res. 1998;12:166–169. doi: 10.1177/08959374980120011401. [DOI] [PubMed] [Google Scholar]
- 31.Payne JB, Reinhardt RA, Nummikoski PV, Golub LM. Abstract title. Doxycycline effects on oral bone loss in postmenopausal women. J Dent Res. 2001;80:55. [Google Scholar]
- 32.Looker AC, Bauer DC, Chesnut CH, 3rd, et al. Clinical use of biochemical markers of bone remodeling: Current status and future directions. Osteoporos Int. 2000;11:467–480. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 33.Sorsa T, Golub LM. Is the excessive inhibition of matrix metalloproteinases (MMPs) by potent synthetic MMP inhibitors (MMPIs) desirable in periodontitis and other inflammatory diseases? That is: ‘Leaky’ MMPIs vs excessively efficient drugs. Oral Dis. 2005;11:408–409. doi: 10.1111/j.1601-0825.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 34.Golub LM, Lee HM, Stoner J, et al. Bone turnover markers in postmenopausal-osteopenic women with periodontitis (POWP): Subantimicrobial-dose-doxycycline (SDD) J Dent Res. 2008;87(Spec Issue B) Abstract No. 3491. [Google Scholar]
- 35.Kim BJ, Yu YM, Kim EN, Chung YE, Koh JM, Kim GS. Relationship between serum hsCRP concentration and biochemical bone turnover markers in healthy pre-and postmenopausal women. Clin Endocrinol (Oxf) 2007;67:152–158. doi: 10.1111/j.1365-2265.2007.02853.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 37.Sapadin AN, Fleishchmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791–802. doi: 10.1016/j.jaad.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Skidmore R, Kovach R, Walker C, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- 40.O’Dell JR, Elliott JR, Mallek JA, et al. Treatment of early seropositive rheumatoid arthritis: Doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54:621–627. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]