Abstract
In the majority of human tumors, expression of the c-MYC oncogene becomes constitutive. Here, we report that c-MYC directly regulates the expression of AP4 via CACGTG motifs in the first intron of the AP4 gene. Induction of AP4 was required for c-MYC-mediated cell cycle reentry of anti-estrogen arrested breast cancer cells and mitogen-mediated repression of the CDK inhibitor p21. AP4 directly repressed p21 by occupying four CAGCTG motifs in the p21 promoter via its basic region. AP4 levels declined after DNA damage, and ectopic AP4 interfered with p53-mediated cell cycle arrest and sensitized cells to apoptosis induced by DNA damaging agents. AP4 expression blocked induction of p21 by TGF-β in human keratinocytes and interfered with up-regulation of p21 and cell cycle arrest during monoblast differentiation. Notably, AP4 is specifically expressed in colonic progenitor and colorectal carcinoma cells. In conclusion, our results indicate that c-MYC employs AP4 to maintain cells in a proliferative, progenitor-like state.
Keywords: colorectal cancer, DNA damage, p53, progenitor cells, TGF-beta
The proto-oncogene c-MYC is commonly activated in human cancer by gene amplification, viral promoter insertion, or chromosomal translocation but also because of mutations of upstream regulators (reviewed in ref. 1). c-MYC is highly expressed in proliferating cells and down-regulated when cells cease to proliferate, e.g., during differentiation. Deregulated c-MYC expression promotes cell proliferation and causes resistance to antimitogenic stimuli (2). Furthermore, constitutive expression of c-MYC sensitizes towards apoptosis (reviewed in ref. 3). The c-MYC gene encodes a transcription factor of the basic helix–loop–helix leucine-zipper (bHLH-LZ) class that binds to the E-box motif CACGTG (reviewed in ref. 4). However, the mechanisms that underlie the mitogenicity of c-MYC are only partially understood. It seems likely that the combined actions of multiple genes regulated by c-MYC contribute to the effects of c-MYC on proliferation (5).
The AP4 protein is a member of the bHLH-LZ subgroup of bHLH proteins, exclusively forms homodimers and binds to the E-box motif CAGCTG (6). Initially AP4 was shown to activate transcription (7). More recent studies documented that AP4 also represses viral and cellular genes (8–10). AP4 expression declines during murine brain development (9).
Here, we identified the AP4 gene as a direct transcriptional target of c-MYC, characterized the central cell cycle regulator p21 as an AP4 target gene and determined the cellular effects of AP4 activation.
Results
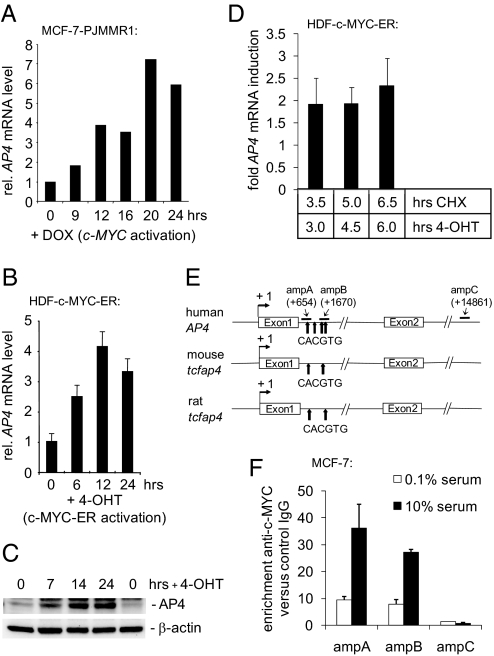
To identify genes regulated by c-MYC in human epithelial cells, we performed a microarray-based gene expression analysis 12 h after activation of c-MYC in MCF-7 breast cancer cells that had been arrested in the G1 phase by treatment with the anti-estrogen ICI182,780/Fulvestrant (ICI) (P.J. and H.H., unpublished results). Using this approach, we detected a 3.4-fold (P = 0.0027) induction of AP4 mRNA (data not shown), which was confirmed by quantitative real-time PCR (qPCR) (Fig. 1A). The increase in AP4 expression was also observed at the protein level [supporting information (SI) Fig. S1]. AP4 mRNA and protein were also induced after activation of a fusion protein consisting of c-MYC and the hormone-binding domain of the estrogen receptor (ER) in serum-starved human diploid fibroblasts (HDF) (Fig. 1 B and C). Furthermore, c-MYC-ER activation induced AP4 mRNA in the presence of the translation inhibitor cycloheximide, indicating that AP4 is directly transactivated by c-MYC (Fig. 1D). The regulation of AP4 by c-MYC is conserved among species, because AP4 expression was induced after activation of a c-MYC-ER fusion protein in serum-deprived RAT1 fibroblasts (Fig. S2). The first genomic intron of human AP4 contains a cluster of four canonical c-MYC-binding sites (CACGTG), two of which are conserved in mouse and rat (Fig. 1E). Stimulation of MCF-7 cells with serum, which increased c-MYC levels (see Fig. S3C), enhanced binding of c-MYC to a region containing three of the four E-boxes in the first intron of AP4 (ampA+B), as determined in a quantitative ChIP (qChIP) analysis (Fig. 1F). A minor binding of c-MYC to this region was detected in serum-starved MCF-7 cells, which express low levels of c-MYC (Fig. 1F; see also Fig. 3C), whereas a region (ampC) located ≈13 kbp downstream of the transcriptional start site in intron 6 of AP4 did not display occupation by c-MYC. Taken together, these findings establish that AP4 is an evolutionarily conserved direct c-MYC target gene.
Fig. 1.
Characterization of AP4 as a direct c-MYC target gene. (A) Quantification of AP4 mRNA after activation of c-MYC. MCF-7-PJMMR1 cells were treated with ICI (1 μM) for 60 h before activation of c-MYC by addition of doxycycline (DOX, 1 μg/ml) for the indicated periods, and RNA was subjected to qPCR analysis. (B) Quantification of AP4 mRNA after c-MYC activation. HDF-MYC-ER cells were serum-deprived for 48 h. After addition of 4-OHT (200 nM), total RNA was isolated at the indicated time points from biological triplicates. AP4 mRNA expression was determined by qPCR analysis. Error bars indicate standard deviations. (C) AP4 protein expression after c-MYC activation. Protein lysates were prepared from HDF-MYC-ER cells at the indicated time points. Expression of AP4 and β-actin was determined by immunoblotting. (D) HDF-MYC-ER cells were grown to confluence and treated with 4-OHT (200 nM) and CHX (70 nM), as indicated. The expression level of AP4 after combined CHX/4-OHT treatment was normalized to cells treated with CHX alone. Expression of AP4 and, for normalization, β-actin mRNA was determined by qPCR. Analyses were performed in triplicates. Error bars indicate standard deviations. (E) Comparison of the mouse, rat, and human AP4 promoter regions. “+1” indicates the transcription start site. “amp” indicates PCR amplicons used for qChIP analysis with their positions relative to the transcription start site. Arrows indicate the approximate positions of canonical c-MYC-binding sites (CACGTG). The positions of these sites relative to the transcription start site (“+1”) are +660, +1262, +1645, and +1766 for human AP4; +560 and +1620 for the mouse tcfap4; and +666 and +1725 for the rat tcfap4, respectively. (F) Detection of c-MYC at the AP4 promoter. MCF-7 cells were serum-starved (0.1% serum) for 48 h or restimulated (10% serum) for 12 h. Chromatin was cross-linked and subjected to qChIP analysis with a c-MYC-specific antibody and, as a control, rabbit IgG. qPCR analysis was performed with primers flanking three of the four canonical E-boxes in the first AP4 intron (“ampA” and “ampB”; see also Fig. 1C) or a control primer pair (“ampC”) localized in the last intron of AP4. For normalization, a fragment not containing E-boxes from chromosome 16q22 was used.
Fig. 3.
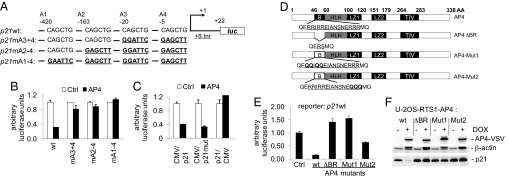
AP4 directly represses p21 expression via E-box motifs. (A) Schematic presentation of putative AP4-binding sites (A1-A4) and their position in the p21 promoter region relative to the transcriptional start site (+1). Wild-type and mutant p21 promoter constructs used in transient reporter assays are depicted. Mutated AP4-binding sites are represented in bold and underlined. The initiator (Inr) element (TCAGTTCCT) localizes to position + 8 to + 16 relative to the transcription start site (“+1”) (13). luc: ORF encoding the firefly luciferase. (B) Determination of p21 reporter activity in H1299 cells. Cells were transfected in 12-well plates with wild type or the indicated mutant p21 reporter plasmids, pcDNA3-AP4-VSV plasmid or equimolar amounts of pcDNA3-VSV backbone. Shown are the median expression values and standard errors of two independent transfection experiments. p21 prom. wt, mA3 + 4, mA2–4, and mA1–4: reporter plasmids encoding for the p21 promoter sequence with wild-type or mutant AP4-binding sites (see Fig. 3A). (C) The CMV/p21 reporter (nucleotides −49 to +16 of p21), a mutant version (CMV/p21mut) harboring substitutions of two nucleotides within three potential Miz1-binding sequences, or the p21/CMV reporter (nucleotides −94/-50 of p21) containing one E2F and four Sp1/3-binding sites (13) were cotransfected with pcDNA3-AP4-VSV plasmid or equimolar amounts of pcDNA3-VSV backbone in H1299 cells. Shown are the median expression values and standard errors of two independent transfection experiments. (D) Schematic representation of AP4 mutants. B: Basic region, HLH: helix–loop–helix, LZ1/2: leuzine zipper motif 1 and 2, TIV: conserved motif of unknown function containing the amino acid sequence TIV. The amino acid sequence of the basic region (underlined) and flanking residues are indicated for the wild type and mutant AP4 versions. Altered residues are represented in bold. (E) Effect of AP4 variants on p21 reporter activity in H1299 cells. Cells were transfected in 12-well plates with wild-type p21 reporter plasmid, pcDNA3-AP4-VSV plasmids (wild type or mutant AP4 versions) or equimolar amounts of pcDNA3-VSV backbone. Shown are the median expression values and standard deviations of three independent experiments. (F) Effect of AP4 variants on endogenous p21 expression. Expression level of AP4-VSV, p21, and β-actin proteins was detected by immunoblot analysis 24 h after induction of conditional wild-type or mutant AP4 alleles in U-2OS cells.
It was reported that ectopic expression of c-MYC abrogates a cell cycle arrest induced by the anti-estrogen ICI in MCF-7 cells (11). We could reproduce this observation, because c-MYC activation increased the fraction of cells in S-phase from ≈7% to ≈16% in the presence of ICI (Fig. 2A). When AP4 expression was down-regulated by RNA interference (RNAi) concomitantly with activation of c-MYC, the number of cells reentering the cell cycle and performing complete cell divisions was reduced (Fig. 2A, Fig. S3). Therefore, the induction of AP4 contributes to the c-MYC-mediated proliferation in the presence of ICI. Because the CDK inhibitor p21 is known to mediate G1-arrest induced by ICI (12), we determined whether expression of p21 is modulated by AP4. Indeed, down-regulation of AP4 by RNAi resulted in increased p21 levels in MCF-7 cells (Fig. 2B). Furthermore, serum stimulation of MCF-7 cells rapidly induced the expression of endogenous c-MYC followed by an increase in AP4, whereas expression of p21 was strongly suppressed (Fig. 2C). RNAi-mediated down-regulation of AP4 prevented the repression of p21 by serum addition (Fig. 2D). Therefore, induction of AP4, which is presumably mediated by c-MYC, is required for the down-regulation of p21 expression after serum stimulation of MCF-7 cells.
Fig. 2.
Effects of AP4 on cell cycle progression and p21 expression. (A) Flow cytometric analysis of ICI (1 μM)-treated MCF-7-PJMMR1 cells after siRNA-mediated down-regulation of AP4. c-MYC was activated by addition of DOX (1 μg/ml) for 22 h. The depicted diagram shows the percentage of cells in S-phase after treatment with ICI alone or in combination with c-MYC overexpression (ICI+MYC). The experiment was performed in duplicate. The standard error is depicted. (B) MCF-7 cells were transfected with two different siRNAs targeting AP4 or a nonsilencing control siRNA. Expression of AP4, p21 and β-actin was detected by immunoblot analysis. (C) MCF-7 cells were serum-deprived for 48 h, restimulated with 10% serum for the indicated periods, and analyzed by immunoblotting. (D) MCF-7 cells were transfected with siRNAs targeting AP4 or a nonsilencing siRNA. Thirty-six hours later, cells were serum-starved for 30 h. After restimulation with 10% FBS-containing medium for the indicated periods, cell lysates were subjected to immunoblot analysis. (E) Expression of AP4-VSV, p53, p21, and β-actin proteins was detected by immunoblot analysis after induction of a conditional VSV-tagged AP4 allele in U-2OS cells. (F) Quantification of p21 mRNA after activation of AP4 in U-2OS cells by qPCR analysis. Expression of p21 was normalized to β-actin expression. (G) The proximal promoter region of the human p21 gene contains four AP4 binding sites (CAGCTG). “+1” indicates the transcription start site. “amp:” PCR amplicons used for qChIP analysis with their position relative to the transcription start site. Positions of two p53-binding sites (p53BDS) are indicated. The approximate positions of four putative AP4-binding sites (arrows) and the initiator (Inr) element (TCAGTTCCT) (filled square) are indicated (their precise positions relative to the transcription start site of p21 is depicted in Fig. 3A). (H) qChIP analysis of AP4 at the p21 promoter. A conditional VSV-tagged AP4 allele was induced by addition of DOX (100 ng/ml) for 16 h in U-2OS cells. Genomic DNA coprecipitated with an anti-VSV or mouse IgG antibody was analyzed by qPCR. For normalization, a fragment on chromosome 16q22, not containing E-boxes was used.
Interestingly, the induction of a conditional AP4 allele decreased the amount of p21 mRNA and protein (Fig. 2 E and F and Fig. S4). The p21 promoter contains four E-boxes (5′-CAGCTG-3′) in the vicinity of its transcriptional start site, which were occupied by AP4 in vivo (Fig. 2 G and H). Expression of AP4 drastically reduced the activity of a wild-type p21 reporter construct, whereas mutation of the two proximal AP4-binding sites A3 and A4 was sufficient to largely alleviate the repressive effects of AP4 (Fig. 3 A and B). Mutation of all four binding sites completely prevented repression by AP4. Interestingly, mutation of putative Miz1-binding sites within a c-MYC-responsive p21 promoter region, which was reported to largely alleviate the positive effect of Miz1 on p21 expression (13), did not affect repression of p21 by AP4 (Fig. 3C, Fig. S5A). A p21 promoter construct containing one E2F and four Sp1/3-binding sites but no E-box (13), was not responsive to AP4 (Fig. 3C). Moreover, deletion or mutation of the AP4 basic region (Fig. 3D) rendered AP4 unable to repress a p21 reporter (Fig. 3E, Fig. S5B) and endogenous p21 protein in U-2OS cells (Fig. 3F). These results show that repression of p21 by AP4 occurs directly via the E-box motifs in the p21 proximal promoter and is not mediated by Miz1 or Sp1/3.
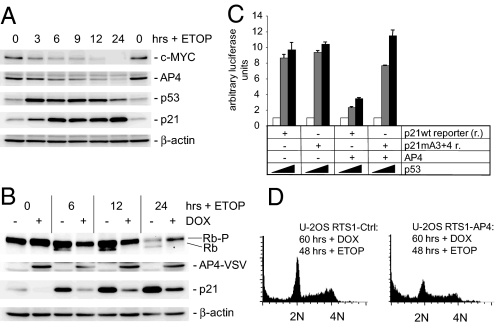
Because p21 is a central mediator of cell cycle inhibition by p53, we studied the potential involvement of AP4 in the DNA damage response. Treatment of MCF-7 cells with the topoisomerase II inhibitor etoposide reduced c-MYC and AP4 protein levels, whereas p53 and p21 levels increased (Fig. 4A). Induction of ectopic AP4 strongly interfered with p53-mediated transactivation of p21 after DNA damage and, presumably as a consequence of the diminished CDK inhibition, prevented the formation of hypophosphorylated pRb (Fig. 4B, Fig. S6A), which inhibits cell cycle progression by binding to E2F family members. Furthermore, AP4 suppressed the p53-mediated induction of a p21 reporter construct (Fig. 4C). This inhibitory effect of AP4 was alleviated by mutation of the two putative AP4-binding sites A3 and A4 in the p21 promoter (Fig. 4C), demonstrating that the suppression of p53-mediated induction of p21 by AP4 involves direct binding to E-boxes. Simultaneous treatment with etoposide and ectopic AP4 expression sensitized cells to apoptosis, as evidenced by the accumulation of sub-G1 cells (Fig. 4D, Fig. S6B). Furthermore, AP4 expression allowed cells to enter S phase in the presence of etoposide (Fig. 4D). Interestingly, these cells displayed extensive γ-H2AX staining after treatment with etoposide (data not shown). Therefore, the AP4-mediated repression of p21 presumably allowed cells to continue DNA replication in the presence of DNA damage. It was previously shown that loss of p21 allows cells to continue DNA replication in the presence of DNA damage (14–16), which could explain the sensitization of cells to apoptosis observed here. These results show that down-regulation of AP4 is a requirement for a coordinated DNA damage response.
Fig. 4.
Role of AP4 in the DNA damage response. (A) Effect of DNA damage on AP4 expression. MCF-7 cells were treated with etoposide (ETOP, 20 μg/ml) and cell extracts were obtained at the indicated time points. Expression of the indicated proteins was determined by immunoblotting. (B) Ectopic AP4-VSV was induced in U-2OS cells for 12 h by addition of DOX (100 ng/ml). Then ETOP (20 μg/ml) was added for the indicated periods. Expression of the differentially phosphorylated retinoblastoma protein (Rb-P/Rb), AP4-VSV, p21 or β-actin was detected by immunoblotting. (C) p21 reporter activity was determined in H1299 cells transfected with the indicated plasmids. Increasing p53 expression was achieved by transfection of 0, 50 or 200 ng of plasmids (indicated as  ). Shown are the median expression values and standard errors of two independent transfection experiments. p21 mA3 + 4: see Fig. 3A. (D) AP4 was induced by DOX for 12 h before treatment of cells with ETOP (20 μg/ml) for 48 h. Then cells were analyzed by flow cytometry. Depicted are exemplary histograms representing 10,000 cells. 2N: cells in G1, 4N: cells in G2/M.
). Shown are the median expression values and standard errors of two independent transfection experiments. p21 mA3 + 4: see Fig. 3A. (D) AP4 was induced by DOX for 12 h before treatment of cells with ETOP (20 μg/ml) for 48 h. Then cells were analyzed by flow cytometry. Depicted are exemplary histograms representing 10,000 cells. 2N: cells in G1, 4N: cells in G2/M.
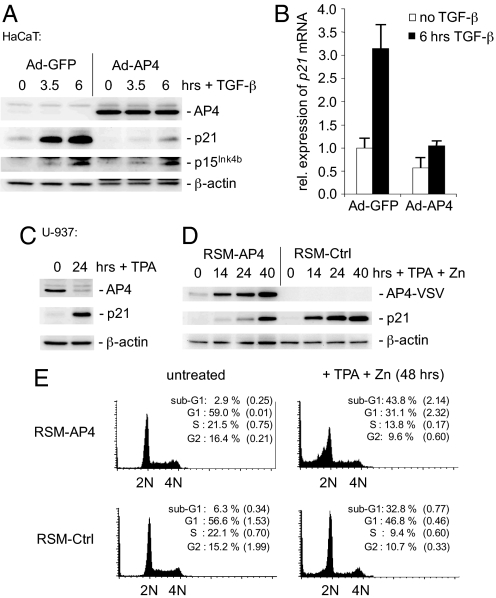
Constitutive expression of c-MYC blocks the induction of p21 by all members of the TGF-β superfamily (17). Therefore, we determined whether AP4 interferes with the TGF-β/Smad-mediated induction of p21. In HaCAT cells ectopic AP4 efficiently suppressed the increase of p21 protein and mRNA after exposure to TGF-β, whereas the induction of the CDK inhibitor p15Ink4b was not affected (Fig. 5 A and B). Similar results were obtained with an AP4-ER fusion protein (Fig. S7). Therefore, AP4 represents a candidate mediator of resistance to TGF-β caused by oncogenic activation of c-MYC.
Fig. 5.
AP4 antagonizes TGF-β and TPA mediated p21 induction. (A) HaCaT cells were infected with adenovirus encoding AP4 and eGFP (Ad-AP4) or eGFP alone (Ad-GFP). Twenty-four hours later, cells were treated with human, recombinant TGF-β (5 ng/ml) for the indicated periods. Expression of AP4-VSV, p21, p15Ink4b and β-actin was determined by immunoblotting. (B) Quantification of p21 mRNA in HaCaT cells infected with adenoviruses encoding either AP4 and eGFP or eGFP alone. Twenty-four hours after infection, cells were treated with human recombinant TGF-β (5 ng/ml) for 6 h. mRNA expression of p21 and β-actin was determined by qPCR analysis. The experiment was performed in duplicate. Error bars indicate standard errors. (C) U-937 cells were treated with 10 nM TPA (10 nM) for 24 h, and expression of AP4, p21, and, as a control for equal loading, β-actin was determined by immunoblotting. (D) U-937 RSM-AP4 or RSM-Ctrl cell pools were treated with 10 nM TPA and 100 μM zinc sulfate for the indicated periods. Expression of AP4-VSV, p21 and β-actin was determined by immunoblotting. (E) U-937 RSM-AP4 or RSM-Ctrl cells were treated with 10 nM TPA and 100 μM zinc sulfate for the indicated periods, and analyzed by flow cytometry. The experiment was repeated twice, and exemplary histograms representing 15,000 cells are shown. The percentage cell cycle distribution represent the average of two independent experiments. Standard errors are indicated. 2N: cells in G1, 4N: cells in G2/M.
Ectopic v-myc expression prevents 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced differentiation of the myelomonoblast cell line U-937 (18). Interestingly, c-MYC has been reported to block differentiation by interfering with the induction of p21 expression (13). Treatment of U-937 cells with TPA reduced the expression level of endogenous AP4 (Fig. 5C), presumably by down-regulation of c-MYC expression (SI Text and Fig. S8A). Simultaneously, the expression of p21 was induced. Interestingly, ectopic expression of an AP4 allele under control of a zinc inducible metallothionein-1 promoter (19) in U-937 cells interfered with TPA-mediated induction of p21 (Fig. 5D). However, at later time points a minor induction of p21 was observed (Fig. 5D). U-937 cells ectopically expressing AP4 failed to stably arrest in the G1-phase after TPA treatment and instead underwent apoptosis to a larger extent than control cells (Fig. 5E). Moreover, ectopic AP4 increased the fraction of U-937 cells undergoing DNA synthesis after treatment with TPA (Fig. S8B). Taken together, these results indicate that ectopic AP4 interferes with the cell cycle arrest, which is part of the terminal differentiation program of myelomonoblasts.
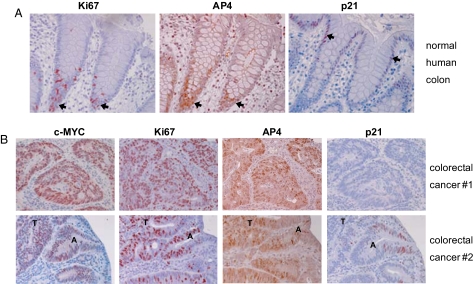
Immunohistochemical analyses revealed that the expression of AP4 protein is restricted to the base of human colonic crypts, which is populated by nondifferentiated, proliferating stem and progenitor cells, as evidenced by the proliferation marker Ki67 and the absence of p21 expression (Fig. 6A). The expression pattern of AP4 was identical to the pattern described for c-MYC (20, 21). In the differentiated upper part of colonic crypts AP4 expression was not detectable, whereas p21 expression increased toward the top of the crypts, which contains terminally differentiated cells (21–23) (Fig. 6A). These results suggest that AP4 may be involved in maintaining cells in a proliferative progenitor-like state. Primary colorectal carcinomas derived from 12 patients showed strong expression of AP4, which correlated with c-MYC and Ki67 protein expression in all cases analyzed (Fig. 6B, Fig. S9). Given the results described above, AP4 may represent an important mediator of c-MYC's oncogenic effects in colorectal carcinomas.
Fig. 6.
AP4 expression in human colon and colorectal cancer. (A) Section of an endoscopic biopsy derived from a normal colonic region. Consecutive paraffin sections were stained with antibodies directed against Ki67, AP4 or p21 (arrows indicate the presence of positive cells). Magnification: ×200. (B) Sections of endoscopic biopsies derived from primary tumors of two patients are depicted. Consecutive paraffin sections were stained with antibodies directed against c-MYC, Ki67, AP4, or p21. Identical results were obtained with colorectal carcinoma biopsies from 10 additional patients (see Fig. S9). A, adenomatous polyp (dysplastic); T, tumor. Magnification: ×200.
Discussion
In summary, our results establish AP4 as a c-MYC-inducible repressor of p21. In colorectal cancer c-MYC is generally deregulated because of mutations in the APC/β-catenin pathway (21, 24). Down-regulation of p21 consistently occurs during colorectal carcinogenesis (20, 25). Therefore, the AP4-mediated repression of p21 may have an important role in colorectal carcinogenesis. Interestingly, repression of p21 by c-MYC was also shown to play a critical role in anti-estrogen resistance during breast cancer therapy (26, 27).
The AP4 transcription factor forms a complex with geminin and the co-repressor SMRT that represses the human PAHX-AP1 gene through recruitment of histone deacetylase 3 (HDAC3) (9). Other studies indicate that AP4 may block access of the TATA-box-binding protein (TBP) to the TATA box (8, 28). However, further studies are warranted to determine the molecular mechanism through which AP4 represses transcription.
Several previous studies addressed the molecular basis of repression of p21 by c-MYC and multiple alternative mechanisms have been proposed (reviewed in ref. 29). One mode of repression of p21 by c-MYC occurs via interference with the transcription factor Miz1 (30). Because the c-MYC transactivation domain, which is dispensable for binding of c-MYC to Miz1, is essential for repression of p21, other factors beside Miz1 have been proposed to participate in the c-MYC-mediated repression of p21 (13). The c-MYC responsive region in the p21 promoter has been mapped between −49 and +16 (13) and overlaps with two of the four AP4-binding sites characterized here. Here, these two sites (A3+A4) were sufficient for p21 repression by AP4, and their mutation rendered the p21 promoter largely unresponsive to AP4. Mutations within putative Miz1-binding sites did not abolish p21 repression by AP4, therefore suggesting a Miz1-independent mechanism. Other authors provided evidence that repression of p21 by c-MYC may be mediated via interactions with Sp1/Sp3 and independently of the Inr sequence (31). Our results show that AP4 is not able to repress p21 via Sp1/Sp3 transcription factor-binding sites.
TGF-β signaling provokes cell cycle arrest in the G1-phase by inhibition of c-MYC and induction of the CDK inhibitors p15Ink4b and p21 (reviewed in refs. 32 and 33). We could demonstrate that AP4 interferes with TGF-β-mediated induction of p21. However, AP4-mediated repression of p21 did not prevent the TGF-β-mediated cell cycle arrest (data not shown). Presumably, regulation of other genes by TGF-β, as p15Ink4b or CDC25A (32, 34) is sufficient to mediate cell cycle arrest in HaCaT keratinocytes. Other functions normally mediated by TGF-β-induced p21, such as terminal differentiation (35), may be affected in cells with deregulated c-MYC and AP4 genes. Moreover, deregulation of AP4 may contribute to the morphogenetic changes observed during colon cancer formation by antagonizing the TGF-β pathway (36).
Down-regulation of c-MYC is required for terminal differentiation of many cell types (37). Elevated expression of c-MYC in the midgestational mouse is indicative of proliferation, whereas proliferative arrest and the onset of differentiation are accompanied by down-regulation of c-MYC (38). Decreased expression of c-MYC presumably explains the down-regulation of AP4 observed after TPA-mediated differentiation of myelomonoblasts and might also account for the decline of AP4 levels during the development of mouse brain observed by Kim et al. (9). Constitutive c-MYC expression blocks TPA-induced differentiation of U-937 cells by repressing p21 (13, 18). p21 itself plays an important role in monocytic differentiation and supports survival of differentiated cells by maintaining a stable cell cycle arrest (39, 40). In agreement with these findings, ectopic AP4 interfered with a stable G1-arrest and increased the fraction of TPA-treated cells in S-phase. This effect of AP4 is likely due to repression of p21 and the resulting increased CDK activity.
As p21 is a potent inhibitor of cyclin-dependent kinases; its repression by AP4 may contribute to the ability of c-MYC to activate CDKs (41–43). c-MYC-mediated repression of p21 was shown to modulate the response to DNA damage by favoring the initiation of apoptosis vs. cell cycle arrest (44). In line with this observation, we found that expression of AP4 sensitizes cells toward DNA damaging agents, which are commonly used in cancer therapy. Further analyses of the processes regulated by AP4 may allow to selectively increase the sensitivity of AP4-expressing cancer cells to therapeutic agents in the future.
Materials and Methods
The analyses were performed as described previously (45–48). For details, see the SI Text. This contains paragraphs describing plasmids and siRNAs, cell lines, cell culture and reagents, generation of cell lines, Western blot analysis and antibodies, cell-based reporter assays, qPCR, ChIP assays, BrdU labeling for detection of DNA synthesis, DNA content analysis by FACS, proliferation assay, indirect immunofluorescence, tissue samples and immunohistochemistry, and generation of recombinant adenoviruses and infection of target cells. Furthermore, tables listing oligonucleotides used for site-directed mutagenesis (Table S1), qPCR (Table S2), or qChIP analyses (Table S3) are provided.
Supplementary Material
Acknowledgments.
We thank Andrea Sendelhofert (Ludwig Maximilians University, Munich, Germany) for excellent assistance with immunohistochemistry; Dimitri Lodyguin (Max Planck Institute of Biochemistry, Martinsried, Germany), and Berlinda Verdoodt (Ruhr University Bochum, Germany) for technical help; Carme Gallego (University of Lleida, Spain), Lars Gunnar Larsson (Karolinska Institute, Stockholm), Georg Bornkamm (Helmholtz Center, Munich, Germany), and Dirk Eick (Helmholtz Center, Munich, Germany) for plasmids; Carla Grandori, Axel Ullrich (Max Planck Institute of Biochemistry, Martinsried, Germany), Lars Gunnar Larsson, and Dirk Eick for cell lines; Goeffrey Greene (University of Rhode Island) for antibodies; and Stephan Hahn (Ruhr University Bochum, Germany) and Manfred Köller (Ruhr University Bochum, Germany) for access to equipment. This work was supported by the Deutsche Krebshilfe and the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801773105/DCSupplemental.
References
- 1.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrow MG, Kawabata M, Aakre M, Moses HL. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor beta 1. Proc Natl Acad Sci USA. 1995;92:3239–3243. doi: 10.1073/pnas.92.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol. 2006;16:275–287. doi: 10.1016/j.semcancer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Dang CV, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Berns K, Hijmans EM, Koh E, Daley GQ, Bernards R. A genetic screen to identify genes that rescue the slow growth phenotype of c-myc null fibroblasts. Oncogene. 2000;19:3330–3334. doi: 10.1038/sj.onc.1203639. [DOI] [PubMed] [Google Scholar]
- 6.Hu YF, Luscher B, Admon A, Mermod N, Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990;4:1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- 7.Mermod N, Williams TJ, Tjian R. Enhancer binding factors AP-4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature. 1988;332:557–561. doi: 10.1038/332557a0. [DOI] [PubMed] [Google Scholar]
- 8.Imai K, Okamoto T. Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J Biol Chem. 2006;281:12495–12505. doi: 10.1074/jbc.M511773200. [DOI] [PubMed] [Google Scholar]
- 9.Kim MY, et al. A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. Proc Natl Acad Sci USA. 2006;103:13074–13079. doi: 10.1073/pnas.0601915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujimoto K, et al. Regulation of the expression of caspase-9 by the transcription factor activator protein-4 in glucocorticoid-induced apoptosis. J Biol Chem. 2005;280:27638–27644. doi: 10.1074/jbc.M501304200. [DOI] [PubMed] [Google Scholar]
- 11.Venditti M, Iwasiow B, Orr FW, Shiu RP. C-myc gene expression alone is sufficient to confer resistance to antiestrogen in human breast cancer cells. Int J Cancer. 2002;99:35–42. doi: 10.1002/ijc.10269. [DOI] [PubMed] [Google Scholar]
- 12.Varshochi R, et al. ICI182,780 induces p21Waf1 gene transcription through releasing histone deacetylase 1 and estrogen receptor alpha from Sp1 sites to induce cell cycle arrest in MCF-7 breast cancer cell line. J Biol Chem. 2005;280:3185–3196. doi: 10.1074/jbc.M408063200. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003;22:351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- 14.Brugarolas J, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 16.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 17.Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204:260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- 18.Larsson LG, et al. Phorbol ester-induced terminal differentiation is inhibited in human U-937 monoblastic cells expressing a v-myc oncogene. Proc Natl Acad Sci USA. 1988;85:2638–2642. doi: 10.1073/pnas.85.8.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen RD, et al. Metal-dependent binding of a factor in vivo to the metal-responsive elements of the metallothionein 1 gene promoter. Mol Cell Biol. 1987;7:3574–3581. doi: 10.1128/mcb.7.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansom OJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 22.Polyak K, Hamilton SR, Vogelstein B, Kinzler KW. Early alteration of cell-cycle-regulated gene expression in colorectal neoplasia. Am J Pathol. 1996;149:381–387. [PMC free article] [PubMed] [Google Scholar]
- 23.Doglioni C, et al. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: Its relationship with differentiation. J Pathol. 1996;179:248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 25.Sansom OJ, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 26.Carroll JS, Swarbrick A, Musgrove EA, Sutherland RL. Mechanisms of growth arrest by c-myc antisense oligonucleotides in MCF-7 breast cancer cells: implications for the antiproliferative effects of antiestrogens. Cancer Res. 2002;62:3126–3131. [PubMed] [Google Scholar]
- 27.Mukherjee S, Conrad SE. c-Myc suppresses p21WAF1/CIP1 expression during estrogen signaling and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2005;280:17617–17625. doi: 10.1074/jbc.M502278200. [DOI] [PubMed] [Google Scholar]
- 28.Ou SH, Garcia-Martinez LF, Paulssen EJ, Gaynor RB. Role of flanking E box motifs in human immunodeficiency virus type 1 TATA element function. J Virol. 1994;68:7188–7199. doi: 10.1128/jvi.68.11.7188-7199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 30.Herold S, et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell. 2002;10:509–521. doi: 10.1016/s1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 31.Gartel AL, et al. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci USA. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 33.Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Iavarone A, Massague J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 35.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 36.van den Brink GR, Offerhaus GJ. The morphogenetic code and colon cancer development. Cancer Cell. 2007;11:109–117. doi: 10.1016/j.ccr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Freytag SO. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988;8:1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid P, Schulz WA, Hameister H. Dynamic expression pattern of the myc protooncogene in midgestation mouse embryos. Science. 1989;243:226–229. doi: 10.1126/science.2911736. [DOI] [PubMed] [Google Scholar]
- 39.Asada M, Yamada T, Fukumuro K, Mizutani S. p21Cip1/WAF1 is important for differentiation and survival of U937 cells. Leukemia. 1998;12:1944–1950. doi: 10.1038/sj.leu.2401228. [DOI] [PubMed] [Google Scholar]
- 40.Asada M, et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steiner P, et al. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermeking H, Funk JO, Reichert M, Ellwart JW, Eick D. Abrogation of p53-induced cell cycle arrest by c-Myc: Evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene. 1995;11:1409–1415. [PubMed] [Google Scholar]
- 43.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 44.Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 45.Jung P, et al. Induction of cullin 7 by DNA damage attenuates p53 function. Proc Natl Acad Sci USA. 2007;104:11388–11393. doi: 10.1073/pnas.0609467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarasov V, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 47.Menssen A, et al. c-MYC Delays Prometaphase by Direct Transactivation of MAD2 and BubR1: Identification of Mechanisms Underlying c-MYC-Induced DNA Damage and Chromosomal Instability. Cell Cycle. 2007;6:339–352. doi: 10.4161/cc.6.3.3808. [DOI] [PubMed] [Google Scholar]
- 48.Hermeking H, et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.