Abstract
Remote optical imaging of human tissue in vivo has been the foundation for the growth of minimally invasive medicine. Improvements in endoscopic technology are critical for advancing the field of gastroenterology. In this article, a completely new type of endoscopic imaging has been developed and applied to the human esophagus, pig bile duct, and mouse colon. The technology is based on a single optical fiber that is scanned at the distal tip of an ultrathin and flexible shaft that projects red, green, and blue laser light onto tissue in a spiral pattern. The resulting images are high-quality color video (high-resolution and wide field of view) which is expected to produce future endoscopes that are thinner, longer, more flexible, and able to directly integrate the many recent advances of laser diagnostics and therapies.
1. State of the Art in GI Endoscopy
Since the early 19th century, endoscopic techniques and clinical procedures have developed in synchrony with technological advances and the advent of new medical imaging tools. The use of endoscopic tools and devices invented during this time have allowed for an increasing number of minimally invasive imaging, diagnostic and surgical procedures. A brief timeline outlining key developments of the endoscope is shown in Figure 1, which has been expanded from Berci and Forde [1] and Sivak [2]. The development of the endoscope shows a trend: smaller, more capable devices allowing for improved disease diagnosis and surgical intervention more deeply and into more organs, with the goals of improved patient outcomes at reasonable cost.
Figure 1.
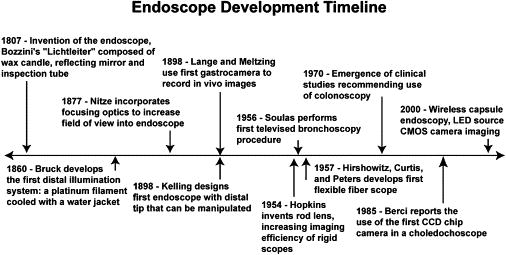
Timeline of technological innovations in endoscopy and minimally invasive surgery
Current techniques for endoscopic disease diagnoses throughout the GI system are based upon the use of white light endoscopy in combination with biopsy. These GI imaging and diagnostic techniques were enabled with innovations from the 1950s. Chief among endoscopic developments at this time were the use of television equipment for the acquisition of in vivo images, the Hopkin’s rod lens system resulting in modern laproscopic imaging devices, and Hirshowitz’s development of the flexible fiberscope for gastro-endoscopy. From these inventions, the combination of in vivo imaging and tissue state diagnosis from biopsy samples has yielded high sensitivity and specificity for disease diagnosis in accessible organs. Sivak notes that following the rapid pace of innovation in the field of endoscopy from the 1950s to the 1980s, the pace of innovation has slowed [2].
The pace of innovation may yet accelerate again as investigators are pursuing advances in four areas: (1) accessing organs that are too small for current endoscopes or are distant from gastrointestinal orifices – such as the pancreas [3, 4], upper bilary tree [5], and small intestine [6]; (2) improving the speed of disease diagnosis and selection of biopsy sites during a clinical imaging procedures using non-invasive optical spectroscopic techniques [7]; (3) improving the ability to deliver therapy during a clinical procedure to diseased tissue and; (4) reducing the cost of endoscopic tools and time from disease diagnosis to surgical intervention for clinical procedures, thus allowing for a reduction of total cost for the patient and health care system and possibly wider availability of this medical care.
The last 20 years have seen the introduction of a number of new endoscopic imaging modalities that have sought to improve the clinician’s ability to diagnose disease and image difficult to access organs. Some of these techniques include: high magnification endoscopy, high definition endoscopy, fluorescence and hyperspectral endoscopy, light scattering spectroscopy, endoscopic optical coherence tomography, narrow band imaging, confocal endoscopy, capsule endoscopy, double balloon enteroscopy, endocytoscopy, and cholangioscopy. These imaging modalities, to be useful, need to achieve diagnostic sensitivity and specificity comparable to that of the current white light imaging and biopsy techniques while being inexpensive and capable of being used by current endoscopic clinicians. The scanning fiber endoscope (SFE) is a new technology developed specifically for clinicians with the objective of matching the high-quality imaging provided by current endoscopes at 1/3 the size. In addition to being able to image previously inaccessible regions of the body, the SFE is a platform on which to build many of the new optical diagnostic techniques listed above. In this paper, the basic technology of SFE imaging is presented along with three examples, (1) tethered-capsule endoscope for unsedated imaging of the esophagus, (2) ultrathin and flexible endoscope for imaging the bile and pancreatic ducts, and (3) proof-of-concept study of combining the SFE with autofluorescence detection of disease in the colon.
2. Scanning Fiber Endoscope (SFE)
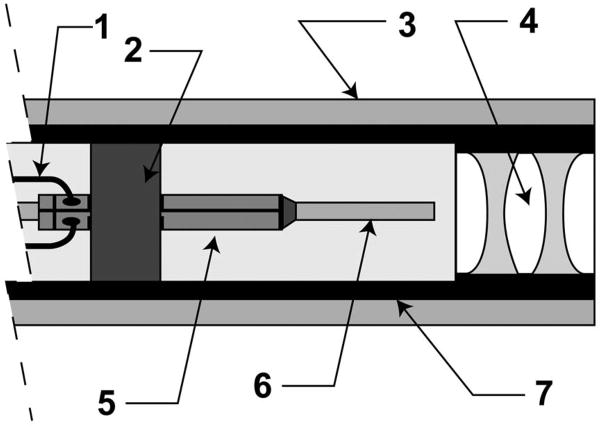
SFE technology shows promise as an ultrathin and flexible endoscope resembling a catheter in shape and size[7, 8]. However, the means for imaging are completely different from all white-light endoscopes which rely on one optical fiber or image sensor per display pixel. Instead, the SFE scans red, green, and blue laser light that is focused onto the tissue and the backscattered light is collected by several optical fibers and the image is generated by a computer one-pixel-at-a-time. Although only narrow bands of light are projected onto the tissue, the backscattered red at 635 nm, green at 532 nm, and blue at 440 nm provides excellent color fidelity. The distal tip of the SFE probe is shown as a schematic drawing in Figure 2, consisting of a cantilevered single-mode optical fiber, a tube piezoelectric actuator at the base of this cantilever and focusing lenses distal to the tip of this cantilevered optical fiber. The scanned illumination light is collected by multi-mode optical fibers surrounding the fiber scanner and lens assembly.
Figure 2.
Distal tip of a SFE probe in cross-section view (vertical expanded 2X more than horizontal for viewing): 1. tube piezoelectric drive signal wiring, 2. piezoelectric mount collar, 3. ring of light collection multimode optical fibers, 4. illumination lens package, 5. piezoelectric tube actuator, 6. scanning single mode optical fiber as a base-excited cantilever, 7. stainless steel tube enclosure. Current size of the rigid distal tip is 1.2 mm in outer diameter and 8 to 10 mm in length for fiber scanning at 30 frames per second at 500-lines per color image. Side-viewing is possible by adding 90-degree reflection at the tip.
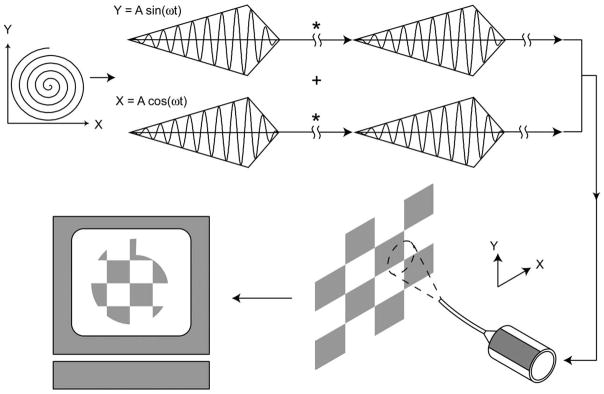
Light is projected from the cantilevered optical fiber while it is actuated at its first mode of mechanical resonance to create a scan. Two dimensional (2D) scan patterns are generated in a SFE using a piezoelectric tube actuator with quartered electrodes. A circular scan can be generated when the horizontal and vertical resonant vibrations of the scanning fiber have the same frequency, but are 90 degrees out of phase. With axially-symmetric scanning fibers, actuating a fiber at its resonant frequency allows for large fields of view in two dimensions. Modulating the amplitude of the drive signal allows for creation of a spiral scan pattern and 2D image acquisition. An illustration of a simplified spiral scan system for image acquisition is shown in Figure 3. The projected light is focused with a lens system onto an imaged surface. Light backscattered or fluorescing from the imaged surface is detected pixel-by-pixel to form an image. An example of a sequence of detected images is shown in Figure 4. These images demonstrate that by changing the amplitude of the drive signal input into the piezo scanner, the field-of-view (FOV) of the SFE can be altered. Decreasing the FOV of the scanning can increase the display resolution of the SFE. An advantage of this method of image acquisition is that the resolution of the acquired image is not limited by the number of detectors (optical fibers or camera sensor array) in the flexible endoscope. This allows for small device size, large FOV and resolution that is limited by the lens assembly and not the number of sensor elements.
Figure 3.
Scan method of the SFE. A piezoelectric tube is driven with a sinusoid where the X and Y axes are 90 degrees out of phase while the signal amplitude is modulated. This results in a space-filling spiral scan. Backscattered light measured by the detector at each pixel location is assembled to form an image displayed on a screen. Between frames (*) the fiber scanner is brought to rest and a spectroscopic measurement can be made to diagnose tissue or high-power laser light can be turned on for laser therapy in a frame-sequential manner.
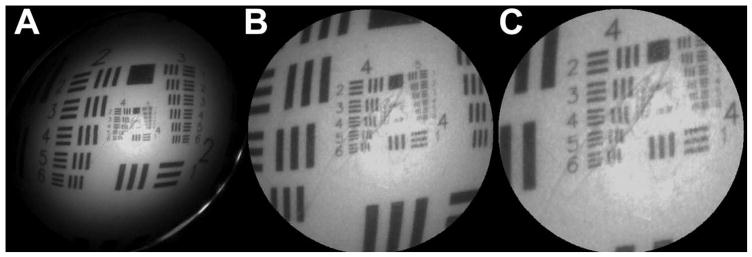
Figure 4.
Images of a US Air Force standard resolution target acquired with a monochrome SFE. Figures 4A–C shows an image was acquired with a 30Volt, 15V and 5V scan, respectively. The scanning fiber system acquired these images at 30 Hz, at 250 spirals per scan or 500 lines per image. The SFE used to acquire these images is 1.2 mm in diameter, with a single ring of 50 μm glass multimode fibers used to collect the backscattered light to photomultiplier detectors, and a PENTAX lens assembly was used to focus laser light projected from the scanning fiber. The bar-space patterns on the image are separated by 88.3 μm at location 2–4, 27.9 μm at location 4–2, and by 13.9 μm at location 5–2, top right in 4C. Note there is an ovular indentation marked on the test target that is visible in the magnified images.
3. Tethered-Capsule Endoscopic (TCE) Imaging of Human Esophagus
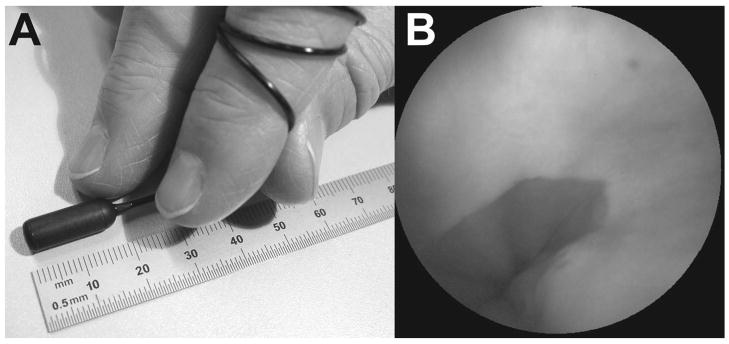
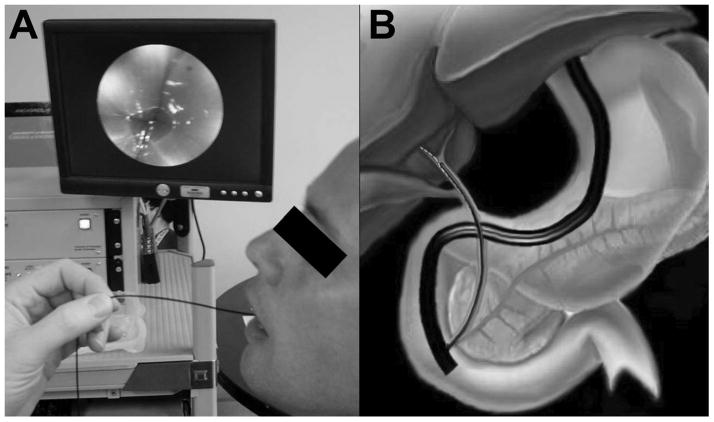
To allow the patient to swallow the SFE probe for unsedated imaging of the esophagus, a capsule 18 mm in length and 6.4 mm in diameter was placed around the rigid distal tip of the SFE probe, as shown in Figure 5A. The 1.4 mm diameter tether is extremely soft and flexible allowing the patient to swallow the TCE probe with a cup of water, see Figure 6A. One resulting image of the gastroesophageal junction is shown in Figure 5B. The maximum FOV of this forward viewing device was measured to be 118 degrees. A detailed description of the TCE probe and imaging system for the purpose of screening for esophageal cancer and Barrett’s esophagus is given by Seibel et al. [9]. The long term goal of this project is to provide a low-cost technology solution to the projected high cost of screening programs for reducing mortality from esophageal cancer. The TCE may also have a role in screening patients with cirrhosis for esophageal varices or in the evaluation of patients with esophageal symptoms. The issue of reusability of the TCE probe is currently being investigated, though disposability is also an option as the SFE components are relatively low in cost.
Figure 5.
5A. TCE probe 5B TCE probe color image of the author’s gastroesophageal junction.
Figure 6.
6A. TCE probe being withdrawn from the first human subject with live video image being displayed above the TCE base station in background. 6B Illustration of a duodenoscope launching a SFE probe into the human bile duct, artwork by Duff Hendrickson.
4. Ultrathin Endoscopic Imaging of Pig Bile Duct
The small size of the SFE makes is suitable for optical imaging inside the biliary tree and main pancreatic ducts. Though several endoscopic devices have been developed for direct optical imaging of these organs, these devices have yet to gain widespread acceptance because of their inferior image quality, expense, and fragility (e.g. minimum bend radius of fiberoptic image bundles is typically greater than 30 mm). To make the SFE probe compatible with endoscopic retrograde cholangiopancreatography (ERCP) guidewire insertion, the SFE probe tip had two small wire loops to hold the 0.0225 inch diameter guidewire and the flexible shaft was extended to four meters in length. Figure 6B shows an illustration of a SFE probe launched from the working channel of a duodenoscope into the bile duct of a human.
4.1 Bile Duct Imaging Experimental Protocol
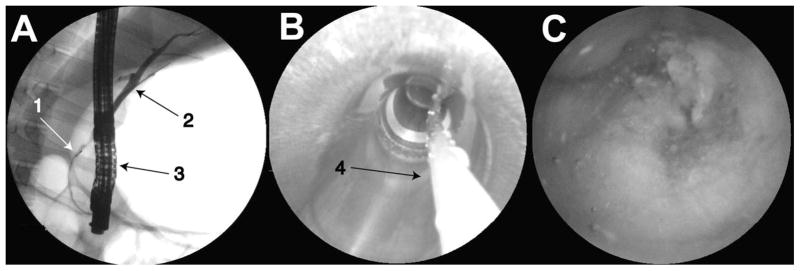
A therapeutic duodenoscope (ED-3470TK, PENTAX Corp., Tokyo, Japan) was introduced into the esophagus of an anesthetized farm pig and passed into the duodenum. The bile duct orifice was located and cannulated with a sphincterotome. This procedure was completed by manipulating a sphincterotome (D.A.S.H., Cook Medical Inc., Bloomington, IN) powered with an electrocautery unit (Bard 3000, C. R. Bard Inc., Murray Hill, NJ) into the entrance to the bile duct, and performing a sphincterotomy using pure cut current. Following the cutting operation, we inserted a guide wire and injected a radio dye contrast agent, iothalamate meglumine (Conray-60, Mallinckrodt Medical, St. Louis, MO), into the bile duct. While holding the guidewire in place, the sphincterotome was removed and the SFE probe was launched over the guide wire through the duodendoscope’s working channel into the bile duct. Care was taken to avoid putting pressure on the tip of the SFE with the elevator of the duodenoscope. Imaging was performed by slow withdrawal of the SFE probe from the bile duct, while modifying its position within the organ by making small adjustments of the duodenoscope. Figure 7 shows representative images acquired during this procedure. We believe that the SFE can be a useful tool for clinicians to optically image inside the bile and main pancreatic ducts. This tool is compatible with current endoscopic imaging practices and techniques inside these organs.
Figure 7.
7A. Image from fluoroscope showing (1) SFE probe, (2) bile duct, and (3) duodenoscope, 7B SFE image of the duodenoscope working channel and (4) guide wire, 7C SFE image of the pig bile duct.
5. Laser-Induced Fluorescent Imaging
In recent years, laser induced fluorescent (LIF) imaging has been a popular technique for tissue diagnosis. LIF imaging uses low wavelength light as an excitation source, stimulating autofluorescent emission from endogenous fluorophores in tissue. Detection of the autofluorescent light provides insight into the biochemistry and microarchitecture of imaged tissues and can be used to distinguish between healthy and diseased tissue in both single-point acquisition and imaging devices. Investigators have used various UV or visible light sources, illumination fibers, dichroic filters and beam splitters, and fibers or CCD arrays for real-time in vivo endoscopic imaging [10–14]. These systems have been used primarily to image the human colon and central bronchial tree due to the large size of the optics and hardware housed in the endoscope. This technique is a popular choice for tissue diagnosis because it relies upon well known technologies, is similar to currently employed endoscopic imaging procedures, and does not require the addition of exogenous reporter dyes to acquire diagnostic information about tissue state. Due to the sharp bending of the shaft required to reach the pancreatic and bile ducts, semi-flexible fiberoptic imaging bundles are not a clinical option, while the SFE may be an ideal solution. An SFE with LIF has been developed based upon ex vivo LIF spectroscopic analysis using an inflammatory bowel disease (IBD) model for the detection of pre-cancerous to cancerous progression [15].
We have extended the capability of the SFE from a RGB reflectance imaging device to a diagnostic tool by imaging laser induced fluorescence (LIF) in tissue, allowing for correlation of endogenous fluorescence to tissue state. Design of the SFE for diagnostic imaging was guided by a comparison of single point spectra acquired from an IBD model to tissue histology evaluated by a pathologist.
5.1 Experimental protocol for correlating LIF with disease state of colon
LIF spectra were acquired by illuminating tissue with a 405 nm light source and detecting intrinsic fluorescence with a multimode optical fiber. The IBD model used in this study was mdr1a−/− mice, where IBD was modulated by infection with Helicobacter bilis. It has previously been shown that infection of mdr1a−/− mice with Helicobacter can trigger IBD, and this mouse model has been used as an animal model of ulcerative colitis, a form of IBD afflicting humans [16, 17]. Though mdr1a−/− mice have been shown to spontaneously develop colitis, a species of Helicobacter that infects rodents - H. bilis - has been used to accelerate the development of IBD in our mouse model. The chief advantages of this mouse model are that it develops gradations of inflammation and dysplasia throughout the length of its colon as soon as three weeks following an infection modulated with H. bilis. IBD lesions in the mouse model ranged from mild to marked hyperplasia and dysplasia, from the distal colon to the caecum. Figure 8A illustrates a dissected mouse colon and caecum while Figure 8B shows the location of the spectra samples in the mice.
Figure 8.
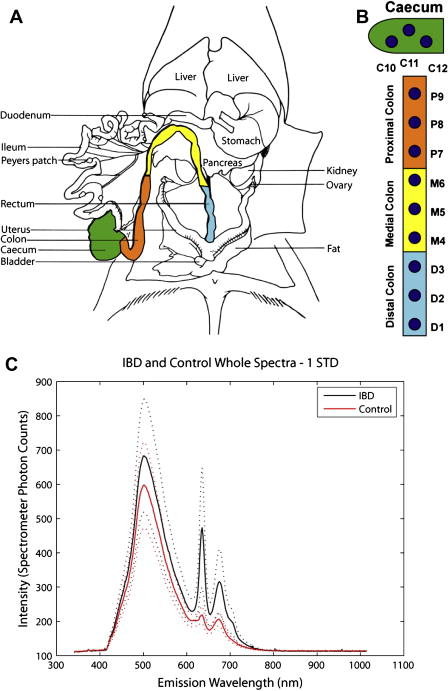
8A. Illustration of dissected mouse abdominal cavity, showing distal, medial, and proximal colon as well as caecum 8B. Sample sites along mouse colon 8C. Average IBD and control tissue spectra. Note that every 5th variable is plotted in this figure, and the dashed lines represent one standard deviation of acquired spectral data.
A principle components analysis (PCA) was conducted on single point spectra of control and IBD tissue. PCA allowed for differentiation between healthy and dysplastic tissue, indicating that emission wavelengths from 620 – 650 nm were best able to differentiate diseased tissue and inflammation from normal healthy tissue. The difference between the average IBD sample and the average control sample can be seen in Figure 8C. Note the difference in average peak heights in the 600–700 nm spectral region. The data indicates that detected fluorescent intensity can change as much as 10X between spectral samples, and generally there are three fluorescent peaks, a broad beak between 420–600 nm, and sharper peaks between 620–650 nm and 650–700 nm.
PCA results show that increased fluorescent intensity in the spectral region from 620–650 nm indicates a higher probability of diseased tissue while fluorescent intensity from 420–600 nm is relatively independent of tissue state [15]. We are currently designing a SFE device to image fluorescent light in both spectral regions, and compare the returned fluorescent intensities of these two spectral regions to acquire an image of tissue disease state in vivo.
6. Discussion and Conclusions
To summarize these preliminary results of testing the SFE technology, the TCE imaging of the human esophagus demonstrates the potential for a low-cost screening application for esophageal pathology, such as Barrett’s esophagus or esophageal varices. In the bile duct of the pig, the 4-meter long SFE probe demonstrates the potential for a longer and more flexible babyscope, extending the use of endoscopy to previously inaccessible regions of the body. At 1.2 mm in outer diameter and a shaft of calibrated stiffness, the SFE probe could simply be a “guidewire with eyes” and replace the step of inserting a metal guidewire and possibly eliminate the requirement for fluoroscopy. In both imaging applications, no working channel was developed for the SFE probe. However, if the SFE probe is used like a catheter then interventional tools can be co-axial or side-by-side with the guidewire, such as a cannula brush, a cannula needle, or a cannula-style forceps.
With the advent of many different laser diagnostics, the SFE provides a platform to add image-guided disease diagnosis and selection of biopsy sites. The integration of LIF in an ultrathin SFE probe allowed autofluorescence spectroscopy of a mouse model of disease. Using the feature of interrupting the fiber scanning in a frame-sequential manner as illustrated in Figure 3, a spot optical biopsy measurement can be acquired at the center of the imaged field. Previously, we have demonstrated that high intensity laser light - 35 mW at 405 nm - can be delivered through our single-mode optical fiber and focused to perform hole drilling [18]. This indicates that future versions of the SFE may prove useful for delivery of in vivo laser therapy such as hole drilling, laser cutting, and photodynamic therapy as well as laser diagnostics in a frame sequential manner with imaging. Thus, the SFE technology promises to image of both high-resolution and wide field of view and provide real-time magnification while also being able to be thinner, longer, more flexible, and possibly manufactured at lower cost than current endoscopes. The SFE laser scanned imaging technology is compatible with many of the advanced imaging technologies that have emerged since the start of the 21st century, and may improve patient outcomes and both expand and reduce the cost of endoscopic procedures in the years ahead.
Acknowledgments
Special thanks to the SFE Team of engineers at the University of Washington who have designed and built the prototype systems: Rich Johnston, Dave Melville, Cameron Lee, Ryland Bryant, Janet Crossman-Bosworth, and Bernie Murray. Animal testing was conducted in the laboratories of Dr. R. Glenny and Dr. L. Maggio-Price. Funding has been provided by the National Cancer Institute through grants CA094303 and CA110184, and the PENTAX Corporation, Tokyo, Japan.
Financial support was provided to Eric Seibel by NIH/NCI and the PENTAX Corporation, Tokyo, Japan. Chris Brown was supported only by NIH NCI grants CA094303 and CA110184. Jason Dominitz was not supported and Michael Kimmey was supported in part by NIH NCI CA094303, PI-Eric Seibel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eric J. Seibel, University of Washington, Box 352600, Seattle, WA 98195, USA, voice: (206) 616-1486, fax: (206) 685-8047, eseibel@u.washington.edu, http://www.me.washington.edu/people/faculty/seibel/.
Christopher M. Brown, University of Washington, Box 352600, Seattle, WA 98195, USA, voice: (206) 616-5743, fax: (206) 685-8047, seebrown@u.washington.edu.
Jason A. Dominitz, University of Washington School of Medicine, Director, Northwest Hepatitis C Resource Center, VA Puget Sound Health Care System, 1660 S. Columbian Way (111-Gastro), Seattle, WA 98108, (206) 764-2285, fax (206) 277-4495, Jason.Dominitz@va.gov.
Michael B. Kimmey, UW Medical Center, Clinical Professor Medicine, University of Washington, Seattle, WA, USA, (206) 543-4404, FAX: 206 685-8684, kimmey@u.washington.edu.
References
- 1.Berci G, Forde KA. History of endoscopy: what lessons have we learned from the past? Surg Endosc. 2000;14(1):5–15. doi: 10.1007/s004649900002. [DOI] [PubMed] [Google Scholar]
- 2.Sivak MV. Gastrointestinal endoscopy: past and future. Gut. 2006;55(8):1061–4. doi: 10.1136/gut.2005.086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kodama T, Tatsumi Y, Sato H, Imamura Y, Koshitani T, Abe M, Kato K, Uehira H, Horii Y, Yamane Y, Yamagishi H. Initial experience with a new peroral electronic pancreatoscope with an accessory channel. Gastrointest Endosc. 2004;59(7):895–900. doi: 10.1016/s0016-5107(04)01272-6. [DOI] [PubMed] [Google Scholar]
- 4.Nichols MT, Russ PD, Chen YK. Pancreatic imaging: current and emerging technologies. Pancreas. 2006;33(3):211–20. doi: 10.1097/01.mpa.0000227912.71202.2c. [DOI] [PubMed] [Google Scholar]
- 5.Brugge WR. Endoscopic techniques to diagnose and manage biliary tumors. J Clin Oncol. 2005;23(20):4561–5. doi: 10.1200/JCO.2005.19.729. [DOI] [PubMed] [Google Scholar]
- 6.Iddan GMG, Glukhovsky A, Swain P. Wireless Capsule Endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 7.Seibel EJ, Johnston RS, David Melville C. Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications VI, Proceedings of SPIE. Vol. 6083. 2006. A full-color scanning fiber endoscope; p. 608303. [Google Scholar]
- 8.Seibel EJ, Smithwick QY. Unique features of optical scanning, single fiber endoscopy. Lasers Surg Med. 2002;30(3):177–83. doi: 10.1002/lsm.10029. [DOI] [PubMed] [Google Scholar]
- 9.Seibel EJ, Dominitz JA, Johnston RS, Melville CD, Lee CM, Seitz SM, Kimmey MB. Tethered Capsule Endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. Trans Biomed Engr. doi: 10.1109/TBME.2008.915680. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Chiyo M, Shibuya K, Hoshino H, Yasufuku K, Sekine Y, Iizasa T, Hiroshima K, Fujisawa T. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer. 2005;48(3):307–13. doi: 10.1016/j.lungcan.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Goujon D, Zellweger M, Radu A, Grosjean P, Weber BC, van den Bergh H, Monnier P, Wagnieres G. In vivo autofluorescence imaging of early cancers in the human tracheobronchial tree with a spectrally optimized system. J Biomed Opt. 2003;8(1):17–25. doi: 10.1117/1.1528594. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda N, Honda H, Hayashi A, Usuda J, Kato Y, Tsuboi M, Ohira T, Hirano T, Kato H, Serizawa H, Aoki Y. Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung Cancer. 2006;52(1):21–7. doi: 10.1016/j.lungcan.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang TD, Crawford JM, Feld MS, Wang Y, Itzkan I, Van Dam J. In vivo identification of colonic dysplasia using fluorescence endoscopic imaging. Gastrointest Endosc. 1999;49(4 Pt 1):447–55. doi: 10.1016/s0016-5107(99)70041-6. [DOI] [PubMed] [Google Scholar]
- 14.Zeng H, Weiss A, Cline R, MacAualy CE. Real-time endoscopic fluorescence imaging for early cancer detection in the gastrointestinal tract. Bioimaging. 1998;6(4):151. [Google Scholar]
- 15.Brown CM, Maggio-Price L, Seibel EJ. Laser induced fluorescence as a diagnostic tool integrated into a scanning fiber endoscope for mouse imaging; Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications VII, Proceedings of SPIE; 2007. p. 64330. [Google Scholar]
- 16.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166(6):1793–806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161(10):5733–44. [PubMed] [Google Scholar]
- 18.Tuttle BW, Seibel EJ. Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications VI, Proceedings of SPIE. Vol. 6083. 2006. Delivery of therapeutic laser light using a singlemode silica fiber for a scanning fiber endoscope system; p. 608307. [Google Scholar]