Abstract
Many behaviors of interest to neurophysiologists are difficult to study under laboratory conditions because such behaviors are often inhibited when an animal is restrained and socially isolated. Even under the best conditions, such behaviors may be sparse enough as to require long duration neural recordings or simultaneous recording of multiple neurons to gather a sufficient amount of data for analysis. We have developed a preparation for chronic, multi-electrode recordings in the auditory cortex of marmoset monkeys, small primates, as well as techniques for neurophysiological recordings when the animals are free-roaming while singly caged in the environment of the monkey colony. In this report, we describe our solutions to overcome the problems associated with chronic recordings in free-roaming animals, where three-dimensional movements present particular challenges.
Keywords: implanted electrodes, multi-electrode arrays, chronic recording, free-roaming, marmoset, auditory cortex
1. Introduction
Much of our current understanding of neural mechanisms in the brain is based on data obtained using acute single-electrode recording techniques. There has been growing interest in the use of chronically-implanted multi-electrode arrays in multiple fields of investigation and the last few years have seen an explosion in the variety and capability of these devices (Nicolelis and Ribeiro, 2002; Buzsaki, 2004). The increased usage of implanted electrode arrays has been due to their numerous advantages, including increased yield and recording stability. More importantly, these devices have also introduced the ability to study ensembles of simultaneously-recorded neurons and the ability to record during complex behaviors.
Our interest in implanted electrode recordings arose primarily from the desire to study the neural basis of vocal behavior in freely-roaming marmoset monkeys. Many animal behaviors of interest, in particular the untrained natural ones, cannot be studied in classical preparations because animals do not perform such behaviors when restrained. Spatial exploration by rats, and the subsequent development of hippocampal place maps, is one such behavior that has taken advantage of implanted electrodes (O’Keefe and Dostrovsky, 1971; Wilson and McNaughton, 1993); song production in songbirds is another example (McCasland, 1987; Fee and Leonardo, 2001). We have been interested in studying the neurophysiology of vocal production in non-human primates (Eliades and Wang, 2003), a social behavior that is severely attenuated when the animal is restrained. The techniques presented here were developed to study the auditory cortex of free-roaming marmoset monkeys (Callithrix jacchus), a small vocal primate species, including: 1) implantable multi-electrode arrays appropriate for use in small, highly mobile animals, 2) methods to protect these arrays from damage during long durations of use, and 3) methods to record from the electrode arrays while the animals moved freely in a cage.
2. Materials and methods
2.1. Animal preparation
Marmosets are a small (~400 g) New World primate species. Natively arboreal, marmosets are particularly active and readily climb about their environment. Marmosets are highly vocal in the wild as well as captivity (Aitkin and Park, 1993; Pistorio et al., 2006), when housed in a colony, but far less so when restrained in the laboratory. Marmosets have been used extensively in our laboratory for acute, single-electrode recordings in awake conditions for a number of years (Lu et al., 2001a,b; Wang et al., 2005). The use of implanted electrode arrays was adapted from these methods.
Prior to placement of electrode arrays, an acrylic head cap is implanted on each animal. The methods used to create this head cap have been previously documented (Lu et al., 2001b), and are briefly summarized here. Under general endotracheal anesthesia, and using aseptic technique, the skin and underlying muscle are excised from the top and sides of the skull, down to the level of the zygoma. Ten small stainless steel screws are inserted along the thickened ridges of the skull. Two custom-machined stainless steel head posts are placed along the midline. These posts will later be used for restraint of the animal. The bulk of the head cap is created using dental acrylic covering the screws and the base of the head posts. A single screw, longer than the rest, is left with its top uncovered and is used as a ground during recording. The dental acrylic is poured to a thickness of approximately 8–10 mm. The areas overlying the auditory cortex, bilaterally, are covered with only a thin (~1 mm) layer of acrylic for easy access later. Following surgery, animals are carefully recovered over a period of 4 weeks and are generally ready for neural recordings after 2–4 weeks.
Animals are fully recovered from the head cap surgery prior to placement of the implanted electrode array. The separation of the two procedures has a number of advantages. First, it separates out the array placement from the riskier head cap surgery, ensuring the animal is healthy before the electrode arrays are implanted into the brain. Second, the separation allows acute recordings to be performed before array implantation. As a result, the target structure (auditory cortex) can be localized physiologically and the array more accurately placed. Third, it allows multiple arrays to be placed at different time delays, or even removed and replaced, with relative ease because such procedures no longer involve major surgeries.
Surgery and recordings were performed under procedures approved by the Johns Hopkins University Animal Care and Use Committee. While the head cap surgery is considered a major recovery procedure, subsequent implantations of electrode arrays are not because they are performed after an animal has recovered from the initial surgery and do not involved sensate tissues such as skin or muscle, only craniotomies through bone that has already been exposed during the head cap placement.
2.2. The implanted multi-electrode array
Although there are a plethora of electrode array designs available, many are unique to a single laboratory or are used only by a small number of groups investigating similar scientific questions. This diversity is because of the need to tailor array designs to specific experiments and animal models. In choosing a suitable array design for studying vocalizing marmosets, we had to weigh several factors including size and weight, type of electrodes, arrangement of electrodes, and movement ability. Because the marmoset is a small primate, the array had to be small and light weight. The best approach to the auditory cortex in marmosets is laterally, so any array would be protruding from the side of the animal’s head, mandating a small array to minimize damage risk as well as to reduce interference with the animal’s mobility. We also desired to use sharp metal microelectrodes because of their ability to penetrate intact dura, increasing recording stability, and because of their better performance in isolating single-units in marmoset cortex. A parallel grid arrangement was chosen in order to sample multiple sites within the auditory cortex simultaneously. Finally, we also desired the ability to move the electrodes once implanted in order to effectively study well-isolated single neurons.
2.2.1. Electrode array design and characteristics
In the present study, we used the Warp16 electrode array (Neuralynx Inc., Bozeman, MT). The conceptual design was based on the large-scale electrode array pioneered in the McNaughton lab for use in rodents and macaques (Hoffman and McNaughton, 2002), but scaled down for use in the marmoset. The Warp16 array (Fig. 1A) is a small implantable multi-electrode array that consists of 16 individually moveable sharp microelectrodes. The array is 17 mm tall, 12×8 mm wide, and has a footprint on the brain of 4×4mm, approximately the size of the marmoset primary auditory cortex. The array weighs approximately 0.8 g.
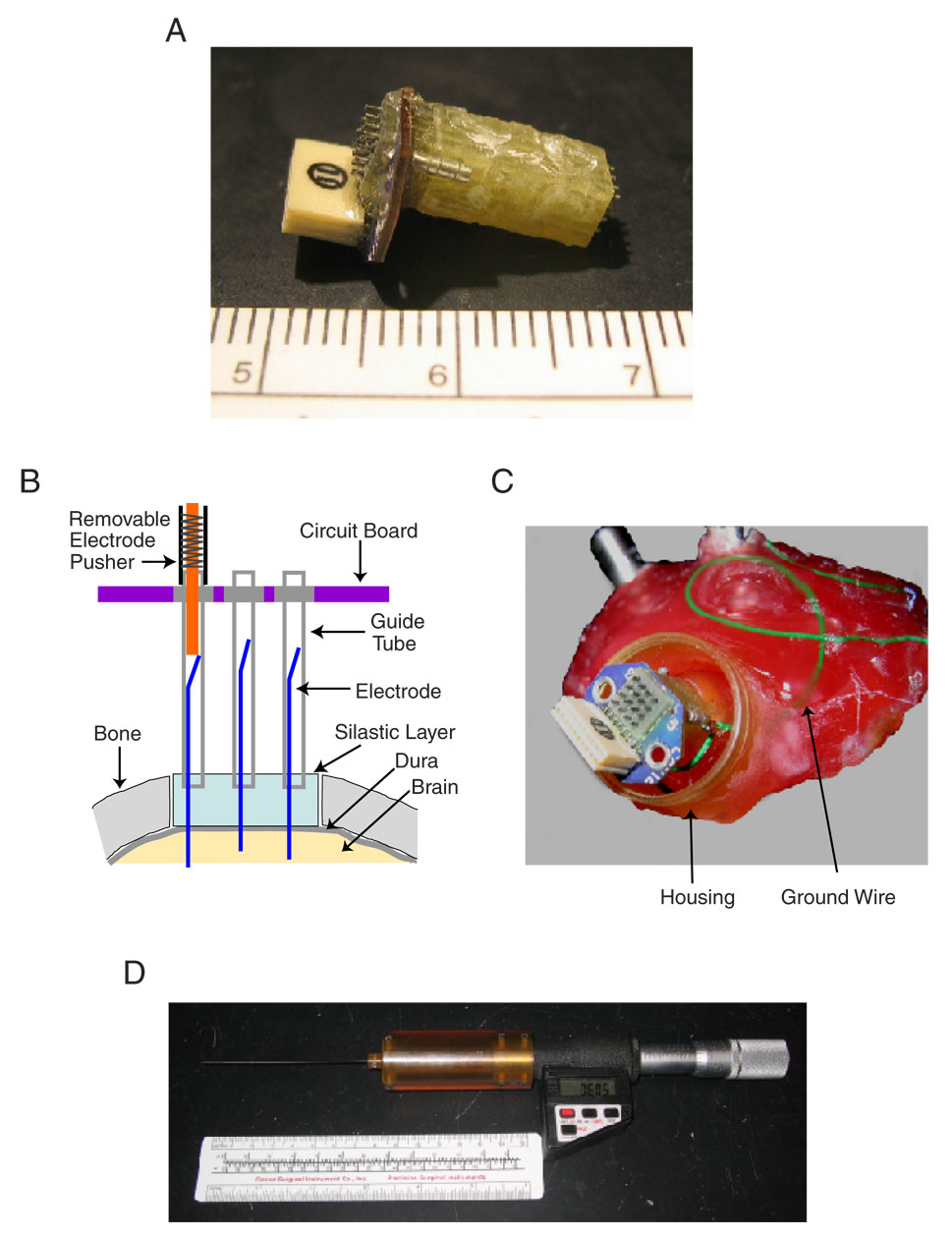
Fig. 1. The Warp16 implanted electrode array.
A: a picture of the electrode array viewed from the side. The maximum dimensions of the array were 17×12×8 mm and its weight is ~0.8 g prior to implantation. B: an illustration of the array design. The array consists of a 4×4 matrix of 15 mm long stainless steel guide tubes (30-gauge) encased in a fiberglass/epoxy matrix and attached to a circuit board. Electrodes are placed in each guide tube and held in place by a ~30° bend in the tail. The bent tail segment lacks insulation and forms the electrical contact between the electrode and the guide tube. A silastic layer fills the space between the array and the dura and serves to both stabilize the electrodes and prevent CSF from leaking back into the guide tubes. C: photograph of the array in-situ. The array is incorporated into the dental acrylic head cap (pink) atop an animal’s skull. A protective housing encases the array and is independently attached to the head cap. When the array was not in use, a small cap was attached to the housing to cover and protect the array. Also visible is a ground wire (green) connecting the array to a skull-anchored grounding screw. D: photograph of the pushing device, modified from a fine caliper, capable of moving electrodes with a resolution of 1 µm. The end of the pusher is a small gauge hollow tube that is lowered until it surrounds the end of a single electrode guide-tube (illustrated in B). A probe wire is then advanced into the guide tube until it makes contact with the tail end of an electrode and then forces the electrode deeper into the brain. Electrode movement is uni-directionally downward; no retraction is possible.
These arrays consist of a 4×4 matrix of 30-gauge stainless steel guide tubes embedded in fiberglass and epoxy matrix, into each of which a single sharp electrode is placed (Fig. 1B). These guide tubes are spaced with approximately 700 µm from center to center. The tubes are connected at their tops to a circuit board, itself connected to a connector block for the removable head-stage amplifier. The electrodes are not firmly attached to the array, but are held in place by a ~30° bend in their tail segments. This bend provides mechanical stability by holding the electrode tails firmly against the walls of the guide tubes, while the absence of a fixed attachment allows the electrodes to be moved vertically down the length of the tubes. Electrical signals from each electrode are conducted to their respective guide tubes through the electrode tails, which have been stripped of insulation. The tubes are insulated from one another by the fiberglass spacers of the array.
The length of the guide tubes is an important design consideration since it limits the maximum length of the electrodes. The guide tubes used in these arrays were custom-made to a 15 mm length. This length was calculated based on maximum depth of structures to be studied (~2 mm), any dead space between the array and the brain (~3 mm), and the need for most of the electrode shaft to remain within the guide tube for stability (between 1/2–2/3 of the total electrode length).
2.2.2. Electrode choice
The Warp16 array has a great deal of flexibility in the specific choice of electrodes to be used. The arrays are manufactured without the electrodes, allowing us to custom tailor to our needs. The minimal requirements for the electrodes are that they have sharp tips to penetrate through an insulating silastic layer (see below), that they be at least 15 mm long, and have a maximum diameter (with insulation) of 75–100 µm to fit within the guide tubes. In the testing of these arrays, several different electrode types were tried including 2 MΩ tungsten electrodes from Frederick-Haer (FHC; Bowdoinham, ME) and Microprobe (Gaithersburg, MD), 4 MΩ tungsten electrodes (FHC), and 2 MΩ platinum-iridium electrodes (Pt-Ir, Microprobe). Most experiments were conducted using epoxy-insulated 2 MΩ tungsten electrodes from FHC.
2.2.3. Array assembly
Implantation of the electrode array first requires a small amount of assembly. Since the arrays and electrodes are separately purchased, the electrodes must be inserted into the arrays. While this could, in theory, be done after implantation, we chose to fully assemble the array and electrodes before implantation. Both the electrodes and lower half of the arrays are first sterilized by soaking in Novalsan (chlorhexidine) solution for an hour. After multiple rinses with sterile saline solution, the arrays and electrodes are allowed to dry overnight. Electrodes are prepared by first inserting them tail first through a 25-gauge hypodermic tube that was 3 mm shorter (12 mm) than the array guide tubes, a spacing designed to expose the appropriate amount of insulation for removal. A small flame source, a cigarette lighter with a length of hypodermic tube inserted to create a more precise flame, is used to burn off the insulation on the exposed tail segment of the electrode. The electrode is then removed and remaining insulation debris manually stripped with fine jeweler’s forceps. Using a micromanipulator (David-Kopf), the electrode is then inserted tail first into the bottom of the array under microscopic visualization. Although the electrodes could be inserted point first, a tail-first placement reduces the risk of damage to the fragile tip due to incorrect alignment. The electrode is inserted until the tip just disappears within the guide tube. The tail end of the electrode is then grasped above the top of the array and pulled an additional 3 mm, retracting the electrode tip deeper into the array. Using wire cutters, the electrode tail is trimmed approximately 3 mm above the end of the guide tube. The electrode is then pushed 0.5–1 mm back into the guide tube, leaving an exposed tail ~2 mm. Finally the tail is manually bent 30° at the point it leaves the tube. Approximately a 30° bend appears to be optimal, more will make the electrode difficult to move, less will result in an unstable electrode or poor electrical contact. After bending, the electrode is advanced into the guide tube until the remaining tail is no longer exposed.
After all electrodes have been inserted, a ground wire is soldered to the two contact points on the array (one is visible on the side of the array in Fig. 1A). A long length of wire is attached and the two contacts cross-connected to ensure durability. This ground wire will later be attached to an exposed screw on the head cap during the implantation procedure.
The final step in assembly is creation of a thin silastic (QWIK-SIL, WPI, Sarasota, FL) layer on the base of the array. The array is positioned bottom-side up and a segment of adhesive tape wrapped around the base to form a well for the silastic to cure. In order to prevent the silastic from filling the guide tubes, a small drop of ointment (triple antibiotic ointment) is applied to the end of each tube using a 1 cc syringe and 30-guage needle. Care is taken so that this applicator does not bump the tube in case the electrode tip protrudes slightly. The silastic, a silicone elastomer, is then poured over the base of the array to a thickness of 1–2 mm and allowed to cure overnight. After curing, the tape is carefully removed by unwrapping, otherwise it can dislodge the silastic layer. If the silastic surface is irregular or the layer to thick, it is carefully trimmed using a fine scissor.
2.2.4. Array implantation procedure
After an animal recovers from the head cap surgery, and before the array implantation is performed, the location of primary auditory cortex (A1) and its tonotopic axis is determined by recording from multiple small (1mm) craniotomies using single-electrode methods (Lu et al., 2001b). If the head cap shape needs to be modified to accommodate the array, a Dremmel with cutting bur is used after the animal is briefly sedated with ketamine (20 mg/kg) and acepromazine (0.75 mg/kg). A craniotomy is then carefully made in the desired location using a custom small drill (with a 1 mm drill bit) attached to a micromanipulator (SM-11, Narishige). The resulting craniotomy is larger than the array footprint, generally a 5×5 mm square. If the animal exhibits any signs of discomfort, it is briefly sedated using ketamine. Any bleeding of the exposed dura is controlled with flushes of 2% lidocaine with 1:100,000 epinephrine, or with a small piece of gelfoam. The completed craniotomy is then filled with a layer of silastic (see Fig. 1B) that is allowed to cure for ~20 min. This silastic layer adheres to the dura, filling in any gaps that would allow tissue growth. The silastic layer also plays two important functions in the array: first, it stabilizes the electrodes as they penetrate through it and, second, it prevents cerebrospinal fluid from leaking back into the guide tubes and shorting the electrodes,
The array is then lowered in place using the micromanipulator. A small custom clamp on the manipulator holds the connector block of the array during placement. The array is positioned roughly perpendicular to the silastic surface and lowered until its base (also a layer of silastic) comes in contact with the silastic covering the dura. Only light pressure is applied by the array to the underlying silastic and dura. The junction between the two silastic layers (dural and array) is then sealed with a small amount of additional silastic. Once positioned, the array is secured to the surrounding head cap with several layers of dental acrylic. To achieve stability, the acrylic application extends at least half way up the array and encompasses all four sides. Care is taken to prevent stray acrylic from entering the tops of the electrode guide tubes or the connector block. The clamp holding the array in place is then removed once the acrylic has hardened for 30–40 minutes. A picture of the array attached to the head cap is shown in Fig. 1C. After array placement, a protective housing (see section 2.3.1) is attached to the head cap with dental acrylic. The ground wire is threaded through one of the holes in the protective housing base and wrapped around the exposed end of one of the head cap screws.
Placement of the head cap on a marmoset generally results in a slight droop in the upper portion of the pinnae, without displacement of the external auditory canals, due to the relaxation of scalp skin tension during surgery. Subsequent placement of the implanted electrode array was not observed to have a further effect on the position of the pinna.
2.2.5. Electrode movement
One of the advantages of the Warp16 design is the ability to independently move each electrode. This movement ability is important for a number of reasons. First, it allows many different neurons to be sampled, from each electrode, across multiple cortical layers. Second, by moving the electrodes, the signal quality of recorded neurons can be optimized. Third, it extends the useful life of the array as new neurons can be sought when others are lost either due to simple brain motion or due to glial scarring around the electrode tip, a common problem for chronically implanted electrodes (Polikov et al., 2005).
A number of past electrode implants have used moveable electrodes, either through screw-based mechanisms (Ainsworth and O’Keefe, 1977; Wilson and McNaughton, 1993; Venkatachalam et al., 1999; Jog et al., 2002; Keating and Gerstein, 2002), small micro-motors (Fee and Leonardo, 2001), or an external device (deCharms et al., 1999; Hoffman and McNaughton, 2002). The Warp16 uses a removable electrode pusher (Neuralynx) to individually move the electrodes (Fig. 1D). This pusher, modified from a micron-scale caliper (Starrett 762XFL), is attached to the end of a guide tube and a probe wire lowered into the tube to advance the electrode (Fig. 1B). Electrodes can be moved with a resolution of 1 µm. A micromanipulator (David-Kopf) and custom attachment are used to steady the electrode pusher while the animal is restrained in a primate chair. The advantages of this design are that each electrode can be independently moved and that the movement apparatus is not attached to the animal, allowing a larger number of electrodes to fit into a small array with higher density. The primary disadvantage of this design is that electrodes can only be advanced downward into the cortical tissue.
After the array is implanted, electrodes are inserted into the brain using the electrode pusher. Initially, an impedance meter (Omega-Tip-Z, WPI) is used to determine the point of dural contact, for each electrode, based on a sudden drop of the measured impedance. Each electrode is then advanced 100 µm and allowed to settle for 10 minutes. A depth reading of the dural surface is taken for each electrode using a digital readout on the pushing device. This depth serves as a reference for all subsequent electrode movements. At the end of each day, the final depths of the individual electrodes are noted and used as the starting point for electrode movements during the following experimental session. This depth corresponds to the point of contact between the pusher and the tail end of the electrode. Positioning of the electrode pusher with its tip around a guide tube and touching the array circuit board (Fig. 1B) allows consistent and precise control of electrode movements.
After the first day, subsequent electrode movements are generally limited to 20 µm per day. This small amount of daily movement is employed to maximize the opportunity to encounter neurons during advancement. Often new neural signals are not found until the day after electrode advancement, suggesting a large degree of overnight settling of the brain tissue surrounding the electrodes. Smaller movements are made when the goal is to optimize existing neural signals. The most stable neural signals are achieved when electrodes are advanced gradually, as movements larger than 20 µm at a time often result in signals that can only be stably recorded for an hour or two. Also to decrease the effects of tissue settling, electrode movements are made in an order such that two adjacent electrodes are never moved immediately following one another. Because of the time necessary to both move all electrodes and perform the experiments, recordings and electrode movements are generally alternated on a daily basis such that the electrodes are moved only every other day.
The general criteria for moving an electrode are the absence of recordable units, the presence of small units that might be optimized through small (5–10 µm) electrode movements, or (rarely) a stable unit at a fixed electrode depth that had already been recoded extensively over many sessions. On average, 6–7 of the electrodes were moved on any given day and 8–9 were left untouched (plus the unmoved reference electrode). Of those electrodes moved, 4 were the larger (20 µm) movements to find new units, while 2–3 were the smaller moves to optimize units already present.
2.3. Free-roaming methods
While free-roaming recording using flexible wire tethers has been used successfully for many years in both rodents (i.e. Wilson and McNaughton, 2003) and birds (i.e. Fee and Leonardo, 2001), it has rarely been used in monkeys. The reason is primarily that monkeys move in three dimensions, including upside-down movements. In the case of the natively arboreal marmosets, they always attempt to reach the top of any enclosure, including climbing up their own tethers. The ideal solution is the use of a radio telemetry system (Grohrock et al. 1997; Nieder and Klump, 1999; Chien and Jaw, 2005; Mohseni et al., 2005; Jurgens and Hage, 2006; Schregardus et al., 2006). However, few of these systems are commercially available and are small enough for use in the marmoset; most are also limited to a small number of channels. A tether system for use in monkeys has been demonstrated previously, but required a rather large enclosure to accommodate all components (Ludvig et al., 2001). We ended up designing and testing two different free-roaming systems for the marmoset experiments reported here.
2.3.1. Tether management and array protection
Unlike implants used in most rodents or songbirds, protecting the array is essential when working with primates (Ludvig et al., 2001; Hoffman and McNaughton 2002; Wilson et al. 2003; Jurgens and Hage, 2006) for a number of reasons, not the least of which is that the animals’ hands are capable of grasping both the tether and the array. Additionally, the lateral approach to study the marmoset auditory cortex makes an array more vulnerable to damage. As a result of these concerns, the tether is protected by encasing it in a flexible silicon tube with an inner diameter of 1/16” (Nalgene, Rochester, NY). Because the tether and head-stage amplifier were already attached by the manufacturer (HS-16, Neuralynx), the tube had to be slit open for the wires to be inserted and was subsequently closed by suture ties placed at regular intervals.
A protective housing was devised to protect the array both during the experiment as well as when not in use. The housing consists of two components, a base surrounding the array and permanently anchored to the head cap (Fig. 1C), and a removable cap that protects the array between experiments and is removed when recording. Both components were custom machined from a strong, heat-stable polymer, polyetherimide (Ultem, McMaster-Carr). Schematics for these two components are shown in Fig. 2B. Independent anchoring of the array and protective housing was chosen to reduce transmission of any impact forces to the array, increasing recording stability.
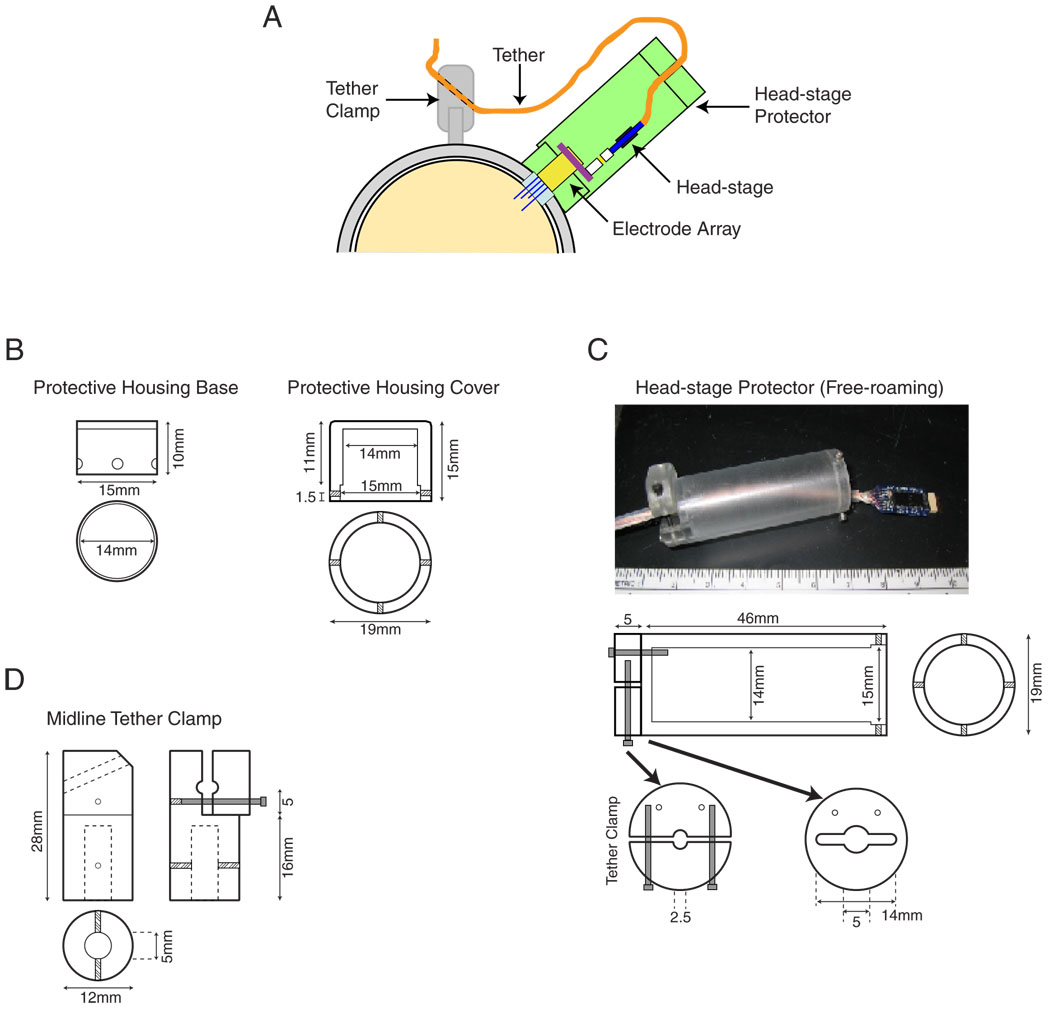
Fig. 2. Tether and head-stage configuration for free-roaming recordings.
Free-roaming experiments are conducted using flexible wire tethers to relay neural signals from the animal to the data acquisition system. In order to protect the fine tethers from damage, they are encased in a small silicone-rubber tube. A: illustration of tether attachments during free-roaming experiments. The electrode array and head-stage amplifier are encased in removable head-stage protector that attaches to the head cap-anchored protective housing base. A split clamp at the end of the cylinder holds the tether to alleviate strain on the head-stage and electrode array. An additional tether clamp is mounted atop one of the head posts along the midlines. B: schematics for the protective housing base (left) that is anchored to the head cap and the cover used overnight between experiments (right). Small set-screws in the cover secure it to a correspondingly placed groove in the housing base. C: photograph of the head-stage amplifier, tether, and head-stage protector (above). Schematics for the head-stage protector with its attached tether clamp are also shown (below). D: schematics for the midline tether clamp are shown. Unlike other components, this clamp was machined of aluminum. The clamp was designed to attach to a head post that sits along the midline of an animal’s head cap. All schematics are shown actual size.
During free-roaming experiments, a protective sleeve is used to protect both the array and head-stage from damage or from being disconnected by animal movement (Fig 2A). This head-stage protector was custom manufactured from Ultem and designed to sit on the protective housing base attached to the head cap (Fig. 2C). The end of the head-stage protector houses a split-clamp that tightens around the tether, preventing any tension in the tether lines from dislodging the head-stage from the electrode array. Additionally, a second tether clamp is placed atop the animal’s head posts, posts normally used to restrain the animal’s head, that further grips the tether (Fig. 2D). By attaching the tether along the midline, this aluminum clamp alleviates strain that could otherwise dislodge the head-stage protector and ensures the tether ascends from the animal along the midline, maintaining balance when the tether is under tension.
2.3.2. Simple free-roaming method
The first of two free-roaming designs was based on the simple tether arrangements of rodent and bird experiments. The animal is placed in a small cage with the tether extending from the animal’s head up to the cage ceiling. The remaining tether is draped down the side of the cage, the weight of which partially counter-balances the tether within the cage and limits the amount of slack tether. Limiting slack within the cage is important because of the potential for entanglement and injury. The small custom cage is made of lexan (polycarbonate, McMaster-Carr) with a metal mesh front and rear. The front mesh is widely spaced to allow the animal to climb; the rear mesh was more closely spaced to prevent climbing. A door in the wall allows animal entry and a small hole in the ceiling allows tether entry. The cage size is approximately 1.5× the animal’s total height to allow a limited degree of climbing.
Although this cage was successfully used during a number of experiments, several problems were encountered from time to time. First, the tether counterbalancing was not always successful, leaving slack within the cage, and the animal would sometimes become entangled. At other times the animal would gain hold of the slack and begin chewing on the tether. Second, the animal’s movement often gradually introduced tangles or coils in the tether, eventually requiring an interruption of recording to remove the accumulated coils.
2.3.3. Advanced free-roaming method
A second, more elaborate, free-roaming recording technique was also developed that compensated the problems of tether coiling and slack (Fig 3). As in rodent and bird experiments, twists in the tether were removed using a commutator, in this case a 16 channel commutator (Neuralynx). Removing the excess slack in the tether was more problematic. This is not normally encountered in rodents or birds, which are generally kept on the cage floor during experiments. Climbing marmosets, on the other hand, require the tether length to adapt quickly as the animal move about its cage. Our attempts to limit marmosets to two-dimensional movement always resulted in the animal climbing up its tether.
Fig. 3. Tether-suspended free-roaming cage design.
A more complicated free-roaming apparatus was developed to address the complications of animal movement. A picture (A) and illustration (B) of this design are shown. This two-part custom cage consists of a lower portion with climbable bars and a solid upper portion within which the animal could not climb. A commutator, located atop the upper cage, is used to alleviate tether torsion. A suspension system allows the animal to move vertically without introducing slack in the tether. The suspension system consists of four lines that connect the tether, via a tether clamp, to counterweights located outside the cage at its corners. C: the tether suspension clamp is shown along with the tether and four suspension lines that clip onto eyelet screws. The clamp contains a circular bearing that allows the tether to rotate without interference by the suspension system. D: schematics for the tether suspension clamp shown actual size.
A novel previous design has used a counterweight system to control the free slack in the tether (Ludvig et al., 2001). This design suspended the counterweight from beneath the commutator, requiring a large apparatus. Building on this concept, we developed a suspension system that would control tether slack but is far more compact (Fig. 3 A,B). The tether is attached to four suspension lines (30-lb recreational fishing line). Each line passes through two pulleys anchored to the cage ceiling at the corners, one inside and one outside the cage. Each line is attached to a counterweight (steel fishing weights) located outside the cage. The total counterweight on all four lines was calibrated to apply only light vertical tension to the tether, enough to alleviate any slack but not enough to affect mobility. By placing the counterweights outside the cage, rather than inside, a much more compact apparatus is possible. The animal is allowed to move freely throughout the lower part of the cage. An upper cage portion (Fig. 3A), made of lexan, prevents the animal from climbing to the cage ceiling and becoming entangled in the suspension system.
The difficulty in combining a commutator and a suspension system is that the two can interfere with one another. The attachments needed to counterbalance the tether can prevent free rotation of the tether. In the past, this was solved by suspending both the tether and counterweight from the commutator. In order to make a more compact design, we developed a tether suspension clamp (Fig. 3 C,D) to couple the tether and suspension wires. The suspension clamp contains a ring bearing that allows the attachment of the suspension system on the outside while the tether freely rotates in the center.
2.4. Neural recordings
2.4.1. Recording hardware
A custom data acquisition system was developed to record neural activities that was mobile and could be used both in the laboratory environment (within a sound-proof chamber) and for free-roaming recordings in the marmoset colony. Neural activities from Warp16 electrode arrays are obtained from the head-stage (HS-16, Neuralynx), a light-weight unitary-gain amplifier that interfaced with a connector block on the implanted array (Fig 3C). These signals are filtered (0.3–6 kHz) and differentially amplified (20,000x gain) using multi-channel hardware (ERP-27 and Lynx-8 amplifiers, Neuralynx). Amplified signals are connected via a custom interface box to a 64 channel data acquisition card (PCI-6071E, National Instruments). Neural signals are digitized at 20 kHz sampling rate, controlled by Matlab software, and stored on a computer hard disk as multi-channel WAVE files for later analysis.
Differential amplification of array signals uses a single electrode per array as a common reference for the remaining electrodes, to reduce noise resulting from movement artifact and other sources, is essential during free-roaming experiments. The same reference electrode is used for all experiments for each array. This electrode, generally located in one of the array corners, is advanced to a depth of 200 µm on the first day of electrode movement, but is not subsequently moved. This electrode is checked at the start of each recording session to ensure absence of unit activity that could contaminate the other electrode channels.
2.4.2. Recording procedures
Experiments were generally conducted in two-day cycles. On the first day, the animal is brought into a sound-proof chamber (IAC-1024, Industrial Acoustics, Bronx NY), placed in a primate chair and the electrodes advanced by the experimenter. During electrode movements, neural signals are monitor via audio speaker and real-time, template-based spike sorting hardware (MSD; Alpha-Omega Engineering, Nazareth, Israel). Electrodes giving good neural signals are often not advanced for days, or even weeks, at a time. On the second day, the animal is brought back to the lab sound-proof chamber and placed in the primate chair. Auditory physiology experiments are conducted, with the animal fully restrained, for 1.5–2.5 hrs. Methods for the auditory stimulus generation and delivery have been previously described (Lu et al., 2001b). After this, the animal and recording apparatus are transferred to the marmoset colony, and recordings resumed. These are performed either with the animal still seated in the primate chair, but with its head freed from restraint, or with the animal free-roaming within a cage (Fig. 4–Fig 5). Colony recordings generally last for 2–2.5 hours, after which the animal is detached from the tether and returned to its home cage. In all experiments, animals are monitored from an adjacent room via a video camera. A subset of recording channels is monitored throughout an experiment to ensure recording quality and absence of noise artifact. In addition to colony recordings, additional experimental sessions were conducted exclusively in the sound-proof chamber, often following electrode movements, to more extensively test auditory physiologic properties of the recorded units.
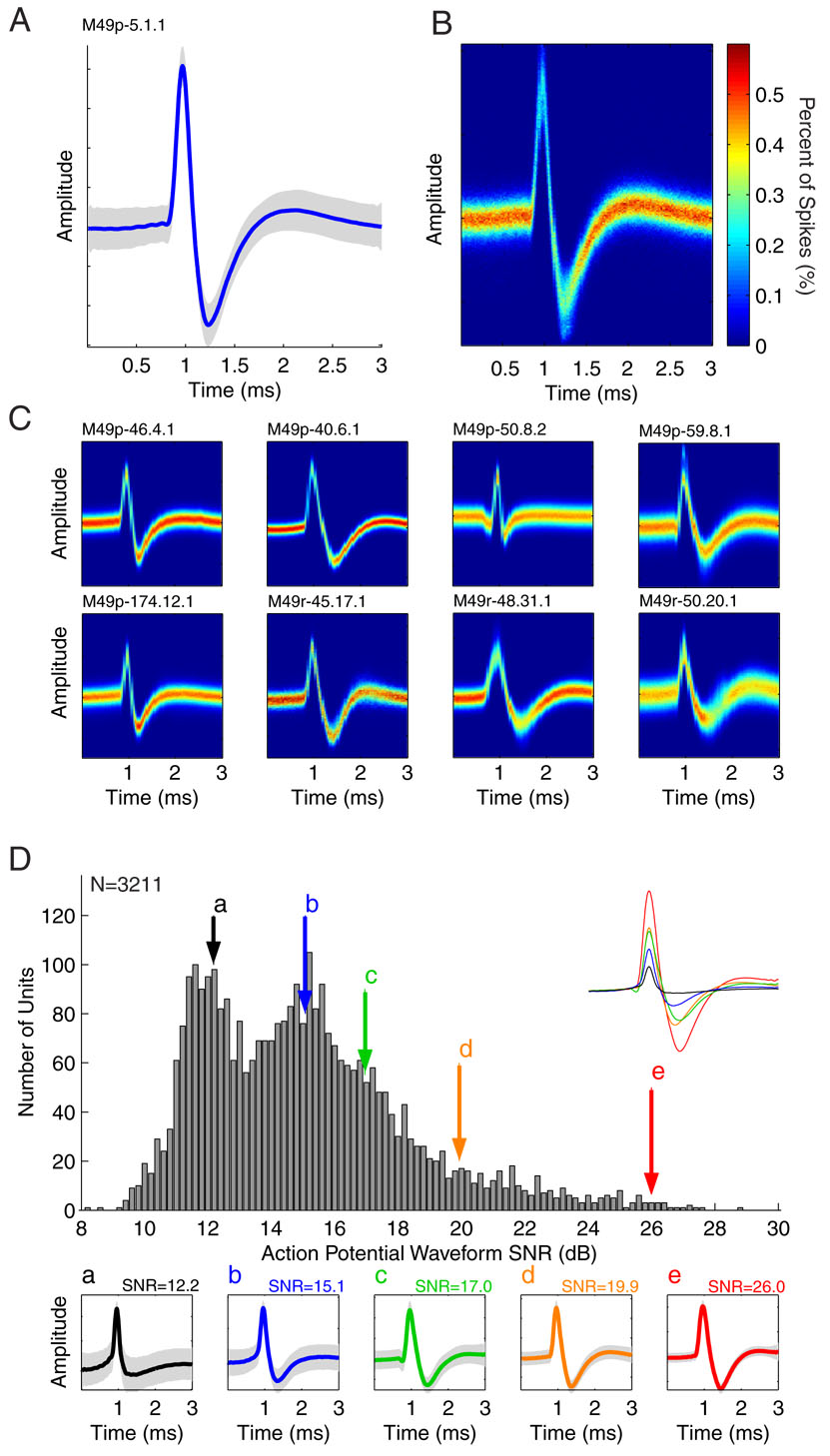
Fig. 4. Sample unit spike waveforms.
Representative spike waveforms from the units recorded are shown. A: the mean spike waveform (solid line) and its STD (shaded region) are illustrated for a sample unit (this unit was the very first one recorded with these implanted electrode arrays). B: A spike waveform density function (SWDF), a two-dimensional histogram of action potentials aligned by the point of maximum amplitude, for the unit shown in A illustrates the mean spike shape and its variance. C: SWDFs from a sample of units illustrating waveform shape variability. All sample SWDFs are shown with a common color scale. D: The signal-to-noise ratio (SNR) was calculated for the mean spike waveform of each of the 3211 recorded units and is illustrated. The SNR was defined as the total height of the mean spike (peak-valley) divided by the mean background STD. Sample spike waveforms (a–e) are shown for units spanning the SNR range, including their mean spike (solid) and waveform STD (shaded). The position of each sample is marked on the SNR distribution, and their mean waveforms are re-plotted using a common amplitude scale (inset) to illustrate the variance of SNR with mean spike size.
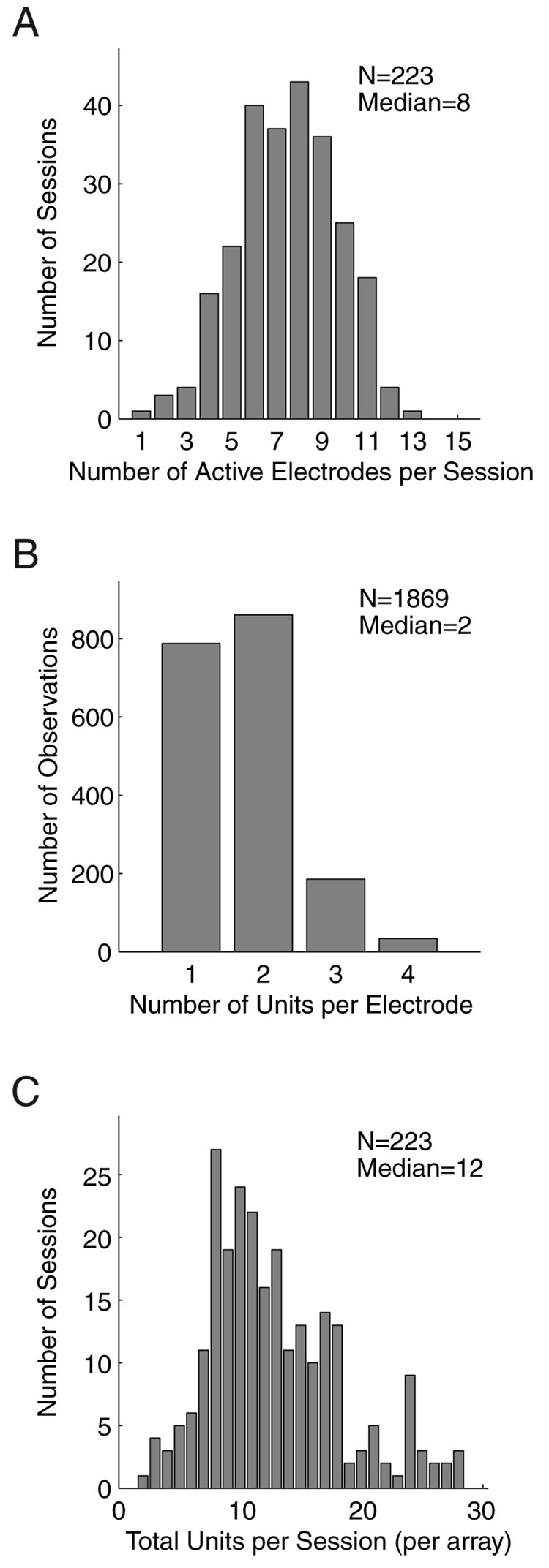
Fig. 5. Signal yield recorded with the implanted electrode array.
A: distribution of the number of active electrodes, electrodes with a recordable signal, per experimental sessions. The median number of active electrodes (8) was slightly over half of those available (15). One electrode per array was used as a local signal reference and was never recorded. B: histogram of the number of units recorded per active electrode. C: distribution of total number of units recorded per session. The median number of simultaneously-recorded units from an electrode array was 12.
2.5. Histology
Following the completion of physiologic recording, lasting between 4–18 months, animals were sacrificed in order to obtain tissue for histological analysis. Electrolytic lesions were made individually on each electrode using a 10 mA DC current presented for 8 sec in each polarity (Digital Midgard Precision Current Source, Stoelting, Wood Dale IL). Immediately following electrolytic lesions, animals were sedated with IM ketamine (20 mg/kg), the arrays removed, and the animals euthanized by a lethal overdose of pentobarbital sodium (Euthasol, 4 ml/kg). Animals were then perfused transcardially with phosphate buffered saline followed by 4% paraformaldehyde in phosphate buffer. Brains were immediately removed, blocked, and sunk in sucrose for ~48 hours before being frozen and stored at −80° C until processing. Frozen blocks were sliced on a sliding microtome at 30 µm thicknesses. Histological slices were made approximately perpendicular to the lateral sulcus for best visualization of the auditory cortex. Slices were stained for both Nissl and cytochrome oxidase (CO).
2.6. Data analysis
2.6.1. Unit separation and classification
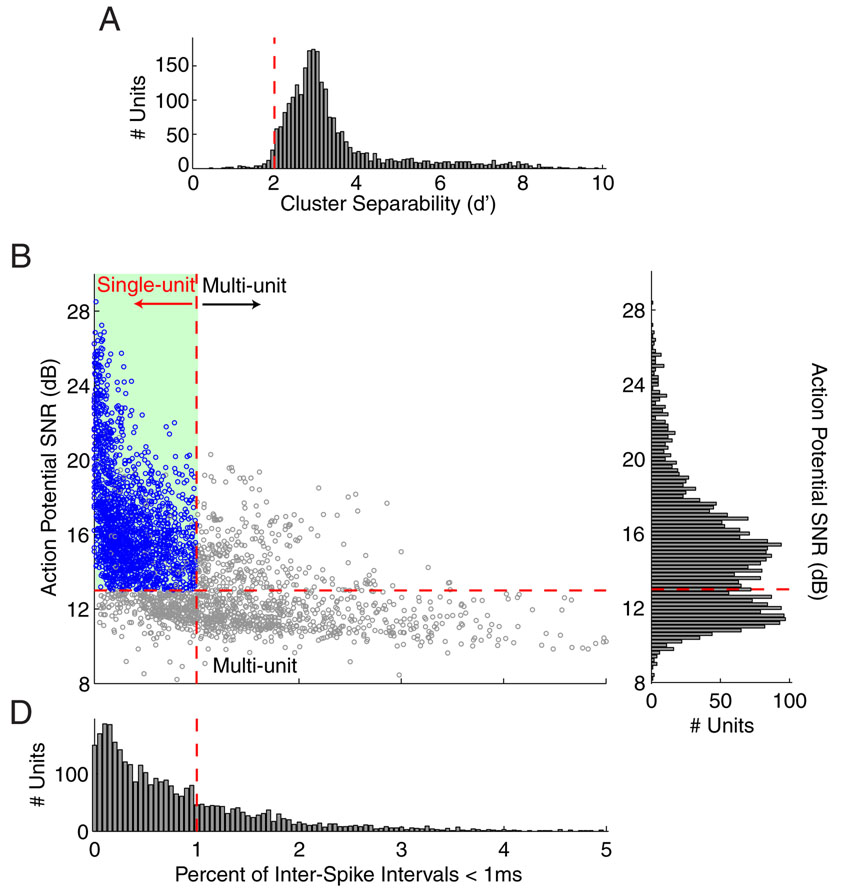
Digitized raw recordings are first bandpass filtered (0.3–7 kHz) and events crossing a manually defined threshold (signal-to-noise ratio, SNR > 2σ) extracted from the raw data as 3 ms segments. Neural events are sorted into individual neuron units based on principle components (PC) of action potential waveforms (Lewicki 1998). A standard set of PCs were pre-calculated and used in sorting all spikes, a method chosen to speed the sorting process. Long recording sessions, lasting several hours, are sorted using a sliding-window method. An initial window containing 5 min of data is first sorted, after which subsequent windows are examined by automatically applying the sorting criteria of the previous time block while allowing for manual updating. Sorting by this blocked method allows gradual adjustment in the sorting criteria to account for slow drifts in spike waveforms. This sorting method also allows verification of recording stability; the same criteria are applied to recordings in the laboratory and initial colony recordings to ensure continuity.
Units are classified as either single-unit or multi-unit based on the separability of action potential PC clusters during spike sorting (single-units d’ =2), action potential SNR (single-units > dB), and the fraction of inter-spike intervals shorter than a 1 ms refractory period (singleunits, maximum of 1%). The cluster separability is calculated, for units recorded simultaneously on a single electrode, using the Euclidian distance between clusters in the PC projection used for spike sorting. Separability is quantified by calculating a d’ statistic comparing separation of clusters and their respective variability. A minimum separation of d’=2 was chosen because this corresponds to a ~3% misclassification error per unit. The SNR is defined as the average action potential peak-to-peak height (maximum-minimum; ‘APheight’) divided by the STD of the background noise over 0.3 ms preceding all spikes (SNR=20*log10[APheight/STD]). The threshold of 13 dB (~ 4.5x) was chosen based on observed trends in the data. Setting a minimum SNR threshold ensures that low amplitude events, less likely to be well isolated single-units, are not included, but may underestimate the number of single-units. The 1% maximum fraction of refractory period violation is a quick criterion to exclude spike events with no refractory period, i.e. those contaminated by action potentials from different neurons. The 1% threshold was chosen after a manual examination of sample units’ autocorrelation functions revealed that refractory period contamination increased above this level.
2.6.2. Analysis of auditory responses
All neurons recorded in these experiments were also tested with auditory stimuli in order to determine their auditory sensory properties. Auditory stimuli tested included tone and band-pass noise stimuli varied in both frequency and SPL, white noise stimuli varied in SPL, a library of different marmoset vocalizations (Agamaite and Wang, 1997; DiMattina and Wang, 2006) and representative samples of four marmoset vocalization types (phee, trilphee, trill, and twitter) presented at different SPLs. Neurons were determined to be auditory responsive if they responded to one or more of the above stimuli as determined by a driven, spontaneous subtracted, firing rate of at least 10 spikes/sec (5 spikes/sec for the vocal stimuli) and a peak driven firing rate p-value <0.05 (Wilcoxon signrank).
3. Results
We implanted a total of four Warp16 arrays in two marmosets, two arrays in each animal, one in each hemisphere. The arrays in the first animal (M49p) were implanted for 447 (left hemisphere) and 205 (right hemisphere) days each. The arrays in the second animal (M49r) were only left in place for 131 (left) and 36 (right) days due to time constraints. A total of 3211 units were recorded from the arrays during these experiments, including all conditions, and are included in the analysis of array performance. In order to test performance and stability, a subset of sessions involved additional recordings in the marmoset colony or when the animal was free-roaming in a cage. Out of the 3211 total units, 2210 units were also studied with the animal in the marmoset colony while either seated in a primate chair, with its head unrestrained, or in a free-roaming cage. Free-roaming methods were used to record 918 units.
3.1. Recording yield and quality
Units recorded with the implanted electrode arrays had clearly isolatable action potential waveforms, separable both from the background noise and each other. Representative action potentials for a well-isolated unit are shown in Fig. 4 (A and B). This example was actually the very first unit recorded from these electrode arrays. Individual action potentials were aligned by the voltage peak and averaged (Fig 4A). A histogram displaying the distribution of this unit’s action potentials, a spike waveform density function (SWDF) is also shown (Fig. 4B). Additional SWDFs for twelve more representative units are shown in Fig 4C.
The isolation quality for each of the 3211 units was quantified by calculating the SNR for their action potential waveforms (Fig. 4D). The SNR for all the recorded units was distributed over a range from ~8 to 29 dB. Most units had an SNR greater than 12 dB, and the distribution featured a prominent tail towards the higher SNR range (better signal quality).
During a given session, quality neural signals were not always present on every electrode channel. However, as electrodes were advanced, eventually nearly every one yielded separable units. The number of electrodes with quality signals was measured for each array during each experiment (Fig. 5A). The maximum daily yield was 13 out of a possible 15 (one electrode on each array served as a reference and was not recorded); the median yield was 8 active electrodes per day. On each active electrode, between 1 and 4 units was isolated (median 2; Fig. 5B). The total number of units per recording session had a median value of 12, but extended as high as 28 (Fig. 5C). When two arrays were implanted in the same animal and recorded simultaneously, it was not uncommon to record from 50 units at a time.
In order to better separate out clearly isolated from poorly isolated units, and classify them as either single-or multi-unit, three parameters were examined for each unit: the action potential cluster separability, waveform SNR, and inter-spike intervals (ISI, Fig. 6). A minimum cluster separability of d’=2 was used to as the first criterion for identifying single-units (Fig. 6A), and 97.1% of the 3211 units exceeded this threshold. A clear trough in the SNR distribution was observed (Fig. 6C) and a threshold was set at this trough (SNR=13 dB) as the second criterion. Most units (67.1%) exceeded this threshold. The third criterion, based on ISIs, separates out units without at least a 1 ms refractory period (Fig. 6D). A maximum of 1% of ISIs violating this 1 ms threshold was allowed for single-units, and 69.7% of units met this criterion. These three criteria were in general agreement, as those units with higher SNRs typically had fewer short ISIs, and those with smaller SNRs had more short ISIs (Fig. 6B). Combining these three criteria, a total of 1801 out of 3211 units (56.1%) were classified as single-units. Those classified as multi-units were usually secondary units with smaller amplitudes recorded on the same electrode where a single-unit was identified (65.9% of multi-units), and less commonly the primary signal recorded from an electrode, although this is biased by the decision of whether or not to record from an electrode during a given session.
Fig. 6. Separation of single and multi-unit data.
In order to classify units as coming from a single well-isolated unit, three criteria were established. First, separability of simultaneously-recorded units was measured using the Euclidian distances between their PC-projection clusters (A). A minimum cluster separability of d’ =2 was chosen for single-units (dashed red line). In order to further separate single-unit recordings from multi-unit clusters, unit SNR and inter-spike interval (ISI) data were compared (B). A minimum threshold SNR (13 dB) was established for single-units by selecting a value between the two peaks of the SNR distribution (C). Single-units were also required to have ISIs greater than a 1 ms minimum, reflecting the refractory period of action potential generation. A maximum threshold of 1% ISIs violating this 1 ms interval was chosen (D). These parameters were related, with the higher SNR (single-units) having few ISI violations, and lower SNR units (multi-units) showing more violations. Dashed red lines: threshold criteria for SNR and ISI; Shaded region: area passing SNR and ISI criteria; Blue markers: classified single-units.
3.2. Auditory responses
Driven auditory responses using the test stimuli were evoked in 2255 of the 3211 recorded units 3211 (70.2%), with 1582 units (49.3%) responding to pure tone stimuli. Out of the 3211 units, 1090 were recorded from putative primary auditory cortex units (A1, see Section 3.4). Driven responses are found in 87.1% of A1 units (945/1090) for all tested stimuli and 63.9% of A1 units (687/1090) for tone stimuli. During any given session, a median of 6 of the 8 recorded electrodes had neurons with responses to auditory stimuli (Fig. 5A; IQR 4–8), or 8 of the 12 recorded neurons (Fig. 5C; inter-quartile range, IQR 5–13). When the auditory analysis is restricted to only well-isolated single-units (Fig. 6), 1127 of 1801 (62.6%) units had driven auditory responses. This fraction of units for which driven auditory responses could be evoked was comparable to our experience recording in marmoset auditory cortex using non-implanted single electrode methods (Wang et al., 2005).
3.3. Recording stability
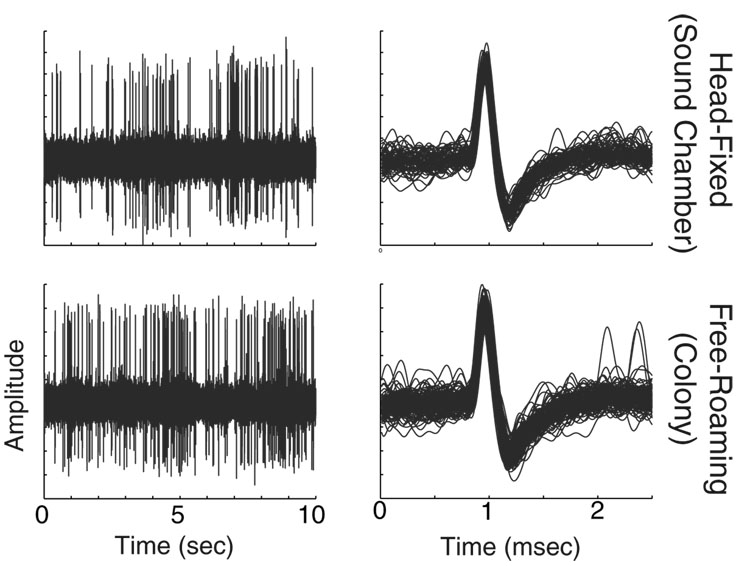
Most units were observed to have stable spike waveforms throughout an experimental session (often >4.5 hours), including both restrained and unrestrained recordings. Figure 7 shows an example of a well isolated unit recorded first in the sound-proof chamber, when the animal’s head was restrained, and then later recorded in the marmoset colony with the animal free-roaming in a cage. This unit’s waveform was stable, and nearly identical, between the two conditions. Such stable recording was possible despite frequent headcap impacts on cage walls or animals’ managing to climb or become entangled in their tethers. Of the 2210 units recorded in both sound chamber and marmoset colony, 123 (5.6%) were subsequently lost over a multi-hour (generally 2–2.5) period of colony recording.
Fig. 7. Sample unit recorded during head-fixed and free-roaming conditions.
Raw data (left) and aligned spike waveforms (right) are shown for a sample unit. Recording stability is compared between head-restrained (above) and free-roaming (below) conditions. The action potentials were nearly identical, indicating that the units were stably held across condition.
3.3. Histology and array localization
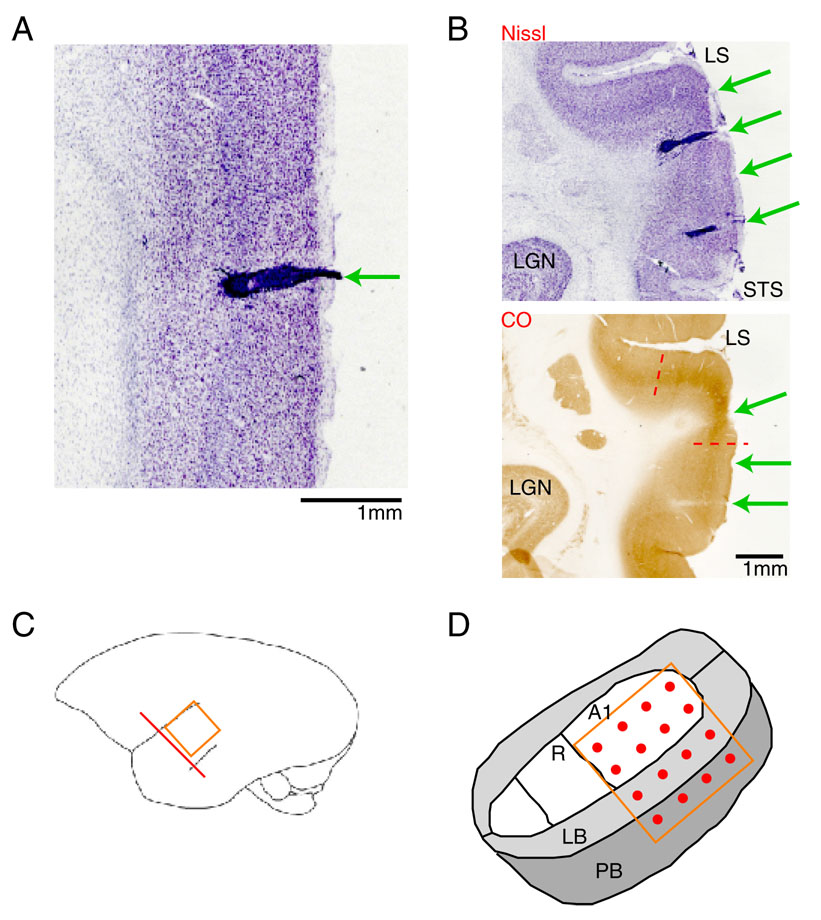
Histological examination of brain tissue after electrolytic lesions and perfusion revealed the presence of localized tissue disruption surrounding each electrode (Fig. 8A), consistent with glial scarring due to the chronically implanted electrode. This damage, however, was limited to a small radius around each electrode shaft. Examination of the dura after array removal, but before perfusion, revealed healthy-appearing dura with minimal abnormal tissue growth. In a few electrodes (<20%), histological examination revealed compression of the upper cortical layers (noted in Fig. 8B, top), possibly caused when electrodes pushed the dura downwards prior to penetration. This occurred primarily in electrodes where the first neural signals were difficult to detect (first spike depths were abnormally deep). The condition of the bone surrounding the craniotomy was also assessed after array removal, and found to be universally intact and healthy. The craniotomy with the array implanted for the longest period (447 days) actually exhibited re-growth of a thin layer of bone over the dura.
Fig. 8. Tissue histology and array localization.
A: Nissl-stained tissue section showing an electrically-lesioned electrode tract (arrow). Disruption of the usual cortical anatomy by scarring is noted, but is limited to a small region around the electrode. B: Adjacent histologic sections of the auditory cortex were stained for Nissl (above) and CO (below) and used to localize the electrode penetrations. Green arrows: position of electrode tracks; Dashed red lines: approximate boundaries of primary auditory cortex (A1). LS: lateral sulcus; STS: superior temporal sulcus; LGN: lateral geniculate. C: illustration of array orientation and approximate position (orange) and orientation of histological sections in A–B (red line). D: illustration indicating position of most electrode arrays. Two rows of electrodes were generally found in A1, another row in lateral belt (LB), and the final row likely in parabelt (PB).
Histology was also used to localize electrode tracks within auditory cortex. Adjacent tissue slices were stained for Nissl and CO (Fig. 8B) in order to separate out core auditory areas, from lateral belt and parabelt areas (Hackett et al., 1998;de la Mothe et al., 2006). Figure 8D illustrates the approximate position of most arrays. The first two rows of electrodes were generally confined to A1, while the third and fourth rows were likely in lateral belt and parabelt areas, respectively. Based on the frequency tuning of the A1 electrodes, the array spanned most of the marmoset tonotopic axis from 1 kHz to 28 kHz, with approximately 0.8–1.5 octaves between each electrode.
4. Discussion
In our attempt to study the neural mechanisms underlying the vocal behavior of marmoset monkeys, we encountered a number of challenges stemming from the fact that, as a general rule, animals will not always perform their natural behaviors under laboratory conditions. We took a three part approach to solve this problem, an implanted multi-electrode array, experiments conducted with an animal freely-roaming within a cage, and experiments conducted in the behaviorally-relevant environment of the marmoset colony. Combining these, we were able to record from several thousand high-quality single-units in the marmoset auditory cortex under a variety of conditions.
4.1. Comparison with existing electrode array designs
The implanted electrode array used in these experiments shares a number of features with another array design previously used to record from marmoset auditory cortex (deCharms et al., 1999). Both arrays use multiple independently-moveable electrodes that conduct electrical signals via the electrode guide tubes. Both also use a silicone base layer to prevent cerebrospinal fluid leakage and both maintain an intact dura. Both are capable of stable neural recordings over several hours. The array used by deCharms et al. (1999) has few advantages over the Warp16 array used in the present study; it has a larger number of electrodes (49 vs. 16) that are spaced more densely (300 vs. 700 µm). The advantages of the Warp16 array is that it is much smaller and lighter (< ~1 g vs. < ~20 g) and simpler in design, using a solid layer of silastic rather than a custom-built perforated gasket requiring adjustments after implantation. Most importantly, the Warp16 is used with an external electrode pusher (Fig. 1D) that can advance electrodes in a precisely controlled manner, allowing an investigator to keep track of the depth of each electrode from day to day. The Warp16 is also commercially available (Neuralynx), useful for research groups lacking sophisticated machining and electronics expertise. We have also shown how the newer design can be adapted for stably recording neurons in animals freely roaming within a cage.
The electrode array recording technique developed for marmosets shares a high degree of similarity with that of Hoffman and McNaughton (2002), upon which it is largely based. These similarities include the conceptual design of the implant electrode array, use of a solid silastic layer to prevent cerebrospinal fluid leak, and use of a removable pushing device for precise electrode control. The array used in the reported experiments was made much smaller and lighter in order to be used in small animals like marmosets. We have also adapted the arrays for free-roaming recordings, including devices to protect the arrays and wire tethers, as well as methods for accommodating the monkeys’ vertical movement.
4.2. Advantages of current methods
The Warp16 electrode arrays used in these experiments combine a number of important features. First they are small and lightweight, necessary both because of the small size of the marmoset and the need for a lateral approach to the auditory cortex. Larger arrays would potentially unbalance the animals’ heads, would be more intrusive to the animals’ natural movement, and would be more prone to damage. Second, the design of the arrays is flexible enough to accommodate a variety of electrodes, allowing configurations specific to experimental constrains, such as the need for well-isolated single-units. Third, the arrays can hold multiple electrodes within a small cortical area, allowing simultaneous sampling of multiple areas of the marmoset auditory cortex. Fourth, because they are implantable, the arrays offer stability that allows both long-duration and free-roaming recordings. Finally, the arrays allow independent movement of each electrode via a removable pushing device. Because the electrodes are moveable, they allow optimization of signal quality by gradual movements, sampling of different cortical layers, and reduction of the effects of long-term glial scarring on array usability. A removable movement apparatus (the external pusher) is also important because it reduces the size and weight of the array and allows more electrodes to be placed in closer proximity. These arrays are easily implanted and can be recorded from for well over a year each.
However, selection of an appropriate implanted electrode array is only the first step. It must be combined with recording techniques to elicit the behavior of interest. First, we developed devices to protect the arrays from damage during experiments and when animals live in their home cages. This is particularly important because marmosets have hands capable of grasping and damaging implants. Furthermore, because marmosets spend so much time climbing their cage walls, the implants come into frequent high-velocity collision with the walls. Second, methods had to be developed to allow stable recording from a free-roaming animal. Because marmosets are natively arboreal they climb and the tether had to be protected by keeping it out of the animals’ grasp, a problem not generally encountered with non-primate animal models.
4.3. Design limitations and potential solutions
The design of the electrode array and the free-roaming methods presented here have some limitations. First, the density of the electrode spacing is lower than that of other array designs (i.e. Nicolelis et al., 2003), although the current spacing (~700 µm) does allow simultaneous sampling of multiple auditory cortical fields. This larger spacing between electrodes is required by the need to attach guide tubes to the circuit board. A denser array may be possible if the design is adapted to use bent or converging, rather than straight and parallel, guide tubes with an array footprint on the brain smaller than at the top end. Such designs have been used in previous arrays (deCharms et al., 1999; Sinha and Moss, 2007). If sampling a larger cortical area is desired, the number of electrodes in the Warp16 array design could be increased by simply adding additional rows of electrodes.
Another limitation of this design is that electrode movements, though a major advantage, can only be directed downward. As no retraction is possible, this limits electrode movements to small increments to avoid passing by neurons. Furthermore, the depth of electrode penetration is generally limited by the length of the array guide tubes. However, the tube length can easily be made longer than what was used in the present study. Ideally, electrodes should be advance no more than 1/3rd of the tube length, and certainly no more than half that length, in order to maintain stability. When the thickness of the silastic layer is factored in, this limits the cortical penetration to ~4 mm for the guide tube lengths used in the present study. Deeper penetrations are possible if the array design were changed to incorporate longer guide tubes, though this will increase the array size.
The yield of recordable units also leaves room for improvement. During any given session, only about half the electrodes had usable signals, though isolated units, once present, were generally stable throughout the session. The yield of recordable units could be increased by increasing the number of electrodes and the recording quality from those electrodes. Further work is still needed to determine the optimal type of electrodes used in the array as well as the best strategy for seeking and isolating new units.
A final limitation of the present free-roaming recording technique is the necessity of tether-based recording methods and the complications that result from this method, including entanglement, animals climbing their tethers, and the need for single housing during recordings. Ultimately, the solution will be the use of radio telemetry, though current technology is either too large for a small animal or lacks the necessary bandwidth for multi-electrode recordings.
Acknowledgements
The authors would like to offer our most sincere thanks to Dr. Bruce McNaughton for kindly demonstrating his multi-electrode techniques to us. We thank the entire team at Neuralynx, which designed and built the Warp16 arrays to our specifications and continue to adapt the design to our ever-changing needs. We would also like to thank Ashley Pistorio for assistance in animal care and training. This work was supported by NIH grant DC005808 (X.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agamaite JA, Wang X. Quantitative classification of the vocal repertoire of the common marmoset (Callithrix Jacchus Jacchus) Association of Research in Otolaryngology Abs. 1997;20:573. [Google Scholar]
- Ainsworth A, O'Keefe J. A lightweight microdrive for the simultaneous recording of several units in the awake, freely moving rat. J Physiol. 1977;269:8–10. [PubMed] [Google Scholar]
- Aitkin L, Park V. Audition and the auditory pathway of a vocal new world primate, the common marmoset. Progress in Neurobiology. 1993;41:345–367. doi: 10.1016/0301-0082(93)90004-c. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Chien CN, Jaw FS. Miniature telemetry system for the recording of action and field potentials. J Neurosci Methods. 2005;147:68–73. doi: 10.1016/j.jneumeth.2005.03.011. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. A multielectrode implant device for the cerebral cortex. J Neurosci Methods. 1999;93:27–35. doi: 10.1016/s0165-0270(99)00087-4. [DOI] [PubMed] [Google Scholar]
- DiMattina C, Wang X. Virtual vocalization stimuli for investigating neural representations of species-specific vocalizations. J Neurophysiol. 2006;95:1244–1262. doi: 10.1152/jn.00818.2005. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Fee MS, Leonardo A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J Neurosci Methods. 2001;112:83–94. doi: 10.1016/s0165-0270(01)00426-5. [DOI] [PubMed] [Google Scholar]
- Grohrock P, Hausler U, Jurgens U. Dual-channel telemetry system for recording vocalization-correlated neuronal activity in freely moving squirrel monkeys. J Neurosci Methods. 1997;76:7–13. doi: 10.1016/s0165-0270(97)00068-x. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Jog MS, Connolly CI, Kubota Y, Iyengar DR, Garrido L, Harlan R, et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J Neurosci Methods. 2002;117:141–152. doi: 10.1016/s0165-0270(02)00092-4. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Hage SR. Telemetric recording of neuronal activity. Methods. 2006;38:195–201. doi: 10.1016/j.ymeth.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Keating JG, Gerstein GL. A chronic multi-electrode microdrive for small animals. J Neurosci Methods. 2002;117:201–206. doi: 10.1016/s0165-0270(02)00115-2. [DOI] [PubMed] [Google Scholar]
- Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 1998;9:R53–R78. [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. (a) [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Neural representations of temporally asymmetric stimuli in the auditory cortex of awake primates. J Neurophysiol. 2001;85:2364–2380. doi: 10.1152/jn.2001.85.6.2364. (b) [DOI] [PubMed] [Google Scholar]
- Ludvig N, Botero JM, Tang HM, Gohil B, Kral JG. Single-cell recording from the brain of freely moving monkeys. J Neurosci Methods. 2001;106:179–187. doi: 10.1016/s0165-0270(01)00348-x. [DOI] [PubMed] [Google Scholar]
- McCasland JS. Neuronal control of bird song production. J Neurosci. 1987;7:23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni P, Najafi K, Eliades SJ, Wang X. Wireless multichannel biopotential recording using an integrated FM telemetry circuit. IEEE Trans Neural Sys Rehab Eng. 2005;13:263–271. doi: 10.1109/TNSRE.2005.853625. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Ribeiro S. Multielectrode recordings: the next steps. Curr Opin Neurobiol. 2002;12:602–606. doi: 10.1016/s0959-4388(02)00374-4. [DOI] [PubMed] [Google Scholar]
- Nieder A, Klump GM. Adjustable frequency selectivity of auditory forebrain neurons recorded in a freely moving songbird via radiotelemetry. Hear Res. 1999;127:41–54. doi: 10.1016/s0378-5955(98)00179-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus) J Acoust Soc Am. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Schregardus DS, Pieneman AW, Ter Maat A, Jansen RF, Brouwer TJ, Gahr ML. A lightweight telemetry system for recording neuronal activity in freely behaving small animals. J Neurosci Methods. 2006;155:62–71. doi: 10.1016/j.jneumeth.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Sinha SR, Moss CF. Vocal premotor activity in the superior colliculus. J Neurosci. 2007;27:98–110. doi: 10.1523/JNEUROSCI.2683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam S, Fee MS, Kleinfeld D. Ultra-miniature headstage with 6-channel drive and vacuum-assisted micro-wire implantation for chronic recording from the neocortex. J Neurosci Methods. 1999;90:37–46. doi: 10.1016/s0165-0270(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Ma YY, Greenberg PA, Ryou JW, Kim BH. A microelectrode drive for long term recording of neurons in freely moving and chaired monkeys. J Neurosci Methods. 2003;127:49–61. doi: 10.1016/s0165-0270(03)00122-5. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]