Abstract
In most vertebrates studied, males have more vasopressin (VP) cells in the bed nucleus of the stria terminalis, or homologous vasotocin cells in nonmammalian species, than females. Previous research excluded differential cell birth and migration as likely mechanisms underlying this difference, leaving just differential cell death and phenotypic differentiation of existing cells. To differentiate between these remaining possibilities, we compared VP cell number in wild-type mice vs. mice overexpressing the anti-cell death factor, Bcl-2. All animals were gonadectomized in adulthood and given testosterone capsules. Three weeks later, brains were processed for in situ hybridization to identify VP cells. Bcl-2 overexpression increased VP cell number in both sexes but did not reduce the sex difference. We repeated this experiment in mice with a null mutation of the pro-cell death gene, Bax, and obtained similar results; cell number was increased in Bax−/− mice of both sexes, but males had about 40% more VP cells, regardless of Bax gene status. Taken together, cell death is unlikely to account for the sex difference in VP cell number, leaving differentiation of cell phenotype as the most likely underlying mechanism. We also used immunocytochemistry to examine VP projections in Bcl-2-overexpressing mice. As expected, males showed denser VP-immunoreactive fibers than females in the lateral septum, a projection area of the bed nucleus of the stria terminalis. However, even though Bcl-2 overexpression increased VP cell number, it did not affect fiber density. Thus, a compensatory mechanism may control total septal innervation regardless of the number of contributing cells.
DURING DEVELOPMENT, sex differences in gonadal hormone levels cause a multitude of sex differences in neurotransmitter systems, which presumably contribute to differences in neural function and behavior (1,2,3,4,5). In principle, two fundamentally different sets of processes could cause these differences: those that determine the absolute number of cells capable of expressing a specific neurotransmitter (such as the birth, death, or migration of cells), or processes that act on preexisting cells to alter their phenotype (4,6,7). It has been surprisingly difficult to disentangle these two possibilities, in part because gonadal hormones often trigger sexual differentiation before the neurons of interest assume their final phenotype.
A case in point is the sexually dimorphic vasopressin (VP) innervation of the brain. This innervation shows one of the most consistently found neural sex differences among vertebrates (8), with males having more VP neurons in the bed nucleus of the stria terminalis (BNST) and medial amygdaloid nucleus and denser projections from these areas than do females across many mammalian species (8). Nonmammalian vertebrates show similar sex differences in homologous vasotocin projections (8,9,10). This sex difference has been well studied in rats, where exposure to gonadal steroids during perinatal life determines the number of VP cells found in adults (11,12). Differential cell birth and migration are very unlikely to contribute to the sex differences in VP expression, because VP cells are born on embryonic d 12 and 13 (13,14) at least a week before gonadal hormones trigger their sexual differentiation (11,12). This leaves differential cell death or phenotypic differentiation as the two most likely causes.
Mice may be especially useful for differentiating between these two possibilities, because there are several genetically engineered strains in which cell death is altered (15). One would predict that mice overexpressing cell death-reducing factors would show no, or reduced, sexual differentiation if differentiation depended on cell death. The same would be true for mice lacking proteins required for neuronal cell death. Here, we compare VP expression in wild-type mice vs. two genetically altered strains: those that overexpress the antiapoptotic factor Bcl-2, specifically in neurons (16), or mice with a null mutation in the gene encoding the cell death factor Bax (17). In both mutants, neuronal cell death is markedly reduced throughout the brain, and sex differences in cell number are abolished in several brain areas (18,19). We report that these mutations do indeed increase the total number of cells that produce VP. Critically, however, the sex difference in cell number remains intact.
Materials and Methods
Animals
Wild-type and transgenic mice overexpressing human Bcl-2 under the control of the neuron-specific enolase promoter [Bcl-2-overexpressing (Bcl-2-OE) mice] (16) were generated by mating Bcl-2-OE males with B6D2F1 females (The Jackson Laboratory, Bar Harbor, ME). The line was subsequently backcrossed to B6D2F1 for at least 10 generations. In these mice, the human Bcl-2 transgene is expressed in neurons from embryonic d 13 through adulthood at levels that far exceed expression of the endogenous mouse Bcl-2 (16). Wild-type (Bax+/+) and Bax knockout (Bax−/−) mice were generated by mating mice heterozygous for the Bax gene deletion (Bax+/−; The Jackson Laboratory). The knockout was originally on a mixed C57BL/6 × 129 background (17) but subsequently was backcrossed to C57BL/6 for at least eight generations. Offspring were genotyped by PCR amplification of tail DNA using published primer sequences (20,21). Mice were housed in groups of three to four in a 12-h light, 12-h dark cycle at 24 C. Food and water were available ad libitum.
Gonadectomy and hormone treatments
Sexual differentiation of VP cell numbers is defined by the observation that the number of VP-expressing cells is higher in males, even when males and females are treated with equivalent levels of testosterone in adulthood (22,23). Thus, we gonadectomized all mice as adults (4–9 months old) under ketamine-xylazine anesthesia and implanted them with 5-mm SILASTIC brand capsules (1.57 mm inner diameter, 3.18 mm outer diameter; Dow Corning, Midland, MI) filled with crystalline testosterone (Sigma Chemical Co., St. Louis, MO). Three weeks later, mice were asphyxiated with carbon dioxide and rapidly decapitated. Brains were removed and cut midsagittally. One side was processed for in situ hybridization (all groups), and the other was used for immunostaining (Bcl-2-OE mice and their wild-type littermates only). All procedures conformed to National Institutes of Health guidelines and were in accordance with a protocol approved by the University of Massachusetts, Amherst, Institutional Animal Care and Use Committee.
In situ hybridization
Brain tissue was quickly frozen in dry ice-cooled 2-methylbutane and stored at −80 C until sectioning. Tissue was cut in 25-μm coronal sections, which were thaw-mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80 C until used. Slides were postfixed in 4% paraformaldehyde for 5 min and rinsed in 0.1 m PBS (pH 7.4) for 2 min at 4 C. The in situ hybridization closely followed a procedure used previously (23), except that each slide was exposed to about 106 dpm of a mixture of two oligonucleotide probes complementary to the mouse VP mRNA bases 383–426 and 430–477, which code mainly for the glycopeptide region at the carboxyl end of the VP precursor peptide. The probes were labeled at the 3′ end with [35S]dATP (NEN Life Science Products, Boston, MA) using terminal deoxynucleotidyl transferase (Life Technologies Inc., Gaithersburg, MD). After the hybridization procedure, slides were dipped in Kodak NTB two-track emulsion under a safelight and stored desiccated in light-tight boxes at 4 C. Four weeks later, the slides were developed, lightly counterstained with methyl green, and coverslipped with Cytoseal (Fisher Scientific).
Cell counts and silver grain density
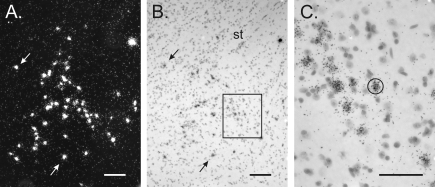
All analyses were performed on slides coded to conceal the sex and genotype of the animal. VP mRNA-labeled cells were examined in every third section through the BNST of each mouse. Labeled cells were identified under dark-field illumination (Fig. 1) and counted only if examination under bright-field illumination confirmed a methyl green-stained nucleus underneath the silver grains.
Figure 1.
In situ hybridization for VP mRNA in a Bcl-2-OE male. A and B, Dark- and bright-field views, respectively, of a section through the BNST. Counts of VP cells were made under dark-field microscopy. Arrows point to the same cells in A and B. A magnocellular VP neuron, easily distinguishable from the parvocellular BNST neurons of interest, can also be seen in the upper right corner. St, Stria terminalis. C, Higher-magnification view of the boxed area in B showing sliver grains over individual VP-positive neurons. A standard circle, as shown here, was centered over each cell for automated grain counting. Scale bars, 100 μm (A and B) and 50 μm (C).
For estimating the average number of silver grains over labeled cells, the section containing the greatest number of VP mRNA-expressing cells was identified for each subject. Images of all labeled cells in that section (approximately 50 cells per animal) were obtained with a ×40 objective and analyzed using the Image version 1.44 program developed by Dr. Rasbaud (National Institutes of Health, Bethesda, MD). The light intensity and camera settings were kept constant to standardize measurements. Grains were counted by computerized gray-level thresholding (24). A standardized circle (∼20 μm in diameter) was centered over each cell, and the number of pixels covered by grains was determined (Fig. 1). This number was converted into number of grains by dividing the total number of pixels per cell by the average number of pixels representing one silver grain (this last number was determined by analyzing 32–66 randomly chosen grains in three subjects of each experimental group).
Immunocytochemistry
VP fibers in the lateral septum were examined in Bcl-2-OE mice. Brain hemispheres were submerged in 5% acrolein in phosphate buffer (0.1 m, pH 7.6) for 2 h. Fixed brains were transferred to 30% sucrose in phosphate buffer and stored at −20 C until sectioning. Thirty-micrometer-thick sections were cut with a freezing microtome and immunostained for VP using anti-VP (ICN Immunobiologicals, Irvine, CA), diluted 1:16,000 in Tris-Triton, as described previously (11). VP antibodies were visualized using the ABC Elite kit (Vector Laboratories, Burlingame, CA) followed by incubation in a 0.05% 3,3′-diaminobenzidine solution, 0.0005% glucose oxidase, 0.15% β-d-glucose, and 0.03% nickel ammonium sulfate in Tris-NaCl for 20 min. After rinsing in Tris buffer, sections were mounted on glass slides, air dried, and coverslipped.
The density of VP-immunopositive fibers in the lateral septum was analyzed in two consecutive sections at the level containing the highest fiber density. Computerized gray-level thresholding (24) was performed using the National Institutes of Health Image program, as described previously (11). Light intensity and camera settings were kept constant across the sections to standardize measurements. The density was expressed as the area covered by VP-immunoreactive fibers in a 0.5- × 0.3-mm sampling area immediately bordering the lateral ventricle midway between the dorsal and ventral extent of the ventricular wall.
Statistical analyses
Differences between groups were analyzed separately for each mutant using two-way ANOVA with sex and genotype as between-subjects variables. Differences were considered significant if P < 0.05.
Results
VP cell number and mRNA expression
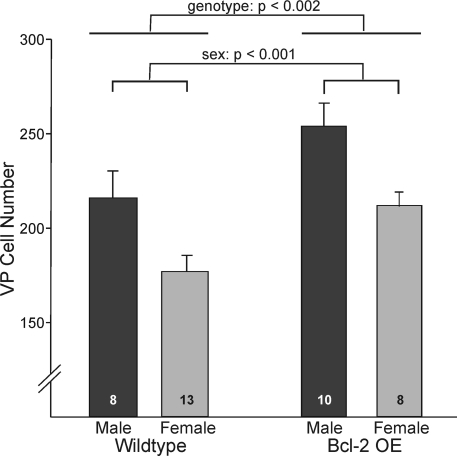
Male rats have more VP cells in the BNST cells than do females, even when both sexes are treated with identical hormone capsules in adulthood (23). In this study, we confirm that mice show a similar sex difference, albeit of smaller magnitude than is seen in rats. In the first experiment with Bcl-2-OE mice and their wild-type littermates, males had about 25% more VP cells than did females [F(1,35) = 14.570; P < 0.001]. We also observed a main effect of genotype, such that Bcl-2-OE mice had more VP cells than did wild-type mice [F(1,35) = 11.7; P < 0.002; Fig. 2]. The increase was observed in both sexes, and no sex-by-genotype interaction on VP cell number was seen [F(1,35) = 0.02; P > 0.8].
Figure 2.
Number of VP neurons in the BNST of Bcl-2 OE mice and their wild-type littermates. Bcl-2 overexpression increased the number of VP cells, but did not reduce the sex difference in cell number. Lines over bars indicate main effects of sex and genotype. There was no sex-by-genotype interaction.
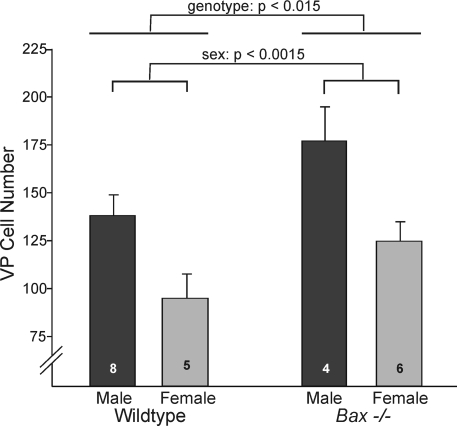
Bax−/− mice and their wild-type (Bax+/+) controls displayed the same pattern. Males had 44% more VP cells than did females irrespective of genotype [F(1,19) = 13.92; P < 0.0015; Fig. 3]. As was seen with Bcl-2-OE mice, we also found that Bax−/− animals had about 30% more VP cells than did wild-type mice [F(1,19) = 7.18; P < 0.015; Fig. 3]. There was no sex-by-genotype interaction [F(1,19) = 0.72; P > 0.7], confirming that the magnitude of the sex difference was not affected by Bax gene status. Thus, based on both strains of mice, there is a significant sex difference in VP cell number, and VP cell number is subject to Bax- and Bcl-2-dependent cell death. Importantly, however, the sex difference in VP cell number is not affected in the cell death mutants.
Figure 3.
Number of VP neurons in the BNST of Bax knockout mice (Bax−/−) and their wild-type siblings. Bax gene deletion increased the number of VP cells but did not reduce the sex difference in VP cell number. Lines over bars indicate main effects of sex and genotype. There was no sex-by-genotype interaction.
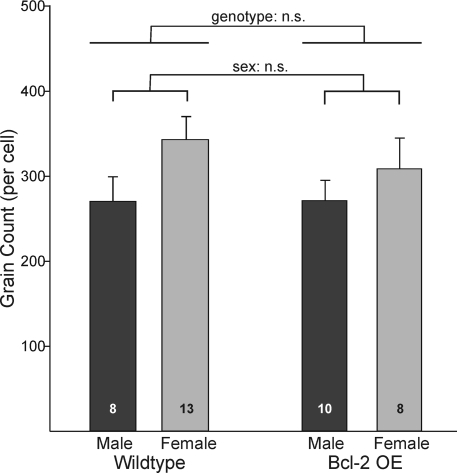
We also quantified the number of silver grains over each positive cell in Bcl-2-OE and WT mice (Fig. 1). We found no effect of genotype on average number of silver grains per cell [F(1,35) = 0.32; P > 0.5]. Thus, Bcl-2 overexpression does not affect VP mRNA levels in positive cells. There was also no effect of sex on this measure and no sex-by-genotype interaction (Fig. 4). Thus, males have more VP cells than do females, but mRNA expression per cell did not differ significantly between sexes. This differs from what has been found in rats, where both cell number and mRNA content per cell are greater in males (23). If anything, the number of silver grains per cell was slightly (but nonsignificantly) higher in female mice (Fig. 4).
Figure 4.
The number of silver grains per cell after in situ hybridization for VP mRNA in the BNST of Bcl-2-OE and wild-type mice. There were no significant differences between groups.
VP innervation of the septum
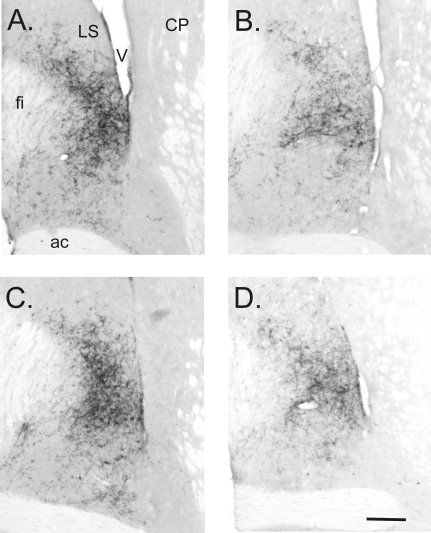
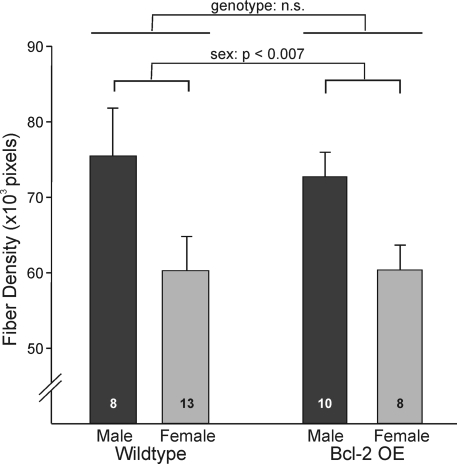
The greater number of VP cells in Bcl-2-OE mice, together with evidence that expression per cell did not differ by genotype, suggested that Bcl-2-OE mice might have a denser innervation of target sites by VP-immunoreactive nerve terminals. We assessed this by performing immunocytochemistry for VP in the lateral septum, a major projection site of BNST VP neurons (8). The sex difference in VP cell number was matched by an equally large sex difference (male more than female) in the density of VP-immunoreactive fibers in the lateral septum [F(1,34) = 8.47; P < 0.007; Figs. 5 and 6]. This was true for both wild-type and Bcl-2-OE mice [i.e. there was no interaction between sex and genotype; F(1,34) = 0.09; P > 0.7]. Surprisingly, however, despite the larger number of VP-expressing cells in Bcl-2-OE mice, VP innervation of the septum was not distinguishable from wild-type controls [F(1,34) = 0.08; P > 0.7; Figs. 5 and 6].
Figure 5.
VP immunoreactivity in the lateral septum of Bcl-2 OE and wild-type mice. A, Wild-type male; B, wild-type female; C, Bcl-2-OE male; D, Bcl-2-OE female. Medial is to the left in all views. VP immunoreactivity was greater in males than in females and was not influenced by genotype. ac, Anterior commissure; CP, caudate putamen; fi, fimbria; LS, lateral septum; V, lateral ventricle. Scale bar, 200 μm.
Figure 6.
Quantification of VP immunoreactivity in the lateral septum of Bcl-2-OE mice and their wild-type siblings. Fiber density (expressed as labeled pixels per 1.5 × 105 μm2) was greater in males than in females (P < 0.007). There was no effect of genotype and no sex-by-genotype interaction on this measure.
Discussion
The sex difference in VP innervation of the forebrain has received considerable attention in recent years and has been linked to sex differences in the modulation of autonomic functions, learning and memory, and social or reproductive behaviors in several species (2,8,10,25,26). Despite the intense interest in this neuropeptide, the cellular basis for the sex difference has not been established. Previous work tested whether differential neurogenesis contributes to sexual differentiation of VP expression. Birth dating shows that VP neurons are among the very earliest cells generated in the region, with well over 80% born on embryonic d 12 and 13 and virtually none born after embryonic d 16 in either sex (13,14). Gonadal hormones, however, act during the first 2 wk postnatally to determine the number of BNST cells that will express VP in adulthood (11,12). Similarly, cell migration into the BNST appears complete prenatally (27), before gonadal hormones determine the final number of VP cells, and there is no evidence for differential placement of VP cells in males and females. This leaves the differentiation of cell phenotype or differential cell death as possible mechanisms underlying the sex difference in VP number.
Prior evidence in rats favors the differentiation of cell phenotype. Essentially all VP cells in the BNST coexpress the neuropeptide galanin, but not all galanin cells coexpress VP (28). Because the total number of galanin cells does not differ between males and females, it was hypothesized that, during development, higher levels of testosterone act on existing galaninergic cells to increase the percentage that will coexpress VP (29). In support, VP and galanin neurons in the BNST and medial amygdaloid nucleus of rats show the same unusual birth profile, with both types of neuron born days earlier than most surrounding cells (14), consistent with the idea that these neurons belong to a single pool.
The hypothesis that testosterone directs pluripotent cells to become vasopressinergic was based on circumstantial evidence, however, and is difficult to test directly. Gonadal steroid hormones determine VP cell number soon after birth (11,12), yet the vast majority of presumptive VP neurons do not begin expressing VP until days (in males) to weeks (in females) later (30). Thus, one cannot identify the cells of interest during the time the sex difference in their number is determined. It has also been difficult to rule out a role for cell death. In mice, the number of galanin cells in the BNST may itself be sexually dimorphic (31), which is problematic for a model that supposes that testosterone acts on a sexually undifferentiated pool of galaninergic precursor cells to produce the sex difference in VP cell number. In rats, it remained possible that testosterone increases the death of galanin-only cells while decreasing the death of cells coexpressing galanin and VP. The current observations, however, rule out cell death as a likely factor.
We find that sex differences in VP cell number persist in Bcl-2-OE and Bax−/− mice. We previously used these same mutants to examine the cellular basis of sex differences in several other neural systems (18,19,32). In each case, and in contrast to results presented here, sex differences in overall cell number were eliminated by Bax deletion, or significantly reduced by Bcl-2 overexpression, indicating that differential cell death in males and females was responsible for the differences. Here we report that although Bax deletion and Bcl-2 overexpression increase the number of VP cells, neither mutation reduces the sex difference.
Because there are multiple paths to cell death (33), these observations alone would not completely rule out the possibility that cell death contributes to the sex difference in VP cell number, but that in this case death is Bax and Bcl-2 independent. Several considerations make this possibility rather unlikely, however. The Bcl-2 family proteins have emerged as crucial regulators of survival in the developing nervous system (15,16,34), and Bax in particular appears to be required for most cell death in the developing nervous system (21). VP cell number in this study was increased in Bax−/− mice, as is total cell number in the BNST of Bax knockouts (19). Even more important, apoptosis is essentially eliminated in the BNST of perinatal Bax−/− mice of both sexes (35). That is, there is no evidence that in Bax−/− mice, cells in the BNST find another, Bax-independent, road to death. Nonetheless, preventing cell death by Bax deletion does not have any impact on the sex difference in the number of BNST cells that express VP.
Bcl-2 overexpression also increased total VP cell number without affecting the sex difference in VP cells. A previous report suggests that a Bcl-2 null mutation reduced VP synthesis in magnocellular neurons of the paraventricular and supraoptic nuclei in the hypothalamus (36). If so, then overexpression of Bcl-2 might have increased cell counts simply by increasing VP expression in the BNST, thereby enhancing the detectability of individual VP neurons. We can rule out this interpretation, however, because we did not find a difference in VP mRNA levels between wild-type and Bcl-2-OE mice. Thus, the increased number of VP cells in Bcl-2-OE mice is not due to changes in peptide expression and, therefore, detectability of cells.
As argued above, our present and previous studies rule out differential cell birth, migration, and death as likely causes for sexual differentiation of vasopressin innervation, leaving differentiation of neuronal phenotype as the only remaining plausible cause. Numerous other neurotransmitters and neuropeptides (e.g. dopamine, neurotensin, substance P, and enkephalin) show sex differences in cell number (reviewed in Ref. 1), and for none of these has the cellular mechanism of sexual differentiation been established. The search for these mechanisms may be amenable to the same strategy used here. For example, we previously examined dopaminergic neurons in the anteroventral periventricular nucleus, which are much more numerous in females than in males (37). Although neither Bax deletion nor Bcl-2 overexpression eliminated this sex difference (18,19), the interpretation of this finding is muddied by the fact that the total number of dopaminergic neurons in anteroventral periventricular nucleus also was unaffected in the cell death mutants. Thus, the mechanism regulating this sex difference remains unresolved.
Because Bcl-2-OE mice had an increased number of VP cells in the BNST, and mRNA expression per cell did not differ, one might expect denser VP innervation of BNST target sites. Interestingly, our data contradict this; the density of VP fibers in the lateral septum did not differ between Bcl-2-OE and wild-type mice. One possible explanation for this result is that the extra VP neurons in the mutants do not form functional connections with the septum. For example, motoneurons rescued from developmental cell death by Bax gene deletion may not project to target muscles (38). However, these supernumerary motoneurons are also severely atrophic and fail to express cell-specific markers, which does not appear to be the case for the VP cells examined here. Alternatively, a mechanism independent of VP cell number may regulate the density of VP innervation of the septum. Signals from target cells influence axon branching and the density of afferent innervation in many neural systems (39,40,41). Thus, the septum might limit the total input from the BNST. If so, then increasing the number of BNST cells would not be expected to increase the density of VP projections; instead, axon branching and/or synapse number per cell would be reduced. Our differentiation of cell phenotype hypothesis predicts that the magnitude of the sex difference in VP innervation is unlikely to be affected, because this hypothesis posits that the number of cells projecting to targets such as the septum does not differ in males and females; rather, it is the percentage of those cells expressing VP that is differentiated. The fact that the sex difference in VP innervation was preserved in Bcl-2-OE mice is one more piece of evidence in support of this hypothesis.
The next hurdle will be to identify the molecular mechanisms underlying differentiation of VP expression. The permanent effect of testosterone on the potential of cells to express VP suggests that epigenetic mechanisms (e.g. methylation of the gene promoter region or modifications of its associated histones) are likely to be involved (42). A more complete understanding of the sexual differentiation of VP innervation may provide clues to the origin of behavioral disorders such as depression, autism, and schizophrenia (43,44,45,46,47). Interestingly, each of these disorders shows sex differences in occurrence (48,49,50) and has been linked to variability in VP neurotransmission, e.g. elevated VP levels in cerebrospinal fluid or polymorphisms in the VP receptor gene (43,51,52,53,54,55,56). Mechanisms that contribute to differences in VP innervation may therefore explain some of the variation between the sexes found in these disorders.
Acknowledgments
We thank Lynn Bengston for assistance with the figures.
Footnotes
The research was funded by National Institutes of Health Grants KO2 MH01497 and RO1 MH47538 (G.J.d.V.) and KO2 MH072825 and RO1 MH068482 (N.G.F.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 22, 2008
Abbreviations: Bcl-2-OE, Bcl-2-overexpressing; BNST, bed nucleus of the stria terminalis; VP, vasopressin.
References
- De Vries GJ 1990 Sex differences in neurotransmitter systems. J Neuroendocrinol 2:1–13 [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP 1996 Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol 16:325–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM 1998 Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol 19:323–362 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB 2002 Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, eds. Hormones, brain, and behavior. Vol IV. San Diego: Academic Press; 137–191 [Google Scholar]
- Simerly RB 2002 Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- Tobet SA, Hanna IK 1997 Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cell Mol Neurobiol 17:565–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG 2006 Cell death and sexual differentiation of the nervous system. Neuroscience 138:929–938 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC 2006 Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Richardson C, Lowry CA 2000 Sexual dimorphism in numbers of vasotocin-immunoreactive neurons in brain areas associated with reproductive behaviors in the roughskin newt. Gen Comp Endocrinol 117:281–298 [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH 2001 Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev 35:246–265 [DOI] [PubMed] [Google Scholar]
- Wang Z, Bullock NA, De Vries GJ 1993 Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology 132:2299–2306 [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ 2003 Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol 54:502–510 [DOI] [PubMed] [Google Scholar]
- al-Shamma HA, De Vries GJ 1996 Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. J Neurobiol 29:91–98 [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ 1999 Neurogenesis of galanin cells in the bed nucleus of the stria terminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neurobiol 38:491–498 [PubMed] [Google Scholar]
- Lindsten T, Zong WX, Thompson CB 2005 Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist 11:10–15 [DOI] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J 1994 Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017–1030 [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ 1995 Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96–99 [DOI] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG 2003 Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci 23:2357–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ 2004 Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier M, Junier MP, Peschanski M, Dreyfus PA 1996 Bcl-2 sensitivity differentiates two pathways for motoneuronal death in the wobbler mutant mouse. J Neurosci 16:5897–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD 1998 Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18:1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, al-Shamma HA 1990 Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol 21:686–693 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Wang Z, Bullock NA, Numan S 1994 Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci 14:1789–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Lun J, McLean JH 1989 Processing and analysis of neuroanatomical images. In: Neuroanatomical tract tracing methods 2. Heimer L, Záborsky L, eds. New York: Plenum Press; 331–390 [Google Scholar]
- Pittman QJ, Chen X, Mouihate A, Hirasawa M, Martin S 1998 Arginine vasopressin, fever and temperature regulation. Prog Brain Res 119:383–392 [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z 2004 The neurobiology of pair bonding. Nat Neurosci 7:1048–1054 [DOI] [PubMed] [Google Scholar]
- Bayer SA 1987 Neurogenetic and morphogenetic heterogeneity in the bed nucleus of the stria terminalis. J Comp Neurol 265:47–64 [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA 1995 Vasopressin and galanin mRNAs coexist in the nucleus of the horizontal diagonal band: a novel site of vasopressin gene expression. J Comp Neurol 361:48–56 [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA 1995 Sex difference in coexpression by galanin neurons accounts for sexual dimorphism of vasopressin in the bed nucleus of the stria terminalis. Endocrinology 136:727–733 [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM 1993 Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Brain Res Dev Brain Res 73:177–183 [DOI] [PubMed] [Google Scholar]
- Rajendren G, Levenkova N, Gibson MJ 2000 Galanin immunoreactivity in mouse basal forebrain: sex differences and discrete projections of galanin-containing cells beyond the blood-brain barrier. Neuroendocrinology 71:27–33 [DOI] [PubMed] [Google Scholar]
- Jacob DA, Bengston CL, Forger NG 2005 Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci 25:5638–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO 2000 The biochemistry of apoptosis. Nature 407:770–776 [DOI] [PubMed] [Google Scholar]
- Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O 1995 bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci USA 92:4397–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG 2007 Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol 67:355–362 [DOI] [PubMed] [Google Scholar]
- Chernigovskaya EV, Taranukhin AG, Glazova MV, Yamova LA, Fedorov LM 2005 Apoptotic signaling proteins: possible participation in the regulation of vasopressin and catecholamines biosynthesis in the hypothalamus. Histochem Cell Biol 124:523–533 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS 1997 Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA 94:14077–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Gould TW, Vinsant S, Prevette D, Oppenheim RW 2003 Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion. J Neurosci 23:7298–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE 1995 Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature 378:192–196 [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW 1998 Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science 279:1725–1729 [DOI] [PubMed] [Google Scholar]
- Cesa R, Strata P 2005 Axonal and synaptic remodeling in the mature cerebellar cortex. Prog Brain Res 148:45–56 [DOI] [PubMed] [Google Scholar]
- Ma Q 2006 Transcriptional regulation of neuronal phenotype in mammals. J Physiol 575:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA 1998 Double duty for sex differences in the brain. Behav Brain Res 92:205–213 [DOI] [PubMed] [Google Scholar]
- De Vries GJ 2004 Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145:1063–1068 [DOI] [PubMed] [Google Scholar]
- Ring RH 2005 The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des 11:205–225 [DOI] [PubMed] [Google Scholar]
- Landgraf R 2006 The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets 5:167–179 [DOI] [PubMed] [Google Scholar]
- Carter CS 2007 Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res 176:170–186 [DOI] [PubMed] [Google Scholar]
- Altemus M 2006 Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50:534–538 [DOI] [PubMed] [Google Scholar]
- Goldstein JM 2006 Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav 50:612–622 [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S 2006 Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol 21:825–845 [DOI] [PubMed] [Google Scholar]
- Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ 1984 Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci 234:162–165 [DOI] [PubMed] [Google Scholar]
- Gjerris A, Hammer M, Vendsborg P, Christensen NJ, Rafaelsen OJ 1985 Cerebrospinal fluid vasopressin: changes in depression. Br J Psychiatry 147:696–701 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti Jr TD, Listwak SJ, Kling MA 1993 Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150:656–657 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook Jr EH, Insel TR 2002 Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry 7:503–507 [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E 2006 The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav 50:518–528 [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP 2006 Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry 11:488–494 [DOI] [PubMed] [Google Scholar]