Abstract
Kisspeptin is recognized to play a critical role in eliciting the pubertal resurgence of pulsatile GnRH release, the proximal trigger of puberty in higher primates. Expression of the kisspeptin receptor (GPR54) by GnRH neurons indicates a direct action of kisspeptin on the GnRH neuronal network. The purpose of the present study was to examine the distribution of kisspeptin cell bodies in the monkey hypothalamus and to assess the structural basis for the stimulatory action of kisspeptin on the GnRH neuronal network. Three castrated male rhesus monkeys, 39–51 months of age, were deeply anesthetized and their brains perfused transcardially with 4% paraformaldehyde in PBS. Serial 25-μm coronal sections throughout the hypothalamus were prepared, and immunopositive neurons identified using a cocktail of specific primary antibodies (sheep anti-kisspeptin at 1:120,000, and rabbit anti-GnRH at 1:100,000) detected with fluorescently tagged secondary antibodies (antisheep, Alexa Fluor 488; antirabbit, Cy3) in combination with confocal microscopy. Kisspeptin perikarya were found only in the mediobasal hypothalamus (MBH) almost exclusively in the posterior two-thirds of the arcuate nucleus. Surprisingly, kisspeptin-beaded axons made only infrequent contacts with GnRH neurons (kisspeptin and GnRH profiles abutting in a 0.5- to 1.0-μm optical section) in the MBH. In the median eminence, kisspeptin and GnRH axons were found in extensive and intimate association. GnRH contacts on kisspeptin perikarya and dendrites were observed. These findings indicate that nonsynaptic pathways of communication in the median eminence should be considered as a possible mechanism of kisspeptin regulation of GnRH release, and provide an anatomical basis for reciprocal control of kisspeptin neuronal activity by GnRH.
PUBERTY IN MAN and other higher primates is triggered by a robust resurgence in pulsatile GnRH release that has been held in check since infancy by mechanisms that are poorly understood (1). An important role for kisspeptin-GPR54 signaling in activating the pubertal increase in GnRH release emerged in 2003 when it was reported that several members of two large consanguineous families presenting with hypogonadotropic hypogonadism and absent puberty were found to carry homozygous loss of function mutations for GPR54 (2,3). Moreover, administration of a pulsatile regimen of GnRH reversed the hypogonadotropic state in a subject bearing a compound heterozygote mutation of the receptor (3), indicating a hypothalamic locus for the hypogonadotropism associated with inactivating mutations of GPR54.
Subsequent studies of the rhesus monkey have provided further evidence for the view that kisspeptin signaling is a critical component of the hypothalamic mechanism that triggers the pubertal resurgence of GnRH pulsatility. Hybridization histochemistry demonstrated that, as in nonprimate species (4,5,6,7), the gene coding for kisspeptin, KiSS-1, was expressed in the arcuate nucleus of the mediobasal hypothalamus (MBH) of male and female rhesus monkeys (8). Expression of KiSS-1 increased at the time of the pubertal resurgence in GnRH release in both agonadal males and intact females, and an increase in GPR54 expression was observed in females (8). Moreover, administration of brief iv infusions of kisspeptin-10 every h for 48 h to agonadal juvenile males in which pituitary responsiveness to GnRH had been heightened by a priming infusion of synthetic GnRH elicited a sustained train of GnRH-dependent LH discharges comparable to those observed spontaneously in pubertal and postpubertal castrate male monkeys (9).
GnRH neurons in several species including the monkey have been reported to express GPR54 mRNA (10,11,12), indicating that the action of kisspeptin to elicit GnRH release is likely to be exerted directly on the GnRH neuronal network. The anatomical locus of the interaction between kisspeptin axonal terminals and GnRH neurons, however, has received limited attention (13) and has not been studied in the monkey. Theoretically, direct kisspeptin regulation of the GnRH neuron may be achieved via three major interactions, namely axo-somatic, axo-dendritic, and axo-axonal or a combination of these. The purpose of the present study was therefore to describe, in the male rhesus monkey, the overall structural inter-relationship between the two networks of these hypothalamic peptides using double-label immunofluorescence coupled with confocal microscopy. For the initial studies reported here, we selected agonadal males because testosterone has been shown to reduce KiSS-1 expression in the MBH of castrate male monkeys (14) and, in rodents, orchidectomy increases kisspeptin mRNA levels in the MBH and, specifically, in the arcuate nucleus (5,15,16). It was reasoned, therefore, that kisspeptin content also might be correspondingly higher in the castrate situation, facilitating, for the first time, description of the distribution of kisspeptin neurons in the hypothalamus of a higher primate.
Materials and Methods
Animals
Three male rhesus monkeys (Macaca mulatta; 39–51 months old, 5–6 kg body weight) were used. The animals had been bilaterally orchidectomized for otherwise noninvasive studies when they were 5 days, 7 months, and 36 months old, respectively. The interval between castration and perfusion of the brain was 5 months or more. It should be noted that the pubertal resurgence of LH secretion in agonadal male rhesus monkeys occurs between 24–30 months of age, regardless of whether castration is conducted during infancy or the juvenile phase of development (17,18,19): this was confirmed in two of the monkeys by documenting elevated circulating LH concentrations before perfusion.
The animals were maintained under controlled photoperiod (lights on between 0700 and 1900 h) and temperature (approximately 21 C) in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Transcardial perfusion and serial sectioning
Animals were first sedated with ketamine hydrochloride (10–20 mg/kg body weight, im; Ketaject, Phoenix Scientific Inc., St. Joseph, MO) and then deeply anesthetized with sodium pentobarbital (approximately 30 mg/kg body weight, iv; Nembutal sodium solution, Abbott Laboratories, North Chicago, IL). The thoracic cavity was opened, the dorsal aorta clamped, and using a peristaltic pump the brain perfused transcardially at a flow rate of approximately 40 ml/min. The brain was initially perfused with approximately 1 liter of pre-fix (physiological saline containing 2% sodium nitrite and 5000 U of heparin/liter), and this was immediately followed by 2–3 liters of fixative (4% paraformaldehyde in 0.1 m PBS, pH 7.2). After perfusion, the brain was removed from the cranium and the hypothalamus isolated. Coronal cuts were made immediately anterior to the optic chiasm and through the mammillary bodies. A parasagittal cut was made at approximately 4 mm on either side of midline. A final horizontal cut was made immediately dorsal to the anterior commissure. The hypothalamic block was then immersed in fixative for a further 1–2 h. A fragment of cortex was also collected from one animal for use in control studies. Tissues were then transferred to a 30% sucrose solution (in 0.1 m PBS, pH 7.2) for at least 24 h at 4 C. Serial 25-μm sections throughout the entire hypothalamic block were cut on a freezing microtome and placed sequentially into a train of 10 wells so that each well contained a sequence of sections collected at 250-μm intervals from the region of the organum vasculosum of the lamina terminalis to the mammillary bodies. Twenty-five-micrometer sections of cortex were also cut. All sections were stored in an antifreeze cryoprotectant solution at −20 C (20).
Antibodies and blocking peptide
The kisspeptin antibody (GQ2) was raised in sheep against synthetic human (h) kisspeptin-54 and was kindly provided by Dr. Stephen Bloom (Imperial College London, Hammersmith Hospital, London, UK). The specificity of GQ2 has been previously documented by Dhillo et al. (21) for use in RIA. Cross-reactivity with h kisspeptin-54, h kisspeptin-14, and h kisspeptin-10 was 100%, whereas that with other related RF-amide peptides (RFRP1, RFRP2, RFRP3, neuropeptide FF, neuropeptide AF, and prolactin releasing peptide) was less than 0.01% (21). In our study, GQ2 antibody was used at a dilution of 1:120,000. The polyclonal anti-GnRH antibody (LR1, raised against [d-Lys (6)] GnRH in rabbit) was provided by Dr. Robert Benoit (Montreal General Hospital, Montreal, Quebec, Canada). We have previously characterized this antibody (22,23) and, in the present study, LR1 was used at a dilution of 1:100,000. Alexa Fluor 488 donkey antisheep IgG (Invitrogen Corp., Carlsbad, CA) and Cy3-conjugated AffiniPure donkey antirabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used as secondary antibodies to detect kisspeptin and GnRH immunoactivity, respectively.
For control purposes, h kisspeptin-54 (Phoenix Pharmaceuticals, Inc., Burlingame, CA) was used to preadsorb GQ2.
Fluorescence immunocytochemistry
Fluorescence immunocytochemistry for kisspeptin and GnRH was performed on free-floating sections of hypothalamus and cortex. Sections were rinsed at room temperature in 50 mm PBS (pH 7.3, 8 × 15 min), incubated for 30 min in 3% hydrogen peroxide, and rinsed again in 50 mm PBS (6 × 5 min). They were then incubated overnight at 4 C on a shaker in PBS buffer containing 5% normal horse serum (Vector Laboratories, Inc., Burlingame, CA), 0.05% Triton X-100 and 0.1% BSA (Sigma Chemical Co., St. Louis, MO) to block nonspecific binding. For dual label immunofluorescence staining, the sections were incubated for 48 h at 4 C on a shaker in a cocktail of the primary antibodies prepared in the PBS-horse serum buffer, and then washed in 50 mm PBS (4 × 5 min) at room temperature. To detect immunofluorescence, sections were incubated for 1 h in the dark at room temperature in a cocktail of the secondary antibodies in PBS-horse serum buffer, both at a dilution of 1:200. After washing, again in 50 mm PBS (4 × 5 min), sections were mounted on slides (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA) treated with subbing solution (0.1% gelatin and 0.01% chromium potassium sulfate), allowed to dry in the dark, coverslipped using GEL/MOUNT aqueous mounting medium with anti-fading agents (Biomeda Corp., Foster City, CA), and stored at 4 C until analyses.
For single label fluorescence immunocytochemistry, the same procedure was employed, but only one primary antibody (and the corresponding secondary antibody) was used.
The specificity of GQ2 was established by either omitting the primary antibody from the 48-h incubation or by preadsorbing GQ2 overnight with h kisspeptin-54 at a concentration of 5 μg/ml before incubation with tissue. This was performed with both the single- and double-label procedures. As noted above, specificity of LR1 had been previously established for monkey hypothalamus (22,23).
Confocal microscopy
Imaging of fluorescence labeling for kisspeptin and GnRH was performed using an Olympus FV1000 confocal microscope equipped with a four-laser system (Multi AR laser, HeNe G laser, HeNe R laser, and LD405/440 laser diode) with transmitted light, DIC, and complete integrated image analysis software system (Olympus America Inc., Melville, NY). The excitation and emission wavelengths for Alexa Fluor 488 (green) and Cy3 (red) were 488/520 nm and 543/570 nm, respectively. For low- (×10–20) and high-magnification (×40–100) profiles, optical sections along the z-axis were collected at 1-μm and 0.5- to 1.0-μm intervals, respectively. Composite digital images were then converted to TIFF format, imported into Adobe Photoshop (Adobe Photoshop CS2, version 9.0; Adobe Systems Inc., San Jose, CA), and color balance was adjusted for presentation.
Analyses of kisspeptin-GnRH interactions
The analysis of kisspeptin-GnRH interactions was restricted to the MBH because the majority of neuroendocrine GnRH neurons in the monkey are localized in this region of the hypothalamus (24). For each animal, up to 30 GnRH perikarya were identified at ×10–20 magnification and their location [ventral hypothalamic tract (VHT), median eminence, or other] noted. Confocal optical sectioning in the z-axis at 0.5–1.0 μm of each GnRH cell body and associated dendrites was performed at ×40–60 magnification and the composite image captured as a projection and saved as a TIFF format. For each animal, projections of 20 GnRH perikarya and their associated dendrites were then randomly selected and analyzed quantitatively for contacts with kisspeptin fibers. Each of the z-sections comprising the projection of a GnRH neuron was examined sequentially to identify interactions with kisspeptin fibers. For a contact to be recorded, two criteria had to be fulfilled. First, a kisspeptin axonal bead had to be observed abutting a GnRH cell body in the same optical section, and second, the kisspeptin axon on one or both sides of the contact had to be recognized as approaching or running adjacent to the GnRH cell body in the projection of individual optical sections. The number of kisspeptin contacts per GnRH perikaryon and the percentage of GnRH cell bodies contacted by kisspeptin was then determined. The length of dendrite associated with each of the 20 GnRH neurons was also measured, and the number of kisspeptin axo-dendritic contacts determined as described above for the perikaryon. Dendritic contacts were expressed per 10 μm of dendrite and an average (± sem) was calculated for each animal. At the level of the median eminence, representative fields of kisspeptin and GnRH axonal interactions (approximately five per animal) were analyzed at ×100 magnification with confocal optical sections of 0.5 μm. Axo-axonal contacts were not quantified.
Results
Kisspeptin immunopositive perikarya were observed in the arcuate nucleus and, occasionally, in the median eminence, whereas GnRH cell bodies were found throughout the hypothalamus with concentrations in the region of the organum vasculosum of the lamina terminalis in the rostral hypothalamus and in the VHT in the MBH (Fig. 1). The profiles of kisspeptin cell bodies were typically pear shaped with a diameter of 15–20 μm and one major short dendrite (see below). In contrast, GnRH soma was fusiform and typically bipolar. The density of kisspeptin and GnRH immunopositive axons was greatest in the region of the arcuate nucleus and median eminence (Fig. 1). For this reason, sections containing the arcuate nucleus and median eminence were selected for control studies. Preabsorption of GQ2 completely abolished cell body and fiber staining for kisspeptin in the arcuate-median eminence region with both the single and double label procedure (Fig. 2). Not surprisingly, omission of GQ2 from the incubation cocktail of primary antibodies also resulted in loss of all kisspeptin immunoactivity (not shown). Neither kisspeptin nor GnRH immunopositive profiles were detected in cortex (not shown).
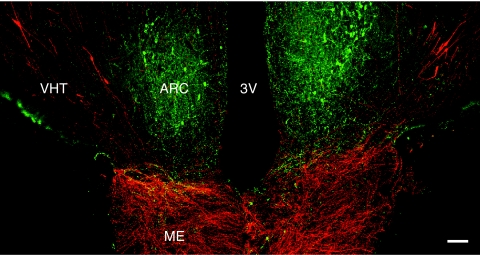
Figure 1.
A confocal projection (×10; 1-μm optical sections) illustrating the distribution of kisspeptin neurons (green fluorescence, Alexa Fluor 488) in relation to the GnRH neuronal network (red fluorescence, Cy3) in a coronal section of the MBH of an agonadal male rhesus monkey aged 4 yr 3 months. Whereas kisspeptin perikarya were confined to the arcuate nucleus (ARC), those of GnRH extended along the VHT, lateral to the arcuate nucleus. GnRH innervation of the external zone of the median eminence (ME) was intense. Beaded kisspeptin axons projected to the median eminence, and at this anteroposterior level GnRH and kisspeptin fibers running in a near horizontal plane were found in close association. 3V, Third ventricle. Scale bar, 100 μm.
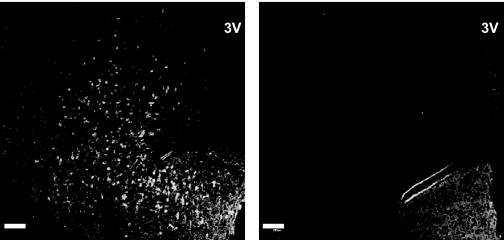
Figure 2.
Confocal projections (×10; 1-μm optical sections) of single immunofluorescence images of hemi-sections taken at the level of the arcuate nucleus from the same full coronal section of monkey MBH immunostained for kisspeptin with the unadsorbed primary antibody, GQ2 (left panel), or the same primary antibody preadsorbed with h kisspeptin-54 (right panel), both as a cocktail with LR1, the primary antibody for GnRH (not shown). 3V, Third ventricle. Scale bar, 100 μm.
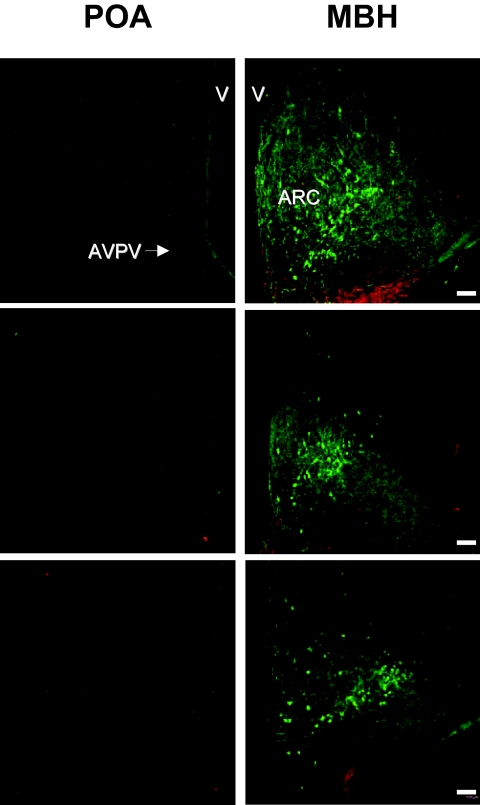
Examination of sections throughout the hypothalamus of each of the three monkeys revealed a circumscribed location of kisspeptin perikarya in the posterior two-thirds of the arcuate nucleus but failed to reveal a single kisspeptin cell body in the preoptic area (POA) that included the anteroventricular periventricular nucleus (Fig. 3). Kisspeptin perikarya were rarely observed in the retrochiasmatic portion of the arcuate nucleus. This kisspeptin cell body poor zone in the anterior arcuate region extended caudally from the posterior boundary of the optic chiasm approximately 500 μm. An occasional kisspeptin cell body was observed in the internal zone of the median eminence.
Figure 3.
Confocal dual immunofluorescence projections of coronal hemi-hypothalamic sections of the POA at the level of the AVPV (left panels) and MBH at the level of the arcuate nucleus (ARC, right panels) stained for kisspeptin (green fluorescence, Alexa Fluor 488) and GnRH (red fluorescence, Cy3) in three castrated male rhesus monkeys each represented by a pair of horizontal panels. Note the abundance of kisspeptin neurons in the arcuate nucleus but the absence of kisspeptin perikarya in the region of the AVPV (shown in top left panel by the arrow) in the POA. The third ventricle (V) and its ependymal lining may be seen on the right and left hand edges of the POA and MBH sections, respectively. Scale bar, 100 μm.
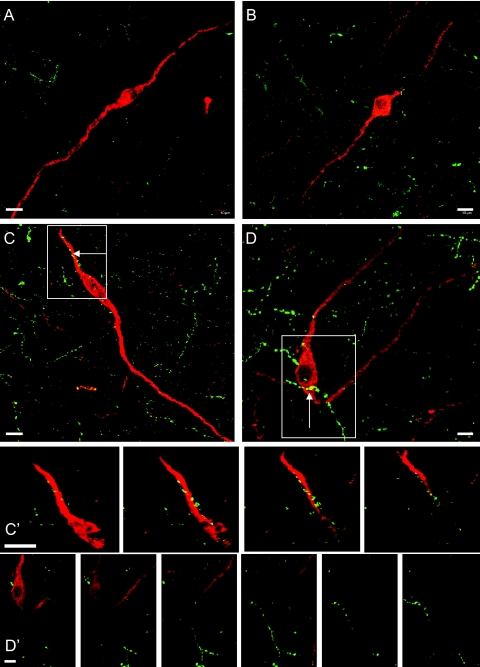
Kisspeptin-beaded axonal projections were found throughout the MBH and to a lesser extent in the POA. Twenty GnRH perikarya and their dendrites primarily located in the VHT were selected for detailed analysis. A striking feature of the double-label confocal projections of these profiles was the absence of evidence for extensive innervation by kisspeptin fibers of either GnRH soma or GnRH dendrites. Four such neurons are shown in Figure 4. Whereas the occasional kisspeptin-beaded axon was observed in the confocal projection to cross or run adjacent to a GnRH profile, examination of sequential 0.5- to 1.0-μm optical sections comprising these projections frequently failed to confirm a kisspeptin contact on the GnRH profile (Fig. 4). The mean number of axo-somatic and axo-dendritic contacts of kisspeptin on GnRH was determined for the 20 MBH neurons for each animal (Table 1). The mean number of axo-somatic contacts per GnRH neuron observed in three monkeys was similar (0.3–0.5 contacts/soma) and between 25–50% of GnRH perikarya were contacted by kisspeptin fibers. The mean number of axo-dendritic contacts ranged from 0.12–0.16 /10 μm of dendrite.
Figure 4.
Confocal dual immunofluorescence images of the structural relationship between kisspeptin beaded axons (green fluorescence, Alexa Fluor 488) and GnRH perikarya and dendrites (red fluorescence, Cy3) in the VHT of the MBH of three castrated male rhesus monkeys. A–D, Confocal projections (×60; 0.5- to 1.0-μm optical sections) of typical GnRH neurons in the VHT from three animals (C and D are from the same monkey). Panels in C′, Contact between kisspeptin axon and GnRH dendrite apparent in panel C (arrow in the white rectangle) was confirmed by examination of four sequential 0.5 μm confocal optical sections. Panels in D′, examination of six sequential 1.0 μm confocal optical sections of the GnRH profile in panel D (arrow in the white rectangle) demonstrates that the beaded kisspeptin axon transversing diagonally does not contact the GnRH soma. Scale bar, 10 μm.
Table 1.
Kisspeptin-GnRH contacts in the MBH of three castrated male rhesus monkeys
| Monkey | Axo-somatic (contacts/perikaryon) | % Perikarya with contacts | Dendrite length (μm)a | Axo-dendritic (contacts/10 μm)a |
|---|---|---|---|---|
| 1 | 0.50 | 50 | 165 ± 13 | 0.12 ± 0.03 |
| 2 | 0.25 | 25 | 70 ± 13 | 0.12 ± 0.04 |
| 3 | 0.30 | 25 | 97 ± 16 | 0.16 ± 0.04 |
Mean ± sem.
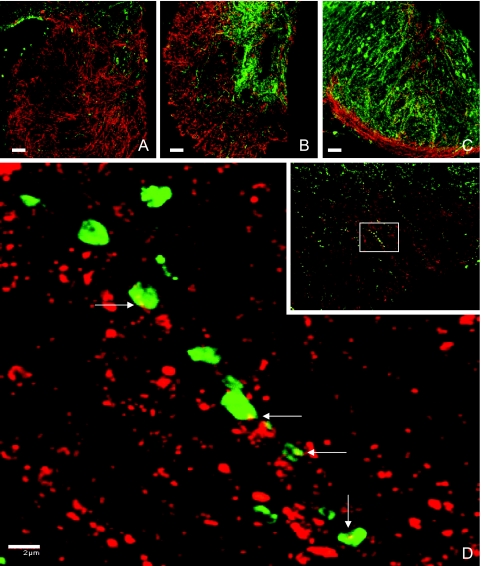
Kisspeptin axons penetrated deep into the internal zone of the median eminence, and this was particularly striking at the mid-tuberal level (Fig. 5B). Kisspeptin axons were also found, but to a noticeably lesser extent, in the external layer of the median eminence (Fig. 5, A–C). More anteriorly, kisspeptin axons were observed pursuing a horizontal path appearing to run in close association with the many GnRH axons projecting ventrally to both the internal and external zones of the median eminence (Fig. 1). At all levels of the median eminence and in the transitional area between the arcuate nucleus and median eminence, kisspeptin-beaded axons and GnRH fibers appeared to be intimately associated, and this was confirmed when selected areas were examined at high magnification (×100). Inspection of individual 0.5-μm optical sections revealed, on occasion, contacts between kisspeptin axonal beads and GnRH fibers (Fig. 5D).
Figure 5.
Confocal dual immunofluorescence images showing interactions between kisspeptin (green fluorescence, Alexa Fluor 488) and GnRH (red fluorescence, Cy3) axons in the median eminence of castrated male rhesus monkeys. Top panels (A–C) (×20, 1.0-μm optical sections): Coronal hemi-sections of median eminence at (A) anterior level through the external zone with predominantly GnRH innervation, (B) mid-tuberal level with heavy kisspeptin innervation of the internal zone and GnRH innervation of both the internal and external zones, and (C) extreme posterior level with GnRH fibers in the external layer and kisspeptin and GnRH fibers in the arcuate nucleus and sub arcuate region. Bottom panel (D), Confocal projection (×100; 0.5-μm optical sections) of kisspeptin-GnRH contacts in a region of the external zone of the median eminence shown in the white rectangle outlined in the inset (×40, 1-μm optical sections). Contacts between kisspeptin axonal beads and GnRH fibers (white arrows) were confirmed by examination of individual 0.5-μm optical sections. Scale bar for A–C, 50 μm. Scale bar for D, 2 μm.
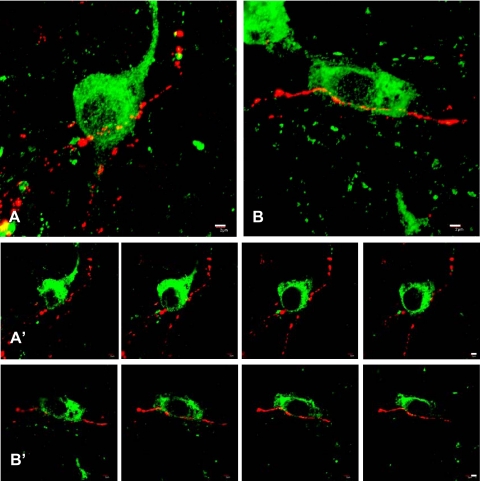
A few GnRH-beaded axons were found in the arcuate nucleus in areas rich in kisspeptin perikarya and the occasional contact between GnRH axonal beads and kisspeptin perikarya and/or dendrites was established by examining serial 0.5-μm optical sections through individual kisspeptin cell bodies (Fig. 6). A semiquantitative analysis in two monkeys indicated that no more than 1% of kisspeptin cell bodies were contacted by GnRH axonal boutons and such neurons were usually found in the ventral arcuate nucleus near the boundary with the median eminence.
Figure 6.
Confocal dual immunofluorescence images of contacts between GnRH beaded axons (red fluorescence, Cy3) and kisspeptin perikarya and dendrites (green fluorescence, Alexa Fluor 488) in the arcuate nucleus of a castrated male rhesus monkey. Panels A and B, confocal projections (×60; 0.5-μm optical sections). Panels in A′ and B′, sequential 0.5-μm optical sections of the kisspeptin neurons shown in panels A and B, respectively, confirming contacts between kisspeptin soma and GnRH axons. Scale bar, 2 μm.
Discussion
To our knowledge, this is the first description of the localization of immunopositive kisspeptin neurons in the hypothalamus of any species of primate. The primary antibody employed, GQ2, was raised in sheep immunized to synthetic h kisspeptin-54 by Dhillo et al. (21), who successfully used GQ2 to measure levels of kisspeptin-54 in the circulation of men. As described earlier, these authors reported that cross-reactivity of GQ2 with several related h RF-amide peptides was less than 0.01%. In the present study, preadsorption of GQ2 with synthetic h kisspeptin-54 completely eliminated the immunocytochemical signal in the monkey MBH at the level of the arcuate nucleus and median eminence. Earlier attempts by this laboratory to use polyclonal antibodies to h kisspeptin-10 to examine hypothalamic kisspeptin in the monkey hypothalamus were far less successful. Although it would be unwise to generalize on antibody performance from these differences, it is clear that GQ2 is a superb antisera that can be used at high dilution to study kisspeptin neurons in the brain of monkeys, and probably other primates including man. The antibody used to study GnRH, LR1, has been long recognized to also be a highly specific polyclonal antisera for examination of the distribution of this peptide in the primate brain (22,24).
In the present study, there was no evidence of coexpression of kisspeptin and GnRH, and we therefore conclude that, in the monkey and probably other primates, these two neuropeptides are generated by unique networks of hypothalamic neurons. Although colocalization of these peptides in neurons of the ovine hypothalamus has been reported (25), this observation has not been confirmed. In contrast to the intact male mouse, where kisspeptin containing neurons were found in the POA (13), kisspeptin-positive perikarya in the castrated male monkey were observed only in the MBH. It should be recognized, however, that the distribution of kisspeptin neurons in the hypothalamus of the intact male monkey may be different. Within the MBH of the castrate male monkey, kisspeptin neurons were found almost exclusively in the arcuate nucleus as has been previously reported for male rodents and female sheep (13,25,26). The present finding of the location of kisspeptin containing neurons in the monkey hypothalamus is consonant with previous studies of KiSS-1 expression in the primate brain. Shibata et al. (14), using ribonuclease protection assay, demonstrated that, in castrated adult male monkeys, kisspeptin mRNA levels were readily detectable in extracts of the MBH, but not in those of the POA. Hybridization histochemistry has shown that kisspeptin mRNA-positive neurons in the hypothalamus of women and of male and female monkeys were similarly restricted to the arcuate nucleus (8,27). Examination of sections throughout the entire hypothalamus in the present study, and in the earlier in situ hybridization study of female cynomolgus monkeys and women (27), indicated that kisspeptin containing neurons in the arcuate nucleus were restricted to the posterior two-thirds of this MBH nucleus. It is therefore interesting to note that selective destruction of the same portion of the arcuate nucleus with radiofrequency current in ovariectomized adult rhesus monkeys resulted in a profound suppression of gonadotropin secretion without a generalized loss of hypophysiotropic control of the pituitary gland (28). The latter finding led Knobil (29) to conclude that the GnRH pulse generator in the monkey resided in the region of the arcuate nucleus. Because the location of kisspeptin perikarya in the arcuate nucleus is separated from the more lateral distribution of the neuroendocrine GnRH neurons in the VHT, the arcuate lesions described earlier would have preserved the GnRH neuronal network but destroyed most kisspeptin perikarya. Thus, it would seem reasonable to propose that kisspeptin neurons in the arcuate nucleus could represent the substrate of the GnRH pulse generator: a notion consistent with the ability of intermittent iv h kisspeptin-10 administration at hourly intervals to elicit pulsatile LH release in the juvenile male monkey in which spontaneous GnRH pulse generator activity is curtailed (9).
On the other hand, multiunit electrical activity (MUA), which provides a robust electrophysiological correlate of GnRH pulse generator activity in several species including the monkey (30), has been recorded throughout the monkey MBH with sites of MUA as far rostral as the suprachiasmatic nucleus (31). In addition, patients with loss of function mutations of GPR54 exhibit low amplitude intermittent LH release at an approximately normal frequency (3,32), suggesting that kisspeptin may function to amplify the activity of the GnRH pulse generator, rather than as a component of the pulse generator. This latter view is consistent with the finding that, in rat, iv administration of kisspeptin elicits a robust discharge of LH without influencing arcuate nucleus/median eminence MUA (33). If the action of kisspeptin to enhance pulsatile GnRH release results from amplification of the intermittent activity of the GnRH pulse generator, then it might be predicted that continuous infusion of kisspeptin, at doses that lead to down-regulation of GPR54 (34), would selectively reduce LH pulse amplitude. In such a study of the adult male rhesus monkey, however, both LH pulse amplitude and frequency were reduced (35). Because reduction in LH pulse amplitude may result in LH increments falling below threshold for detection as an LH pulse, it remains to be confirmed whether the reduction in LH pulse frequency, which generally reflects GnRH pulse frequency (36), was due to a kisspeptin-induced retardation of the GnRH pulse generator. Clearly, the precise role of the arcuate kisspeptin neurons in GnRH pulse generation remains to be established.
The conclusion that arcuate kisspeptin neurons play a role in mediating the negative feedback control of gonadotropin secretion in the male monkey, as they do in the rodent (16), may be inferred with more certainty. Testosterone replacement to castrate male monkeys results in a reduction in kisspeptin mRNA levels in the MBH (14) where, as shown in the present study, the vast majority of kisspeptin cell bodies are located in the arcuate nucleus.
There is a general consensus among those currently studying the role of GPR54 signaling in regulating GnRH neuronal function that kisspeptins represent the most potent GnRH secretagogue discovered to date (16,37,38,39), and evidence is rapidly emerging implicating kisspeptin neurons in hypothalamic pathways mediating the influence of development, nutrition, metabolism, and season on the hypothalamic-pituitary-gonadal axis (16,39). Herbison’s group (40) recently argued that excitatory synaptic input to GnRH neurons had hitherto been greatly underestimated. This proposal was based on the finding, in mouse, of extensive spine density (a putative structural indicator of excitatory synaptic input) on GnRH soma and dendrites. In the adult, means of approximately 25 spines/GnRH perikarya and 1 spine/μm of proximal dendrite were reported (40). Taking together the foregoing considerations, we had anticipated that the present study of the monkey would reveal extensive contacts of kisspeptin axons on GnRH soma and dendrites. It was, therefore, surprising to find that only 25–50% of GnRH perikarya in the MBH of the monkey were contacted by kisspeptin fibers, and, on average, less than one kisspeptin contact per GnRH cell body was observed. Axo-dendritic contacts were also infrequent (approximately one kisspeptin contact per 70 μm of GnRH dendrite), and therefore our findings are more in line with the classic view of the GnRH neuron as a scantly innervated entity (41). Here, it is very interesting to note that, in the male mouse, application of an immunofluorescence approach similar to that employed in the present study revealed that only approximately 10% of GnRH neurons were contacted by kisspeptin axons (13). Whereas the relationship between structural (synaptic density at the EM level, and spines at the light level) and immunocytochemical (contacts) indices of synaptic input need to be rationalized, we conclude from the present findings that, in castrated male monkeys in which the pubertal LH resurgence had been triggered, kisspeptin contacts on GnRH soma and dendrites are relatively rare.
At the level of the median eminence, there was extensive and intimate intermingling of beaded kisspeptin and GnRH axons, and this was particularly marked in the internal zone of this structure. Although GnRH innervation of the median eminence was relatively homogenous, that of kisspeptin was concentrated in, but not limited to, the internal layer. In the mouse, kisspeptin staining was not observed in the external layer of the median eminence (13). Ultrastructural studies of the mammalian median eminence, including that of the monkey, have indicated that synaptic input to GnRH axonal boutons and terminals in this circumventricular organ are rare (23,42). It is therefore not surprising that, despite the intimacy between kisspeptin and GnRH fibers throughout the median eminence of the monkey, only the occasional axo-axonal contact between these peptidergic fibers was observed. Nevertheless, because of the intense and intimate dual innervation of the median eminence of the monkey, coupled with the apparent scant kisspeptin innervation of GnRH soma and dendrites, it is tempting to speculate that a nonsynaptic action of kisspeptin at the level of the median eminence may be an important component of GnRH regulation. This notion is consistent with the generalized ability of iv administered kisspeptins to robustly trigger GnRH release across species (16,39). In addition to regulating GnRH release, kisspeptin axonal projections to the median eminence may also serve a hypophysiotropic function. In this regard, kisspeptin has been reported in ovine hypophysial portal blood (43), GPR54 is expressed in anterior pituitary (44) and a modest ability of kisspeptin to stimulate gonadotropin release has been demonstrated in some studies (44,45,46).
Although the emerging role of kisspeptin as a key regulator of the release of GnRH provided the rationale for the present study, the finding that kisspeptin perikarya and dendrites in the arcuate nucleus were occasionally contacted by beaded GnRH axons raises the possibility that GnRH may exert control of kisspeptin neuronal activity. Here, it may also be noted that the substrate for such reciprocal control between kisspeptin and GnRH also exists at the level of the median eminence (see above).
In summary, the present study describes the application of double-label immunofluorescence to visualize kisspeptin and GnRH neuronal networks in the monkey brain and provides, for the first time, a description of the location of kisspeptin-immunopositive perikarya and their projections in the primate hypothalamus. In the agonadal condition, kisspeptin soma in the hypothalamus of the male monkey were found only in the arcuate nucleus/median eminence region. Axo-somatic, axo-dendritic and axo-axonic contacts of kisspeptin on GnRH were infrequently observed, and nonsynaptic communication at the level of the median eminence is proposed as a pathway contributing to the regulation of GnRH release by kisspeptin. With the methodology validated in the present study, it will now be possible to systematically examine the structural interactions between these two neuropeptide networks during primate development to further examine the role of kisspeptin in dictating 1) the restraint of pulsatile GnRH release during infancy and 2) the resurgence of pulsatile GnRH release at the termination of the juvenile phase of development.
Acknowledgments
We are most grateful to Dr. Stephen R. Bloom (Imperial College London, London, UK) for the generous gift of GQ2, an excellent polyclonal antibody to h kisspeptin-54. We also thank Dr. Judy Cameron’s laboratory for help with transcardial perfusion, and the Primate Core Staff of the Pittsburgh Specialized Cooperative Centers Program in Reproduction and Infertility Research and Ms. Carolyn Phalin for expert technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants HD 13254 (T.M.P.), HD 08160 (T.M.P.), and ISIORR022515 (R.B.G.) and by an Endocrine Society Summer Research Fellowship to Ms. Kathryn Guerriero.
Current address for K.A.G.: Wisconsin National Primate Research Center, University of Wisconsin-Madison, 1223 Capitol Court, Madison, Wisconsin 53715-1299.
Disclosure Statement: S.R., K.A.G., R.B.G. and T.M.P. have nothing to declare.
First Published Online May 29, 2008
Abbreviations: GPR54, Kisspeptin receptor; h, synthetic human; MBH, mediobasal hypothalamus; MUA, multiunit electrical activity; POA, preoptic area; VHT, ventral hypothalamic tract.
References
- Plant TM, Witchel SF 2006 Puberty in non-human primates and humans. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, eds. Knobil and Neill’s physiology of reproduction. 3rd ed. San Diego: Elsevier; 2177–2230 [Google Scholar]
- De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Apaicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BE, Crowley Jr WF, Seminara SB, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss 1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ 2007 KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley Jr WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, DiPietro MJ 2006 Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- Shibata M, Gibbs RB, Shahab M, Plant TM GnRH neurons in the peripubertal male rhesus monkey (Macaca mulatta) express GPR54: implication for the control of primate puberty. Program of the 87th Annual Meeting of The Endocrine Society, San Diego, CA, 2005 (Abstract P1-98) [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Friedman RL, Ramaswamy S, Plant TM 2007 Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinizing hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol 19:432–438 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA 2008 The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Ann Rev Physiol 70:213–238 [DOI] [PubMed] [Google Scholar]
- Plant TM 1985 A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 116:1341–1350 [DOI] [PubMed] [Google Scholar]
- Fraser MO, Arslan M, Plant TM 2005 Androgen and estrogen treatment, alone or in combination, differentially influences bone maturation and hypothalamic mechanisms that time puberty in the male rhesus monkey (Macaca mulatta). Pediatric Res 57:141–148 [DOI] [PubMed] [Google Scholar]
- Mann DR, Bhat GK, Stah CD, Pohl CR, Plant TM 2006 Induction of a hypothyroid state during juvenile development delays pubertal reactivation of the neuroendocrine system governing luteinizing hormone secretion in the male rhesus monkey (Macaca mulatta). J Neuroendocrinol 18:662–671 [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR 2005 Kisspeptin-54 stumulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Thind KK, Perera AD, Plant TM 1994 Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology 134:858–868 [DOI] [PubMed] [Google Scholar]
- Durrant AD, Plant TM 1999 A study of the gonadotropin-releasing hormone neuronal network in the median eminence of the rhesus monkey (Macaca mulatta) using a post-embedding immunolabeling procedure. J Neuroendocrinol 11:813–821 [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Thind KK, Song T, Kim EJ, Boggan JE 1990 Location of the neuroendocrine gonadotropin-releasing hormone neurons in the monkey hypothalamus by retrograde tracing and immunostaining. J Neuroendocrinol 2:157–168 [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Estrada KM, Clarke IJ 2006 Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology 147:804–810 [DOI] [PubMed] [Google Scholar]
- Grieves TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LH, Demas GE 2007 Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148:1158–1166 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E 1978 The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 102:1008–1014 [DOI] [PubMed] [Google Scholar]
- Knobil E 1980 The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88 [DOI] [PubMed] [Google Scholar]
- O'Byrne KT, Knobil E 1993 Electrophysiological approaches to gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod 2:37–40 [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Wilson R, Kesner JS, Knobil E 1986 Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology 44:168–171 [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N 2007 Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab 92:1137–1144 [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT 2008 Effect of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 149:1004–1008 [DOI] [PubMed] [Google Scholar]
- Seminara SB, DiPietro JM, Ramaswamy S, Crowley Jr WF, Plant TM 2006 Continuous human meatastin-45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley Jr WF, Plant TM 2007 Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148:3364–3370 [DOI] [PubMed] [Google Scholar]
- Plant TM 1986 Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocr Rev 7:75–88 [DOI] [PubMed] [Google Scholar]
- Seminara SB 2006 Mechanisms of disease: the first kiss—a crucial role for kisspeptin-1 and its receptor, G protein-coupled receptor 54, in puberty and reproduction. Natl Clin Pract Endocrinol Metab 2:328–334 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2008 New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Campbell RE, Han SK, Herbison AE 2006 Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147:3652–3661 [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Livne I, Witkin JW 1994 The gonadotropin-releasing hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press; 1683–1709 [Google Scholar]
- Kuljis RO, Advis JP 1989 Immunocytochemical and physiological evidence of a synapse between dopamine- and luteinizing hormone releasing hormone-containing neurons in the ewe median eminence. Endocrinology 124:1579–1581 [DOI] [PubMed] [Google Scholar]
- Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ 2008 Kisspetin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology 149:1951–1959 [DOI] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML 2008 KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotropin-releasing hormone. J Neuroendocrinol 20:381–393 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla M, Tena-Sempere M, Malagon MM, Castano JP 2007 Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol 19:521–530 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kadokawa H, Hashizume T 2008 Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Animal Reprod Sci 103:360–365 [DOI] [PubMed] [Google Scholar]