Abstract
Follistatin binds and neutralizes members of the TGFβ superfamily including activin, myostatin, and growth and differentiation factor 11 (GDF11). Crystal structure analysis of the follistatin-activin complex revealed extensive contacts between follistatin domain (FSD)-2 and activin that was critical for the high-affinity interaction. However, it remained unknown whether follistatin residues involved with myostatin and GDF11 binding were distinct from those involved with activin binding. If so, this would allow development of myostatin antagonists that would not inhibit activin actions, a desirable feature for development of myostatin antagonists for treatment of muscle-wasting disorders. We tested this hypothesis with our panel of point and domain swapping follistatin mutants using competitive binding analyses and in vitro bioassays. Our results demonstrate that activin binding and neutralization are mediated primarily by FSD2, whereas myostatin binding is more dependent on FSD1, such that deletion of FSD2 or adding an extra FSD1 in place of FSD2 creates myostatin antagonists with vastly reduced activin antagonism. However, these mutants also bind GDF11, indicating that further analysis is required for creation of myostatin antagonists that will not affect GDF11 activity that could potentially elicit GDF11-induced side effects in vivo.
ACTIVIN, MYOSTATIN, and growth and differentiation factor-11 (GDF11) comprise a branch of the TGFβ superfamily of growth factors that share a common core of signaling components, including type I and II activin receptors and the Smad2 and 3 (Smat mothers against decapentaplegic-2 and -3) second messengers (1,2). Activin has been implicated in cell-fate determination and organogenesis during development, the importance of which is emphasized by the observation that activin-A null mice die from bone and muscle abnormalities shortly after birth (3). In adults, activin influences reproduction (1), wound healing (4), bone formation (5), immune responses (6), and pancreatic β-cell proliferation (7). Myostatin regulates muscle development in embryos and muscle mass in adults (8), and myostatin null mice have substantially enhanced musculature (9). In addition, myostatin influences adipogenesis because both overexpression and genetic disruption of myostatin expression result in reduced fat mass (10). GDF11 null mice have profound defects in pancreatic islet development, leading to increased numbers of islet progenitors but reduced differentiation of mature β-cells (11). Taken together, these findings demonstrate that this group of ligands has critical actions in both embryonic development and adult homeostasis.
Follistatin (FST) and follistatin-like (FSTL)-3 (FLRG, FSRP) form a related group of follistatin domain (FSD)-containing proteins with overlapping biochemical, molecular, biosynthetic, and structure-function attributes, although there are also important differences (12). Neutralization of TGFβ superfamily ligands is their only known biological activity. Both proteins bind and neutralize activin irreversibly and bind myostatin with a 3- to 5-fold lower affinity (13,14,15). FST null mice die immediately after birth due to defects in muscle and bone formation (16), whereas FSTL3 null mice are viable but have altered glucose metabolism and enhanced pancreatic β-cell formation (17). FST overexpression in gonads leads to abnormal gonadal architecture and reduced fertility (18), whereas FSTL3 overexpression results in smaller testes and reduced male fertility (19), suggesting that regulation of activin activity by FST and/or FSTL3 is important for normal reproduction. In addition, transgenic overexpression of FST in muscle leads to increased musculature presumably due to decreased myostatin activity through antagonism by FST (20). These observations indicate that both in vitro and in vivo, FST and FSTL3 function to regulate the activity of activin and myostatin and that their absence or excess leads to abnormal development and/or function.
Experimental states of myostatin excess result in reduced body mass and cachexia (21), a state of body tissue wasting commonly associated with chronic diseases including cancer, AIDS, renal failure, and gastrointestinal disease and contributes to the morbidity and mortality of these conditions (22,23). In addition, circulating myostatin levels were significantly elevated in AIDS patients with more than 10% weight loss (24). These observations have accelerated the search for myostatin inhibitors that might form the basis for novel therapeutic strategies to treat muscle-wasting conditions.
The crystal structure of FST bound to activin was recently reported in which FSD2 plays a prominent role in contact between each FST molecule and one subunit of the activin homodimer (25). Additional analysis of FST structure-function relationships using point and domain mutagenesis has identified numerous residues in FSD1, FSD2, and the N-terminal domain that have important effects on FST’s binding activity (26,27). Moreover, these studies demonstrated that the order of the FST domains is also critical (26). Taken together, these studies demonstrate that specific residues in FST, especially within FSD2, are critical for the high-affinity interaction between FST and activin.
Although direct structural evidence is presently unavailable, it is theoretically possible that critical determinants for activin and myostatin binding to FST and FSTL3 do not completely overlap, suggesting that selective activin or myostatin antagonists might be derived through mutation of the natural protein. To begin to decipher the portions of FST critical for binding and neutralizing myostatin vs. activin, we tested previously prepared FST point and domain mutants with altered activin activity (26,27) for their ability to inhibit myostatin activity in vitro. Our results indicate that mutations in FSD1 have the largest effect on myostatin binding, whereas FSD2 mutations that reduce or eliminate activin binding have little effect on myostatin binding. In addition, given the close structural relationship between myostatin and GDF11, we also examined binding and neutralization of GDF11 by wild-type (WT) and mutant FST. These results confirm the concept that the primary contacts of FST differ for activin and myostatin (28) and demonstrate that selectivity of FST mutants can be altered to reduce activin binding while preserving myostatin neutralization. Our results also indicate that such mutants will bind GDF11 and that further experimentation is required to separate myostatin and GDF11 binding determinants.
Materials and Methods
Materials
Activin A, myostatin, and GDF11 were purchased from R&D Systems (Minneapolis, MN). Radioiodine was obtained from NEN Life Science Products Corp. (Boston, MA), and all electrophoresis materials were purchased from Bio-Rad (Hercules, CA).
Production of FST mutants
Mutations were introduced into the FST288 cDNA as previously discussed (26,27), and mutant sequences were verified by bidirectional sequencing. The complete list of mutants analyzed is shown in Table 1 and were previously described in terms of activin binding and neutralization (26,27). All mutant and WT FST constructs were cloned into pcDNA3.1-myc/his vector (Invitrogen, Carlsbad, CA) and prepared using NucleoBond Maxiprep kits (BD Biosciences CLONTECH, Palo Alto, CA).
Table 1.
FST mutations tested for differential antagonism of activin and myostatin, their published activin antagonistic activity, and the original reference describing the mutation
| Follistatin mutation | Activin antagonism | Reference where first reported |
|---|---|---|
| Structural alteration | ||
| dG1N2 | − | 27 |
| CC(26,27)AA | − | 27 |
| dFSD1 | − | 26 |
| dFSD2 | − | 26 |
| dFSD3 | + | 26 |
| FSD1/1/3 | − | 26 |
| FSD2/1/3 | − | 26 |
| FSD3/1/2 | +/− | 26 |
| N-Domain point mutations | ||
| GN(1,2)AA | +/− | 27 |
| W4A | − | 27 |
| W4F | −/+ | 27 |
| W4D | − | 27 |
| W36A | − | 27 |
| W36D | − | 27 |
| Domain 1 point mutations | ||
| Y110A | +/− | 26 |
| Y110D | − | 26 |
| L116A | +/− | 26 |
| L116D | +/− | 26 |
| L127A | + | 26 |
| L127D | + | 26 |
| V129A | +/− | 26 |
| V129D | +/− | 26 |
| Domain 2 point mutations | ||
| Y185A | +/− | 26 |
| Y185D | − | 26 |
| L191A | − | 26 |
| L191D | − | 26 |
Mutants with substantial differential activity are shown in Fig. 1, and those that retained myostatin inhibition but had reduced activin antagonism were explored further. +, Full or nearly full activity relative to WT FST; +/−, partial activity; −, no or nearly no activity as an activin antagonist.
Activin/myostatin transcription reporter assay
Human embryonic kidney 293 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (Life Technologies, Inc., Rockville, MD). For initial mutant screenings, transient transfections were performed in 24-well trays using Effectene (QIAGEN, Valencia, CA) and a total of 300 ng DNA [80 ng of the Smad2/3-responsive reporter CAGA-Luc, 20 ng pRL-TK (Promega Corp., Madison, WI), and 200 ng of mutant DNA or nonspecific plasmid DNA]. For dose-response inhibition assays, a total of 350 ng DNA was transfected into cells (80 ng CAGA-Luc, 20 ng pRL-TK, and 0–250 ng of mutant DNA + appropriate amount of control plasmid to keep total DNA transfected constant). After a 24-h incubation, media were replaced with RPMI + 0.1% BSA and either 5 ng/ml activin A or 15 ng/ml myostatin or GDF11 and incubated for an additional 24 h, after which the cells were lysed and assayed for luciferase activity using the dual-luciferase reporter assay kit (Promega), with results normalized to Renilla luciferase activity.
Protein production and purification
Human embryonic kidney FreeStyle 293-F cells were maintained in 30 ml of FreeStyle 293 expression medium (Life Technologies) to a density of 1 × 104 cells/ml with a minimum of 85% viability. Plasmid DNA (35 μg per 30 ml culture) was transfected using 40 μl of 293fectin reagent and 2 ml of Opti-MEM (Life Technologies) according to the manufacturer’s protocol. The culture was incubated for 72 h at 37 C, after which the supernatant was collected and circulated over a nickel-Sepharose affinity column (QIAGEN) using a peristaltic pump at 4 C overnight. Purified FST was eluted with imidazole [300 mm (pH 6.8)] and then concentrated and exchanged into Dulbecco’s PBS using an Ultra-15 centrifugal filter unit (Amicon, Bedford, MA).
Quantitation of purified protein
The protein concentration of WT and mutant FST preparations was determined using two immunoassays for FST or the myc tag as previously described (26) and adjusted for results from silver stained gels of the concentrated FST after electrophoresis, which was compared with a standard of known concentration (>90% pure). FST bands were identified by Western blot of SDS-PAGE gels using anti-myc (clone 4A6; Upstate Biologicals, Lake Placid, NY) and detection with goat antimouse IgG linked to horse radish peroxidase (1:15,000; Jackson ImmunoResearch, West Grove, PA).
Solid-phase direct binding assay
Myostatin or activin was plated onto 96-well Immulon-2 (Dynatech Laboratory, Chantilly, VA) strips in carbonate buffer overnight at 4 C at a concentration of 50 ng per 50 μl/well (24). Each well was aspirated and blocked with 200 μl of blocking buffer (10 mm PBS containing 3% BSA) for at least 2 h. Nonspecific binding wells were left in blocking solution, and all other wells were washed three times using Tween 20 and Tris-buffered saline (TTBS) (TBS/0.05% Tween 20). Increasing concentrations of FST WT or mutants, diluted in TBS, were added to the well for 1 h at room temperature. After washing, 100 μl of anti-myc antibody (clone 4A6; Upstate Biologicals) were added at a final dilution of 1:500 in TBS/0.1% BSA and incubated for 1 h at room temperature. After three washes of TTBS, goat antimouse IgG-alkaline phosphatase (Jackson ImmunoResearch) was used at a final dilution of 1:500 as the secondary antibody in TBS/0.1% BSA. The plate was incubated for 1 h at room temperature and washed three times with TTBS. ρ-Nitrophenol phosphate (1 × 15 mg tablet; Sigma, St. Louis, MO) was dissolved in 15 ml of 0.1 m glycine buffer with 1 mm MgCl2 and 1 mm ZnCl2 (pH 10.4). Two hundred microliters were added to each well for 30 min in room temperature. The plate was analyzed on a microplate reader at 405 nm.
Solid-phase radioligand binding assay
Activin was iodinated as previously described (13). Purified WT FST was plated onto 96-well Immulon-2 plates (Dynatech Laboratories) in 0.1 m carbonate buffer (pH 9.6) overnight at 4 C at 25 ng/well (13). After blocking nonspecific sites with 200 μl of blocking buffer (0.01 m PBS/0.05% Tween 20/3% BSA) for 2 h, increasing concentrations of unlabeled activin or GDF11 were added to each well in 100 μl assay buffer (0.01 m PBS/0.05% Tween 20 + 0.1% gelatin). Radiolabeled activin was diluted to 50,000 cpm per 50 μl, and 50 μl were added to all wells. The plate was incubated for 2 h at room temperature. After three washes, the wells were aspirated and counted in a γ-counter.
Data analysis
Reporter activity results were expressed as percent of maximum (no FST) for each ligand. Each experiment also included WT FS 288 as a positive control. Mutants showing significant differences between activin and myostatin inhibition were tested at least three times.
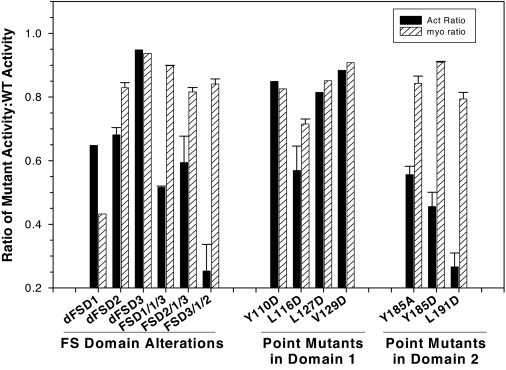
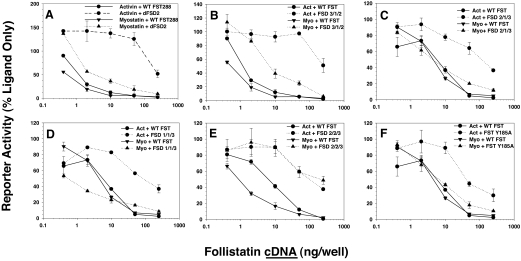
For comparison of activin and myostatin binding activity of FST mutants at 200 ng DNA/well (Fig. 1), the activin and myostatin inhibition by each mutant was normalized to the activity of WT FST in that assay. For assays comparing activin and myostatin inhibition of increasing doses of WT or mutant FST (Fig. 2), the ED50 was estimated at the dose at which 50% of maximal stimulation was inhibited. This point was compared for mutant FST vs. WT FST and to compare myostatin with activin inhibition activity.
Figure 1.
Comparison of activin and myostatin antagonism for FST mutants. FST mutants were tested at a single dose (200 ng/ml) for ability to antagonize activin (5 ng/ml) or myostatin (15 ng/ml) in an in vitro bioassay, and results were expressed relative to WT FST tested in the same assay so that a ratio of 1 indicates identical antagonism to that of WT FST. The first group represents deletion, substation, or rearrangement of whole FST domains, whereas the mutants in the second and third groups represent point mutations in FSD1 or -2, respectively. Mutants in which activin antagonism was compromised but myostatin antagonism activity remained largely intact were investigated further. Shown are representative results from one of at least three experiments.
Figure 2.
Comparison of activin and myostatin antagonism for six FST mutants with largest selectivity for activin. Based on the results from Fig. 1, six mutants with differences between activin and myostatin antagonism or that represent significant alteration of domain order or number were investigated in dose-response assays. Inhibition by WT FST is shown in solid lines and mutant FST in dotted lines. Activin is in closed circles and myostatin is in closed triangles. The dFSD2 mutant (A), in which FSD 2 is deleted, had the greatest difference between myostatin inhibition, which was only slightly reduced from WT FST, and activin inhibition, which was undetectable except at the highest dose. B-F, The other mutants were similarly investigated. Shown are representative results from one of three experiments.
Results
Twenty-six previously described FST mutants (Table 1) containing single-amino acid substitutions in FSD1, FSD2, the N-terminal domain, or mutations that altered the number or order of FSDs were examined for myostatin and activin inhibitory activity. The N-terminal domain mutations that were previously shown to suppress activin inhibition (27) also reduced myostatin activity to a similar degree (not shown) and were thus not investigated further. The remaining 13 mutants retaining antagonistic activity to at least one ligand were screened by in vitro bioassay at a single, maximal dose to compare activin and myostatin antagonism relative to WT FST. We found that outright deletion of FSD1 diminished both myostatin and activin inhibition, indicating that this domain was required for both activities (Fig. 1). However, the remaining FST mutants revealed differential inhibition of activin vs. myostatin, with six mutants, primarily focused on FSD2, retaining substantial or complete myostatin antagonism with reduced activin inhibition (Fig. 1) and were investigated further.
The six differentially active mutants were examined at multiple doses to compare their relative activin and myostatin antagonist activities with WT FST in more detail. Interestingly, outright deletion of FSD2 (dFSD2) had little effect on myostatin inhibition (Fig. 2A; compare triangle, solid vs. dotted lines), whereas inhibition of activin activity was almost completely ablated (Fig. 2A; compare circle, solid vs. dotted lines). Altering the order of the FST domains so that FSD3 preceded FSD1 and -2 (Fig. 2B; FSD3/1/2) had a similar effect on activin activity but also reduced myostatin inhibition by nearly 10-fold. Placing FSD2 before FSD1 (FSD2/1/3) was not as effective at reducing activin inhibition, but it had no effect on myostatin inhibition (Fig. 2C). These results suggest that FSD2 is more critical for activin antagonism, whereas FSD1 appears to be more critical for myostatin inhibition.
We therefore replaced FSD2 with an extra copy of FSD1 (FSD1/1/3), which was more effective than WT FST in antagonizing myostatin but lost the majority of its activin antagonist activity (Fig. 2D). Conversely, replacing FSD1 with an extra copy of FSD2 (FSD2/2/3) reduced myostatin inhibition nearly 10-fold more than the reduction in activin inhibition (Fig. 2E), consistent with FSD2 having greater effect on activin bioactivity, compared with FSD1 as hypothesized. Point mutations in FSD2, such as Y185A, had little effect on myostatin inhibition but reduced activin antagonism more than 10-fold, consistent with this domain being more important for activin inhibition. Taken together, these results indicate that mutations affecting FSD2 have a greater effect on activin antagonism, whereas FSD1 appears to be more critical for inhibiting myostatin activity.
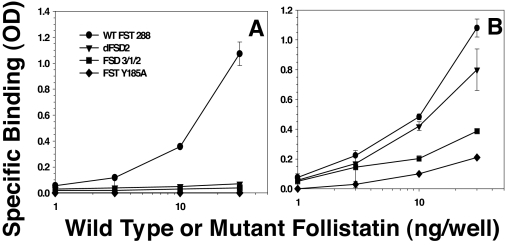
To determine whether the altered activity of the mutants was due to altered binding of the ligands, we investigated three mutants with the greatest difference in activity for direct binding to solid-phase ligand. Although WT FST bound well to solid-phase activin, none of the mutants bound activin, even at the maximal dose tested (Fig. 3A). In contrast, WT FST and all of the FST mutants bound to solid-phase myostatin in rank order, consistent with bioactivity (WT > dFSD2 > FSD3/1/2 > FST Y185A; Fig. 3B). Thus, the differential activity appears to be due to a difference in binding affinity with FSD2 mutants retaining myostatin binding but demonstrating vastly reduced activin binding. These results also indicate that by deleting FSD2, altering its position in the FST molecule, or introducing mutations into FSD2 selectively reduces activin relative to myostatin inhibition, selective myostatin antagonists were created.
Figure 3.
Direct binding of WT or mutant FST to activin and myostatin. A, Activin (50 ng/well) was adsorbed to plates and increasing amounts of WT or mutant FST proteins added for 2 h. None of the mutants bound detectably to activin, whereas WT FST was detectable at all doses. B, Both WT FST and mutants bound to myostatin in rank order consistent with their bioactivity (see Fig. 2), suggesting that differential binding of myostatin by mutants accounts for their differential activity. Shown are representative results from one of three experiments.
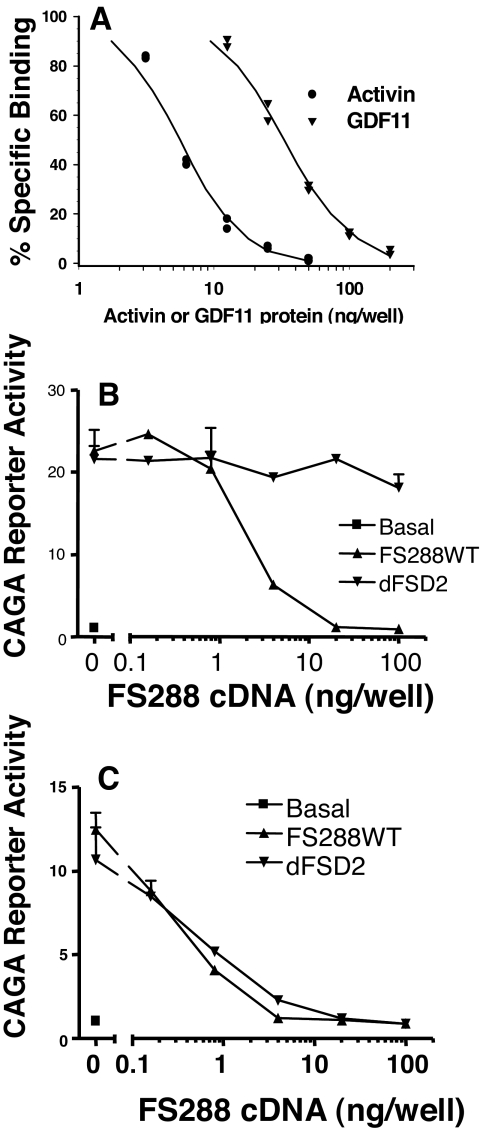
Because the sequences of GDF11 and myostatin are highly conserved, we examined whether FST could bind these two ligands with similar affinity. We were unable to obtain functional radioiodinated myostatin or GDF11, but when compared with activin for competitive inhibition of radiolabeled activin binding to FST, we found that GDF11 activity was approximately 6-fold lower than that of activin (Fig. 4A), equivalent to the difference in FST binding to myostatin relative to activin in a similar assay (15). We then examined whether dFSD2, the FST mutant with greatest differential antagonism between activin and myostatin, would similarly differentially antagonize GDF11. Whereas dFSD2 had vastly reduced activin inhibitory activity (Fig. 4B), this mutant had identical activity to WT FST in inhibiting GDF11 in the same in vitro bioassay (Fig. 4C). Moreover, this differential activity was identical with that seen with myostatin (Fig. 2A). These results indicate that like myostatin, FST mutants with domain 2 deletion are selective for GDF11 relative to activin. Furthermore, they demonstrate that such mutants will antagonize both myostatin and GDF11 similarly and thus not be selective with respect to these two ligands.
Figure 4.
WT and mutant FST binding to GDF11. A, The ability of unlabeled activin or GDF11 to compete with radioiodinated activin binding to solid-phase WT FST was compared. Binding to activin was about 6-fold better than GDF11, but GDF11 binding was similar to that observed for myostatin. B, WT FST inhibited activin activity, whereas the dFSD2 mutant was inactive as observed in Fig. 2A. C, In the same assay, there was essentially no difference between WT and dFSD2 mutant FST for neutralization of GDF11 activity, similar to results for myostatin (Fig. 2A). Thus, myostatin-selective mutants will also bind and neutralize GDF11.
Discussion
Follistatin has been recognized for many years as a high-affinity binding and neutralizing protein for activin (1,15). More recently high-affinity binding and neutralization of other TGFβ family ligands, including myostatin and bone morphogenetic proteins, have been reported, although at lower affinities relative to activin (15,29). However, these affinity differences suggested that the critical contact regions between FST and activin may not be identical for the related TGFβ family ligands. The experiments reported here were designed to test the hypothesis that differences in FST contacts among the TGFβ family ligands most closely related to activin, namely myostatin and GDF11, could lead to identification of FST mutants with selective binding for myostatin relative to activin. Such mutants would be valuable as pharmaceutical agents that could inhibit myostatin activity to promote muscle growth in muscle-deficient states such as cachexia or muscular dystrophy and avoid potential side effects due to simultaneous activin inhibition.
Consistent with the recently solved crystal structure of activin bound to FST that demonstrated extensive contacts between FSD2 and activin (25) and our previous mutational analyses that identified FSD2 as a critical activin binding region (26), the present results indicate that deletion of FSD2, alteration of its position in the FST molecule, or point mutations within FSD2 all reduce activin antagonism. Conversely, mutations that eliminate FSD1 or alter its position usually alter myostatin inhibition. Significantly, those FSD2 mutations that reduce activin antagonism have little effect on myostatin inhibition, demonstrating that indeed these ligands are differentially bound and antagonized by FST. This observation demonstrates that it is possible to create FST mutants that are selective for myostatin inhibition. The most selective mutant was dFSD2 in which the second FST domain was deleted, leaving the N domain and FSD1 followed by FSD3. This mutant antagonized myostatin similarly to WT FST, but its activin-inhibitory activity was reduced more than 250-fold. Moreover, direct binding to activin was undetectable, whereas myostatin binding was reduced only slightly. These results indicate that the dFSD2 mutant might act as a myostatin selective mutant in vivo and therefore constitute a starting point for development of a selective myostatin inhibitor of therapeutic value.
The value of such an antagonist was recently demonstrated by Nakatani et al. (28) in a transgenic model. Overexpression of a FST mutant containing the N domain and two copies of FSD1 (FS I-I) in muscle led to increased muscle mass and strength resulting from both hyperplasia and hypertrophy (28). Moreover, overexpression of this mutant in muscle of mdx mice (a model for Duchene muscular dystrophy) resulted in at least partial rescue from this disease (28). Although this study clearly demonstrated the potential of one selective FST mutant for reversing muscle degeneration, the only other FSD1 or -2 mutant that was investigated was a construct containing two copies of FSD1 and FSD3 (FS I-I-3). This protein was found to bind activin and myostatin similarly to the FS I-I mutation and was not investigated further (28). Interestingly, the FS 1–1-3 mutant appears identical with our FSD 1/1/3 mutant in structure, but in our assay, the FSD 1/1/3 mutant was less selective than our dFSD2 mutant because substantial activin inhibitory activity was retained in FSD1/1/3. Although we used the same reporter, we used human embryonic kidney 293 cells as opposed to A204 cells used by Nakatani et al., which may be one source for the disparate results. Nevertheless, both studies clearly demonstrate that FST mutants can be constructed allowing selective inhibition of myostatin activity with respect to activin binding.
GDF11 was recently shown to be a critical regulator of pancreatic β-cell development and differentiation (11). Given its extensive structural identity with myostatin, we investigated whether FST could neutralize its bioactivity and whether FST mutants selective for myostatin were similarly selective for GDF11. We found that GDF11 inhibited activin binding to FST with approximately 6-fold lower potency, a difference that is comparable with myostatin’s binding relative to activin (15,28). Moreover, WT FST antagonized the bioactivity of both activin (Fig. 4B) and GDF11 (Fig. 4C), and this antagonism was equivalent to that of the mutant dFSD2 (Fig 4C), which also antagonized myostatin (Fig. 2A). However, whereas the binding results suggest greater potency for inhibiting activin relative to GDF11, the bioassay results suggest greater inhibition of GDF11 relative to activin. This discrepancy is likely due to the GDF11 preparation being less than 100% bioactive, thereby decreasing its effective concentration relative to FST or dFSD2 in the bioassay, which would shift the binding curve to the right and the bioassay curve to the left. Nevertheless, these results demonstrate that FST is a binding and neutralization protein for all members of the activin branch of the TGFβ superfamily and mutants selective for myostatin over activin are similarly selective for GDF11. This observation suggests that whereas FST mutants relatively deficient for activin neutralization can be created, they will likely inhibit both myostatin and GDF11. Because the activity of GDF11 in adults is unknown at present, our findings indicate that potential side effects of myostatin-selective FST mutants need further investigation.
In this study, we have demonstrated that the surfaces of FST necessary for binding and neutralizing activin and myostatin are at least partially distinct, with FSD1 being more critical for myostatin binding and FSD2 more important for activin neutralization. The mutant with the greatest decrease in activin antagonism relative to myostatin, dFSD2, had at least a 250-fold greater inhibition of myostatin relative to activin. We also showed that both WT FST and the dFSD2 mutant bind GDF11. Taken together, our results confirm the presence of differential binding sites for activin and myostatin/GDF11 in FST and support the development of selective antagonists with reduced activin inhibition. Although such antagonists may still not be able to differentially neutralize myostatin and GDF11, further mutational analysis of FST antagonists may identify residues or regions that permit their functional discrimination.
Acknowledgments
We are indebted to the expert technical assistance of Leslie Johnson in producing proteins for this study and Amy Mahan for assistance with purification and assay development.
Footnotes
This research was supported by Grant R01DK075058 from the National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases (to A.L.S.) and two sponsored research awards from Pfizer.
Current address for A.L.S.: Pioneer Valley Life Science Institute, 3601 Main Street, Springfield, Massachusetts 01107.
Disclosure Statement: P.A.K. is currently employed by and has equity interest in Pfizer. A.L.S., Y.S., H.K., and P.A.K. are inventors on a U.S. patent application. A.G. and J.L.S. have nothing to declare.
First Published Online June 5, 2008
Abbreviations: dFSD2, Deletion of FSD2; FSD, follistatin domain; FST, follistatin; FSTL, FST-like; GDF11, growth and differentiation factor 11; TBS, Tris-buffered saline; TTBS, Tween 20 and TBS; WT, wild type.
References
- Welt C, Sidis Y, Keutmann H, Schneyer A 2002 Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227:724–752 [DOI] [PubMed] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E 2002 Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev 16:2749–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassilli A, Bickenbach RR, Roop DR, Jaenisch R, Bradley A 1995 Functional analysis of activins during mammalian development. Nature 374:354–356 [DOI] [PubMed] [Google Scholar]
- Sulyok S, Wankell M, Alzheimer C, Werner S 2004 Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol Cell Endocrinol 225:127–132 [DOI] [PubMed] [Google Scholar]
- Shuto T, Sarkar G, Bronk JT, Matsui N, Bolander ME 1997 Osteoblasts express types I and II activin receptors during early intramembranous and endochondral bone formation. J Bone Miner Res 12:403–411 [DOI] [PubMed] [Google Scholar]
- Jones KL, Kretser DM, Patella S, Phillips DJ 2004 Activin A and follistatin in systemic inflammation. Mol Cell Endocrinol 225:119–125 [DOI] [PubMed] [Google Scholar]
- Demeterco C, Beattie GM, Dib SA, Lopez AD, Hayek A 2000 A role for activin A and betacellulin in human fetal pancreatic cell differentiation and growth. J Clin Endocrinol Metab 85:3892–3897 [DOI] [PubMed] [Google Scholar]
- Lee SJ 2004 Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20:61–86 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ 1997 Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ 2002 Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK 2004 GDF11 modulates NGN3+ islet progenitor cell number and promotes β-cell differentiation in pancreas development. Development 131:6163–6174 [DOI] [PubMed] [Google Scholar]
- Schneyer A, Sidis Y, Xia Y, Saito S, Re EE, Lin HY, Keutmann H 2004 Differential actions of follistatin and follistatin-like 3. Mol Cell Endocrinol 225:25–28 [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Rzucidlo DA, Sluss PM, Crowley Jr WF 1994 Characterization of unique binding kinetics of follistatin and activin or inhibin in serum. Endocrinology 135:667–674 [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, Sidis Y, Holtzman DA, Holmes WE, Schneyer AL 2001 Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology 142:3426–3434 [DOI] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A 2006 Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 147:3586–3597 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel HJ, Sellheyer K, Roop DR, Bradley A 1995 Multiple defects and perinatal death in mice deficient in follistatin. Nature 374:360–363 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL 2007 FSTL3 deletion reveals roles for TGF-β family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 104:1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Kumar TR, Woodruff TW, Hadsell LA, DeMayo FJ, Matzuk MM 1998 Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol 12:96–106 [DOI] [PubMed] [Google Scholar]
- Xia Y, Sidis Y, Schneyer A 2004 Overexpression of follistatin-like 3 in gonads causes defects in gonadal development and function in transgenic mice. Mol Endocrinol 18:979–994 [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC 2001 Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ 2002 Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- Jeevanandam M, Horowitz GD, Lowry SF, Brennan MF 1984 Cancer cachexia and protein metabolism. Lancet 1:1423–1426 [DOI] [PubMed] [Google Scholar]
- Lynch GS, Schertzer JD, Ryall JG 2007 Therapeutic approaches for muscle wasting disorders. Pharmacol Ther 113:461–487 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S 1998 Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA 95:14938–14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS 2005 The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9:535–543 [DOI] [PubMed] [Google Scholar]
- Keutmann HT, Schneyer AL, Sidis Y 2004 The role of follistatin domains in follistatin biological action. Mol Endocrinol 18:228–240 [DOI] [PubMed] [Google Scholar]
- Sidis Y, Schneyer AL, Sluss PM, Johnson LN, Keutmann HT 2001 Follistatin: essential role for the N-terminal domain in activin binding and neutralization. J Biol Chem 276:17718–17726 [DOI] [PubMed] [Google Scholar]
- Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, Murakami T, Uezumi A, Takeda S, Noji S, Sunada Y, Tsuchida K 2008 Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J 22:477–487 [DOI] [PubMed] [Google Scholar]
- Tsuchida K 2004 Activins, myostatin and related TGF-β family members as novel therapeutic targets for endocrine, metabolic and immune disorders. Curr Drug Targets Immune Endocr Metab Disord 4:157–166 [DOI] [PubMed] [Google Scholar]