Abstract
The intestine and kidney are linked by a mechanism that increases salt excretion in response to salt intake. The peptide uroguanylin (UGn) is thought to mediate this signaling axis. Therefore, it was surprising to find (as reported in a companion publication) that UGn is stored in the intestine and circulates in the plasma almost exclusively in the form of its biologically inactive propeptide precursor, prouroguanylin (proUGn), and, furthermore, that infused proUGn leads to natriuretic activity. Here, we investigate the fate of circulating proUGn. Kinetic studies show rapid renal clearance of radiolabeled propeptide. Radiolabel accumulates at high specific activity in kidney (relative to other organs) and urine (relative to plasma). The principal metabolites found in kidney homogenates are free cysteine and methionine. In contrast, urine contains cysteine, methionine, and three other radioactive peaks, one comigrating with authentic rat UGn15. Interestingly, proUGn is not converted to these or other metabolites in plasma, indicating that circulating proUGn is not processed before entering the kidney. Therefore, our findings suggest that proUGn is the true endocrine agent released in response to salt intake and that the response of the kidney is dependent on conversion of the propeptide to an active form after it reaches the renal tubules. Furthermore, proUGn metabolites (other than small amounts of cysteine and methionine) are not returned to the circulation from the kidney or any other organ. Thus, to respond to proUGn released from the gut, any target organ must use a local mechanism for production of active peptide.
THE RENAL EXCRETORY response to salt ingestion (often referred to as “postprandial natriuresis”) is more effective than the response to an equivalent iv infusion of saline (1,2,3,4). This has contributed to the concept of an enterorenal axis for solute homeostasis that is thought to preempt the slower volume-dependent responses, such as those mediated by atrial natriuretic peptide (ANP), angiotensin, aldosterone, and renal perfusion pressure. Several mechanisms probably contribute to enhanced postprandial natriuretic activity, among which the most often invoked are neuronal or humoral reflexes initiated by salt sensitive chemoreceptors in the portal vein (5,6,7,8,9), and an endocrine axis in which natriuretic peptides are released into the circulation from the intestine in response to salt intake (10).
In 1993, uroguanylin (UGn), a guanylyl cyclase-activating peptide, was purified from urine (11). Shortly thereafter, closely related forms of this peptide were purified from plasma (12) and from intestinal extracts (13). Several lines of evidence suggest that UGn is a good candidate for mediating the postprandial enterorenal endocrine axis described above. UGn is expressed principally in the intestine (13,14,15) by a specific type of endocrine cell (16,17), where its expression is regulated by orally administered salt (18). The peptide also circulates in blood (12,14,19,20) and is natriuretic when infused into the isolated perfused rat kidney or the anesthetized mouse (21,22,23,24,25). Perhaps most significantly, elimination of endogenous UGn in mice (by targeted disruption of the gene that encodes it) leads to impaired urinary excretion of orally delivered sodium, and modest, chronic hypertension (26). Collectively, these observations provide strong evidence that UGn plays a role in electrolyte homeostasis, particularly with regard to the management of dietary salt.
In a companion publication (27), we report that UGn is stored in the rat intestine and released into the circulation primarily in the form of its propeptide precursor, prouroguanylin (proUGn). We also show that intact proUGn has natriuretic and diuretic effects when infused iv into anesthetized rats. Thus, the processing step for conversion of proUGn to its mature, active form is likely to be a post-secretory event.
To investigate potential post-secretory processing mechanisms that could convert circulating proUGn to active metabolite(s), as well as the means by which the propeptide and/or its metabolites are cleared from the plasma, we infused animals iv with radiolabeled recombinant proUGn. HPLC analysis of tissue extracts, urine, and plasma from these animals has provided a clear picture of the relevant mechanisms. Surprisingly, the data show that the propeptide remains in an intact state throughout its time in the circulation. This suggests that processing of circulating proUGn most likely occurs after the propeptide has been delivered to its target organ(s). Our data also reveal that one well-established target organ (the kidney) is able to effectively metabolize proUGn. The resulting peptides, which include UGn and several other unidentified fragments, are retained within the nephrons and are eliminated in the urine. This is reminiscent of the proposed handling of proUGn in the intestine, where intraluminal digestive enzymes, such as chymotrypsin, are thought to rapidly convert luminally secreted propeptide into active UGn (28).
Materials and Methods
Experimental animals
Male Wistar rats (200–300 g) were purchased from Charles River Laboratories (Wilmington, MA) and maintained on a 12-h light, 12-h dark cycle in an Association for Assessment of Laboratory Animal Care (AALAC)-approved facility with continuously available veterinary care and unimpeded access to water and rat chow. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals weighed between 250 and 350 g at the time of study.
Purification and quantification of native proUGn
Native proUGn was extracted from the rat intestine and purified by a two-step HPLC procedure, as described in our companion paper (27). In plasma recovery studies, proUGn was measured with a quantitative Western blot assay, also described in our companion publication (27).
Production of isotopically labeled recombinant proUGn
The rat proUGn sequence was subcloned into an expression vector optimized for high-yield in vitro translation (pCITE-4a(+); Novagen/EMB Biosciences, Madison, WI). This vector encodes an S-tag peptide and a thrombin cleavage site upstream from the multiple cloning site. The proUGn insert was PCR amplified from a previously cloned cDNA using a forward primer with an added NcoI restriction site (5′-TCATGCCATGGTCCAAGTCCAGCTAGAA-3′) and a reverse primer with an added XhoI restriction site (5′-TGTACGGGCTGCTGACACTCGAGCACGAGC-3′). These primers were chosen to eliminate both the 21-residue signal peptide and an internal NcoI site near the 5′ end of the proUGn coding sequence. In addition, by inserting a methionine residue adjacent to the thrombin cleavage site, this construct enhanced radiolabeling of any peptide fragments that might be cleaved from the amino-terminal region of the propeptide. The PCR product was ligated into linearized pCITE-4a(+), and, after single colony isolation and amplification in bacteria, the sequence and orientation of the insert were confirmed. The insert encodes the amino acid sequence GSMVQVQLESVKKLNELEEKQMSDPQQQKSGLLPDVCYNPALPLDLQPVCASQEAASTFKALRTIATDECELCINVACTGC.
Plasmid DNA was then used as the template for a coupled in vitro transcription/translation reaction (TnT rabbit reticulocyte lysate system; Promega Corp., Madison, WI) in the presence of 35S-cysteine and 35S-methionine (ProMix; Amersham-Pharmacia Biotech/GE Healthcare, Life Sciences, Waukesha, WI). Labeled proUGn was subsequently affinity purified on S-protein-coupled agarose beads (Novagen) and released from the beads by digestion with biotinylated thrombin (Novagen). The thrombin was then bound to streptavidin-agarose beads (Novagen). After removing the beads, the recombinant propeptide in the supernate was applied to a VYDAC 218TP1010 C-18 reverse-phase column [The Separations Group Inc. (Grace Vydac), Hesperia, CA] that had been pre-equilibrated with HPLC grade water containing 0.1% trifluoroacetic acid. The column was eluted at a flow rate of 1 ml/min, with an immediate step increase to 36% acetonitrile, followed by a linear gradient of acetonitrile from 36–45% over 40 min, and ending with a step increase to 100% and maintained at 100% for 30 min. Two closely eluting peaks of labeled material were identified by liquid scintillation spectroscopy. Two lines of evidence demonstrate that the later peak corresponds to 35S-proUGn: 1) its retention time (35–40 min) was identical to that of native proUGn on this gradient, and 2) the material eluted in this second peak behaved like full-length proUGn in a Western blot. After HPLC purification, solvents were removed under vacuum, and the recombinant 35S-labeled propeptide was resuspended in PBS for infusion into anesthetized animals.
Intravenous infusion of labeled, recombinant proUGn
Animals were deprived of food (but not water) overnight, then anesthetized with urethane (1.6 g/kg body weight, ip). To prepare animals for these experiments, surgeries were performed as described for the renal function studies in our companion publication (27). Radiolabeled proUGn was then administered as either: 1) a bolus injection (5 × 106 cpm) through the jugular vein, or 2) a priming dose (6 × 105 cpm) given by bolus injection followed by a maintenance dose (5 × 106 cpm) added to the infusion solution over a long (60–360 min) period. Plasma and urine were collected at intervals, as described in the Results. Plasma samples (heparinized) and urine were stored on ice for the duration of the experiment, then frozen and stored at −80 C before analysis by HPLC (see next section). At the end of the infusion period, tissues were removed and flash frozen on dry ice. Note that most tissues were frozen immediately, but kidneys were flushed with 15 ml 140 mm NaCl before freezing, to clear radioactive materials from both the intravascular and intratubular compartments. All frozen tissues were homogenized in ice-cold homogenization buffer (4 ml/g tissue), using a motor-driven Potter-Elvehjem homogenizer (Thermo Fisher Scientific Inc., Waltham, MA). The homogenization buffer contained 25 mm HEPES (pH 7.4), 0.5 mm EDTA, and the following protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO): 2.5 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride, 38 μm pepstatin A, 35 μm trans-epoxysuccinyl-l- leucylamido(4-guanidino)butane, 100 μm bestatin, 55 μm leupeptin, and 2 μm aprotinin. After homogenization, the extracts were centrifuged at 27,000 × g for 30 min at 4 C in a Ti-50 rotor (Beckman Instruments, Inc., Fullerton, CA), and the pellets were discarded. Supernatant fractions were stored at −80 C before analysis by HPLC (see next section). At the end of the experiment, after all tissues and fluids had been collected, animals were euthanized by anesthetic overdose.
HPLC analysis of radiolabeled proUGn metabolites in liver, kidney, plasma, and urine
Tissue, plasma, and urine samples were passed through a 0.2-μm Acrodisc syringe filter (Pall Corp., Ann Arbor MI), then applied to the C-18 reverse-phase column described previously, after pre-equilibration with HPLC grade water containing 0.1% trifluoroacetic acid. The column was eluted at a flow rate of 1 ml/min, with a linear gradient of acetonitrile from 2–50% over 30 min, followed by a step increase to 100% acetonitrile and maintained at 100% for 10 min. One-milliliter samples were collected, dried in a centrifugal vacuum concentrator (Savant SpeedVac; Thermo Electron Corp., Waltham MA), dissolved in scintillation fluid (Scintisafe; Thermo Fisher Scientific), and analyzed by scintillation spectroscopy to establish the relative amounts of radioactivity incorported into metabolites and determine their retention times on the column.
Inulin and proUGn clearance measurements
Fluorescein isothiocyanate (FITC)-conjugated inulin (Sigma Chemical) was dissolved in isotonic saline at a concentration of 40 mg/ml in a boiling water bath, cooled, and dialyzed against isotonic saline (SpectraPor 6 dialysis membrane, 1000-Da cutoff limit; Spectrum Laboratories, Rancho Domingo, CA). The dialyzed solution was filtered through a 0.2-μm membrane and then administered to anesthetized Wistar rats as a bolus injection of 0.5 ml into the jugular vein. Blood samples were collected through the carotid artery cannula into heparinized hematocrit tubes at 0.25, 2, 5, 10, 20, 40, 60, 100, and 120 min after the bolus injection. The procedure was repeated in a separate group of “anephric” rats 5–10 min after bilateral ligation of the renal pedicles. Plasma samples were assayed for FITC inulin as described in our companion publication (27), based on the method of Lorenz and Gruenstein (29). The fluorescence intensity obtained from the first plasma sample (30 sec after bolus injection) was set at 100%, and subsequent plasma inulin values are expressed as a percentage of this value.
35S-proUGn disappearance curves were generated by a similar procedure in normal and acutely nephrectomized rats, except that the bolus injection contained 5 × 106 counts of 35S-proUGn produced by the in vitro reticulocyte expression system. Plasma samples were taken at 0.25, 5, 10, 30, 60, 90, and 120 min. Fewer samples were collected than in the inulin experiments because a greater volume of plasma was needed at each time point for HPLC analysis. A 100-μl aliquot of plasma from each sample was fractionated on a C-18 reverse-phase HPLC column to identify potential labeled metabolites that might have accumulated in blood over the duration of the experiment. Fractions from the C-18 column were transferred to scintillation vials, dried under vacuum, and reconstituted in Scintiverse (Fisher Scientific, Pittsburgh PA) for liquid scintillation spectroscopy. ProUGn was estimated as the total counts (blank subtracted) summed across the fractions that coincided with the known retention time for rat proUGn. This always corresponded to a distinct peak of radioactivity (see Fig. 2).
Figure 2.
A bolus dose of 35S-proUGn was cleared rapidly from the plasma of a normal animal and much more slowly after renal ablation. A, Representative HPLC analyses of plasma samples obtained at the indicated times from a control animal. Radioactive material eluted in each fraction was measured in a scintillation counter and is expressed as counts per minute recovered per μl plasma applied to the column (mean ± sem for replicate column runs, n = 3). For the sake of clarity, some time points that were included in the study have been omitted from the figure. The dotted line shows the gradient of acetonitrile that was used to elute the column. B, Representative HPLC analyses of plasma samples obtained from an anephric animal, as in A. Again, for the sake of clarity, some time points have been omitted. C, Schematic depiction (not to scale) of proUGn, c-terminally deleted proUGn (CΔ), UGn15, methionine (met), and cysteine (cys), along with their observed retention times on the C-18 column. The retention times are also marked by arrows in A and B. The asterisks indicate the approximate positions of cysteine and methionine residues that would be radioactively labeled in the parent molecule and its metabolites. D, The plasma level of radioactive proUGn is determined by integrating the radioactivity of the proUGn peaks in A and B, then plotted as a function of time after a bolus injection into control animals (white symbols) and anephric animals (black symbols). To correct for animal-to-animal differences in total blood volume and proUGn specific activities, the proUGn levels for each animal were normalized to the amount present at that animal’s earliest (15 sec) time point. Combined data are presented (mean ± sem, n = 3). In the time intervals chosen for sampling, we could not observe any distribution phase corresponding to the equilibration of the infused material within the animal’s circulating volume, presumably because the initial mixing occurred very rapidly. The solid lines represent the best fit of a triple exponential function to the data (see Materials and Methods). E, Inulin disappearance curves obtained under the same conditions as the proUGn disappearance curves shown in panel C. The gray curves (for proUGn) are reproduced from panel C for reference.
A separate group of five rats was cannulated for an infusion of FITC-labeled inulin (Sigma Chemical) at a rate of 100 μg/min in isotonic saline at 30 μl/min. After 1 h equilibration, 0.5-ml blood samples were taken from the carotid artery and left renal vein for analysis of plasma inulin and endogenous (nonradioactive) proUGn concentrations [methods described in the companion publication (27)]. The renal extraction ratios for each substance were calculated from the arteriovenous concentration differences. Given the well-established renal handling of inulin, the inulin extraction ratio represents the filtration fraction, or the proportion of renal plasma flow that is filtered into the nephrons at that moment. Thus, a comparison of the extraction ratios for proUGn and inulin reveals the extent to which peptide removal by the kidneys corresponds to the amount of peptide filtered into the nephrons.
Autoradiographic localization of 35S-labeled metabolites in histological sections of renal tissue
Animals were anesthetized with urethane, and the jugular vein was cannulated with PE10 tubing for infusion, as described previously. A 5 × 106 cpm dose of 35S-proUGn was administered as an iv infusion over 30 min in a volume of 1 ml isotonic saline. Animals were then flushed with ice-cold heparinized saline through the carotid artery. Venous effluent was collected from a PE90 cannula positioned in the inferior vena cava via the femoral vein. After exsanguination the saline was replaced with ice-cold 4% paraformaldehyde, which was infused until fixation was evident. Kidneys were removed, cut into 4-mm cubes, and cryoprotected by immersion in 10% sucrose for 12 h, followed by 30% sucrose until the tissue sank. The tissue was then embedded in optimum cutting temperature (OCT) compound (Tissue-Tek, Miles Inc., Elkhart, IN) and frozen in an isopentane bath cooled in liquid nitrogen. Frozen sections of the embedded tissue were prepared with a cryostat and mounted on Superfrost slides (Erie Scientific Co., Portsmouth, NH). Slides were dipped in photographic emulsion and sealed in light tight boxes for 2 wk before developing the exposed emulsion with standard photographic procedures. Sections were viewed with an inverted microscope (Zeiss Axioscope; Carl Zeiss MicroImaging, Inc., Thornwood, NY) equipped for epifluorescence excitation and detection. Silver grains were visualized by combining a low level of conventional-phase contrast illumination with high-intensity light delivered perpendicular to the axis of viewing (Darklite Illuminator; Micro Video Instruments, Avon, MA). This allows silver grains to be viewed simultaneously with the underlying tubular structures. Fluorescence images of the same field were then superimposed on the phase-contrast image. In parallel studies, autofluorescent tubular structures were shown to stain selectively with an antibody directed against neutral endopeptidase (NEP 24.11), a brush border protease, thus identifying them as proximal segments (data not shown).
Curve fitting
We used curve-fitting procedures in Igor Pro (WaveMetrics, Lake Oswego, OR) and Prism 5 (GraphPad Software Inc., San Diego CA). These procedures use the Levenberg-Marquardt algorithm to adjust the parameter values of a given function iteratively until the weighted sum of the squared residuals (χ2) is minimized. We used this algorithm to define single, double, and triple exponential functions that would approximate the plasma proUGn and inulin disappearance curves. After each fit was optimized, the resulting χ2 values were used to calculate Akaike’s Information Criterion (AIC) for each function: AIC = n × ln (χ2) + 2m; where n is the number of data points, and m is the number of adjustable parameters (30). In each case the lowest AIC value was observed for the triple exponential function, indicating that it is the preferred model, with the better fit to the data.
Results
In a companion paper (27), we have shown that intact proUGn circulates in the blood at a very much higher level than does UGn. This raises two important issues: 1) at what rate, and by what mechanism, is proUGn cleared from the plasma; and 2) do post-secretory processing mechanisms convert circulating proUGn to active metabolite(s), either in the plasma or in a specific target tissue? To investigate these questions, we performed infusion studies in anesthetized rats, using radiolabeled recombinant rat proUGn.
During infusion of radiolabeled proUGn, radioactivity accumulates in liver, kidney, and urine
After 60 min continuous infusion with labeled propeptide, only two tissues (kidney and liver) accumulated significant amounts of radioactivity (Fig. 1, white bars). The specific activity of labeled material in the kidney was approximately 10 times higher than that in liver (Fig. 1A, black bars), and the concentration of radioactivity in urine was approximately 10 times higher than that in plasma (Fig. 1B), consistent with the idea that the kidney plays a dominant role in clearing the propeptide from the circulation, as would be expected for a peptide of this size.
Figure 1.
Distribution of radiolabel in tissues and fluid compartments at the end of a 60-min steady intravascular infusion of 35S-proUGn. A, The bulk of the recovered radioactivity (counts per minute per organ) was found in kidney and liver (white bars). However, specific activity of labeling (cpm/μg protein) in the kidney is far greater than in any other tissue (black bars). Data report the mean ± sem for three independent determinations. B, Radioactivity was substantially more concentrated in urine than plasma (mean ± sem, n = 3).
Circulating proUGn is rapidly removed from the plasma by renal clearance
To explore further the role of the kidney in proUGn clearance, we measured the rate of disappearance of labeled propeptide from plasma after bolus injection into control and anephric animals (the latter was produced by ligating the renal pedicles 5 min before injection). This is equivalent to a classical pulse-chase procedure because, as demonstrated in our companion publication (27), endogenous, nonradioactive proUGn is continuously released from the intestine into the circulation in both intact and anephric animals. Plasma samples obtained at specific times after the bolus injection were fractionated by reverse-phase HPLC (Fig. 2A, control animals; Fig. 2B, anephric animals). At every time point, the radioactivity eluted from the reverse-phase column as a single, well-defined peak, whose chromatographic behavior was indistinguishable from that of authentic proUGn (retention times of HPLC standards are given in Fig. 2C, and marked by the arrows in Fig. 2, A and B). The amplitude of this peak decreased progressively over time in both types of animal. To evaluate this quantitatively, we integrated the area of the peak for each sample and plotted this value as a function of time after infusion (Fig. 2D). In control animals, proUGn was cleared quickly from the circulation, with an overall plasma half-life of 49 sec (Fig. 2D, white symbols). This disappearance curve was best fit by a triple exponential function (Table 1), suggesting that infused propeptide exits the circulation by a minimum of three independent routes. At least one of these routes must involve the kidney because complete nephrectomy increased proUGn’s plasma dwell time by a factor of seven, giving a new overall plasma half-life of 351 sec (Fig. 2D, black symbols). Furthermore, although the time course of propeptide disappearance was again best fit by a triple exponential function, the fit in the nephrectomized animals appeared to lack the slowest clearance component that was normally observed in control animals, and instead included an ultraslow process (τ > 13,000 sec, Table 1) that was difficult to observe under control conditions, due to the exceedingly low levels of proUGn present in the plasma at all time points after 30 min when the kidneys were functional.
Table 1.
Time constants (τ) for the clearance of proUGn and inulin in control and anephric animals
| proUGn
|
Inulin
|
|||
|---|---|---|---|---|
| Control | Anephric | Control | Anephric | |
| τfast (sec) | 27 | 77 | 49 | 66 |
| τintermediate (sec) | 260 | 389 | 210 | 355 |
| τslow (sec) | 4474 | ND | 1604 | ND |
| τultraslow (sec) | ND | 21296 | ND | 13259 |
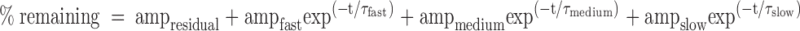 |
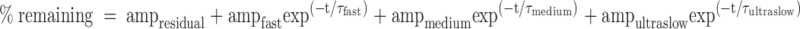 |
ND, Not detectable.
We also performed experiments to measure the rate of disappearance of FITC-labeled inulin after bolus injections into control and anephric animals (Fig. 2E). Inulin has long been used as a filtration marker in renal clearance studies because it is biologically inert, not metabolized by any tissue, freely filtered by the kidney, and neither absorbed nor secreted by the renal tubules. The similar effect of nephrectomy on the time constants for proUGn and inulin disappearance (Table 1) suggests that the two molecules are handled in a similar way, i.e. that the kidney provides a prominent route for clearance of both molecules under normal circumstances. This is supported further by measurements of the renal extraction of inulin and native proUGn. Based on the concentration difference between arterial and renal venous plasma, the renal extraction of inulin from arterial plasma was 26.6 + 3.5% compared with 29.9 + 5.0% for proUGn (mean ± sem, n = 5). These values are not statistically different and suggest that the renal clearance of proUGn is a filtration-dependent phenomenon that parallels inulin clearance.
However, note that proUGn was eliminated from the circulation slightly more effectively than inulin, both in the presence and the absence of the kidneys; compare the inulin curves in Fig. 2E with the superimposed proUGn curves shown in gray and reproduced from Fig. 2D. Thus, at least one clearance mechanism acts on proUGn that does not act on inulin.
Circulating proUGn is not metabolized within the vascular compartment
The HPLC traces in Fig. 2A reveal that essentially all of the radioactivity in plasma was accounted for by intact proUGn at each time point after bolus injection. This remained true even when plasma clearance was greatly retarded by removing the kidneys (Fig. 2B). If there had been significant conversion of proUGn to UGn in the plasma, a radioactive peak should have appeared in the chromatogram at 42–43 min (the retention time of authentic UGn). Any such metabolite would have been heavily 35S-labeled because it would contain the four cysteine residues that are found at the C terminus of the propeptide (Fig. 2C). Furthermore, because we added an extra (nonnative) methionine residue to the N terminus of our recombinant construct, we would also have detected any processing fragments derived from this portion of the molecule. Given the precursor/product relationship between proUGn and any such metabolites, the levels of the latter should have increased as levels of the former decreased. However, no fragments of any sort were observed in any of the chromatograms at any time point, arguing strongly that plasma and endothelial proteases did not process proUGn while it was circulating within the vasculature. Furthermore, within the time frame of these bolus injection experiments, any proUGn metabolites that were generated in tissues were not returned to the plasma in detectable amounts.
For comparison to the results obtained with the radiolabeled recombinant propeptide, we also investigated the plasma stability of native proUGn. For these experiments we generated 2-μl aliquots from a freshly drawn sample of plasma, and added 500 fmol native rat proUGn to each. We then incubated the aliquots at 37 C for varying amounts of time, terminating any potential ongoing proteolytic activity at each specified time point by adding sodium dodecyl sulfate (SDS) sample buffer and immediately boiling the sample. SDS-denatured samples were then applied to polyacrylamide gels, and the relative proUGn content was determined by Western blotting followed by quantitative densitometry, as described in our companion publication (27). We observed no significant degradation of proUGn, even after 60 min incubation, which is more than 70 times longer than the plasma half-life that is normally observed in the intact animal. The propeptide content at 60 min was 92 ± 7% of the control level measured in samples that had been immediately boiled in SDS without any incubation at 37 C (mean ± sem, n = 4).
Circulating proUGn is metabolized by the kidney, and specific metabolites are excreted in the urine
As shown previously (Fig. 1A), a significant amount of labeled material accumulated in the kidney during prolonged iv infusion with radioactive proUGn. However, when analyzed by HPLC, kidney extracts actually contained very little intact proUGn (Fig. 3A). Instead, label was principally associated with two large HPLC peaks eluting at positions corresponding to free cysteine (peak 1) and free methionine (peak 2). These radioactive amino acids were not uniformly distributed within the kidney. When we performed an autoradiographic study of kidneys that were perfusion fixed with 4% formaldehyde 10 min after beginning an iv infusion of radioactive proUGn, we observed silver grains, marking the locations of 35S-labeled metabolites, over a large number of the autofluorescent profiles of proximal tubular segments close to glomeruli, but not over the glomeruli themselves or nonfluorescent distal tubules (Fig. 3B).
Figure 3.
Metabolites accumulating in kidney and urine during prolonged infusion of radiolabeled proUGn. A, HPLC analysis of the 27,000 × g supernatant fraction (cytosol) obtained from kidney homogenates. Material was applied to a C-18 reverse-phase column and eluted with a gradient of acetonitrile (dashed line). Radioactivity eluting in each fraction was measured in a scintillation counter and is expressed as a percentage of the total radioactivity recovered (mean ± sem, n = 4). Retention times of cysteine (cys), methionine (met), UGn and proUGn standards are marked by the arrows. B, Photomicrograph of a representative section of renal cortex prepared from tissue fixed 10 min after iv infusion of 35S-labeled proUGn. The phase-contrast image on the top shows two glomeruli (labeled “G” in the lower panel) and several tubular profiles. The fluorescence image on the bottom shows silver grains and autofluorescence at 650 nm in the same field. Proximal tubules are identifiable by autofluorescence, whereas glomeruli and distal tubular segments are negative for autofluorescence. This was verified in separate experiments (data not shown) that compared the distribution of autofluorescence to that of neutral endopeptidase (NEP 24.11), an enzyme that is present in the brush border membranes of the proximal tubules. Silver grains, indicating the presence of 35S-labeled cysteine and 35S-labeled methionine, were concentrated over some autofluorescent proximal tubular profiles but were never present over distal tubules or glomeruli. Proximal tubules labeled in this way were situated close to glomeruli. C, HPLC analysis of urine (mean ± sem, n = 7), performed as described for A. Retention times of cysteine, methionine, UGn, and proUGn standards are marked by the arrows. Peaks numbered three and four represent unidentified metabolites.
To evaluate the possibility that the absence of significant amounts of intact proUGn in kidney extracts was due to post-homogenization proteolysis of the propeptide, we spiked a homogenized sample with a known amount of native proUGn and measured the recovery of intact propeptide after prolonged incubation. This was similar to the plasma stability studies described in the previous section, except that we also included our standard protease inhibitor cocktail and performed the incubation at 4 C, to parallel the way in which the radioactive samples had been processed. After 60 min we recovered essentially all (92.6 ± 2.6%, mean ± range, n = 2) of the spiked native material in intact form, indicating that proteolysis was not a significant factor in the radiolabeling studies.
In striking contrast to the limited spectrum of labeled metabolites observed in kidney homogenates, radioactivity recovered in the urine was distributed among a wide variety of molecular species (Fig. 3C), including cysteine (peak 1), methionine (peak 2), a small amount of material whose retention time matches that of intact proUGn (peak 6), and three additional metabolites (peaks 3, 4, and 5); note that peak 3 partially overlaps the methionine peak. One of these metabolites (peak 5) had a retention time identical to that of an authentic rat UGn15 standard. The other metabolites (peaks 3 and 4) are as yet unidentified. However, we believe it is likely that they correspond to UGn13 and UGn14 because these alternative proUGn cleavage products were both previously identified (along with UGn15) as prominent bioactive components of opossum urine (11). However, it is difficult to compare previously published and current results on a peak-by-peak basis because substantial differences in pH, ion pairing reagent, and gradient elution profile will have affected the relative retention times of the individual molecules.
The fact that urinary peptide metabolites of proUGn do not gain access to the circulation was further confirmed in samples of blood taken contemporaneously from carotid artery and renal vein after 1–2 h continuous iv 35S-proUGn infusion (Fig. 4A). Consistent with the prolonged nature of this infusion protocol, both plasma samples showed a predominant proUGn peak (peak 6). In addition, and unlike the bolus injection studies previously presented in Fig. 2, modest cysteine- and methionine-like peaks were also detectible (peaks 1 and 2). Presumably, these were generated within the kidney during the processing of proUGn and then returned to the plasma. As would be predicted by this hypothesis, the renal venous plasma contained a significantly higher proportion of the labeled amino acids, relative to intact proUGn, than did the carotid arterial plasma (Fig. 4B). In addition, it is particularly noteworthy that neither the venous nor the arterial plasma sample contained any metabolite of proUGn other than free cysteine and free methionine. Thus, even over prolonged time periods, circulating proUGn does not serve as a source of circulating UGn.
Figure 4.
During prolonged infusion of radiolabeled proUGn, the kidney extracted the propeptide from the plasma, while secreting radiolabeled amino acids back into the circulation. A, HPLC profile of radioactive metabolites in systemic plasma collected from the carotid artery (open symbols) compared with plasma collected from the renal vein (filled symbols), as in Fig. 2. B, Relative recoveries of cysteine (cys), methionine (met), and proUGn. Compared with the carotid arterial plasma (open bars), the renal venous plasma (filled bars) contained a significantly higher proportion of metabolites, and lower proportion of intact proUGn (mean ± sem, n = 6; **, P < 0.01; *, P < 0.05). This is consistent with the loss of some proUGn due to renal extraction, coupled with the return of some cysteine and methionine to the circulation after digestion of the extracted proUGn.
Liver, which is the only other tissue that accumulated significant amounts of radioactivity during prolonged infusion of labeled proUGn (Fig. 1A), was also found, on HPLC analysis, to contain primarily cysteine and methionine (Fig. 5). These labeled amino acids could have been generated locally by a catabolic mechanism resident within the liver itself or could have been generated extrahepatically (e.g. by the kidney) and transported to the liver via the circulation. Thus, in contrast to the kidney, the liver does not metabolize proUGn to UGn, or to any other metabolites more complex than free amino acids. Unexpectedly, the ratio of labeled cysteine to labeled methionine in liver was strikingly lower than in plasma or kidney. This is likely a reflection of the tight control that is known to be maintained over hepatic cysteine levels, allowing the liver to maintain a pool of the amino acid adequate to support large-scale protein synthesis while keeping its levels below the threshold for cytotoxicity (31).
Figure 5.
Metabolites accumulating in the liver during prolonged infusion of radiolabeled proUGn. The figure shows an analysis of the 27,000 × g supernatant fraction (cytosol) obtained from hepatic homogenates and subjected to HPLC, as in Fig. 3. Livers were removed from anesthetized animals after 60 min continuous intravascular infusion (mean ± sem, n = 3). cys, Cysteine; met, methionine.
Liver homogenates also contained very small amounts of intact proUGn (Fig. 5). However, because the livers were not perfused with nonradioactive medium before processing, we consider it most likely that this small peak represents residual proUGn that was present within vascular and/or interstitial spaces. Further experimentation will be required to determine whether hepatocytes can take up intact proUGn.
Discussion
As a general rule, peptide hormones are synthesized as inactive propeptide precursors and converted to smaller, biologically active peptides by specific proteolytic cleavages (32,33). For typical peptide hormones, the most abundant intracellular storage form is the mature, processed peptide. For example, only 5% of gastrin and 20% of cholecystokinin are found in the small intestine in propeptide form (34). However, a few peptide hormones, such as ANP and angiotensin, are stored almost exclusively as inactive propeptides (35,36,37). In such cases, processing occurs as a post-secretory event, mediated by ecto-proteases located at the site of secretion or within the vasculature.
Our studies, presented here and in a companion publication (27), reveal that UGn falls into yet a third category. Like ANP and angiotensin, UGn is stored within tissues primarily in propeptide form, and significant quantities of the intact propeptide are released into the plasma. However, in contrast to pro-ANP and angiotensinogen, circulating proUGn is not processed intravascularly (Figs. 2, A and B, and 4A). Rather, it is cleared from plasma by the kidney (Fig. 2D) and processed intrarenally (Fig. 3C). With the exception of free cysteine and free methionine, the renal metabolites are not returned to the general circulation (Fig. 4A), but rather are excreted in the urine (Fig. 3C).
One or more of these renal metabolites must have biological activity because, as shown in our companion publication (27), infusion of intact proUGn evokes a strong natriuretic response from the kidney. Furthermore, the increases in fractional sodium excretion observed in those renal function studies, coupled with stable blood pressure and glomerular filtration rate (GFR), suggest that the active fragment acts on solute absorption mechanisms that reside within the tubules, rather than hemodynamic mechanisms (systemic or renal vascular resistance) that reside outside the tubules. Thus, our results suggest that a plasma pool of proUGn is secreted from the intestine, to serve as a plasma reservoir of biologically inert material that is converted intrarenally to an active product for delivery to the nephrons. We have not yet explicitly established the identity of the active metabolite(s), but one of the radioactive fragments produced from proUGn by the kidney has the same retention time as UGn15, a bioactive form of UGn that is known to be relatively abundant in urine (11). Intrarenal generation of UGn-related peptides, followed by excretion of these mature peptides in urine, would be strikingly consistent with previous studies demonstrating the presence of large amounts of bioactive UGn in the urine of opossums (11), humans (38), and rats (39). Indeed, it seems likely that the high levels of UGn in urine would be derived from the abundantly circulating propeptide rather than from the extremely limited supply of UGn that is present in plasma.
Our experiments also do not identify the intrarenal site where proUGn processing occurs. Two distinct possibilities can be considered. First, the propeptide could pass intact across the glomerular filtration barrier, for processing within the tubular lumen by proteases residing in the epithelial brush border of the proximal tubules. Alternatively, the propeptide could be absorbed from the plasma into tubular epithelial cells by basolateral endocytosis, then processed within vacuoles for apical secretion (in processed form) into the tubular lumen. Although our data do not unequivocally discriminate between these models, several lines of evidence provide strong support for the former over the latter. First, as reported in our companion publication (27), proUGn circulates in plasma as a free 9.4-kDa peptide, uncomplexed with a carrier protein, and is thus small enough to be readily filterable. This predicts that, at a minimum, approximately 30% of the propeptide entering the kidney at any time will be delivered to the tubular lumen in intact form by filtration. This was confirmed by the equivalence between renal extraction ratios close to 30% for both proUGn and inulin, a classical marker of renal function that is cleared exclusively by glomerular filtration. Second, the time course of clearance of plasma proUGn (Fig. 2D) was very similar to that of inulin, which reinforces the idea that the renal clearance of proUGn is due to filtration rather than secretion. Third, we observed intense radioactive labeling of proximal tubule profiles after iv infusion of labeled proUGn (Fig. 3B), but the tissue content of radioactivity associated with those profiles was comprised almost exclusively of free amino acids (Fig. 3A) rather than the larger peptide metabolites that are found in urine (Fig. 3C). All of these results are more consistent with intratubular processing than with transcytotic intracellular processing.
A working model for the biological roles of proUGn in health and disease
Whole body electrolyte homeostasis requires that dietary salt intake must, over the long term, be matched by renal salt excretion. However, the episodical nature of salt consumption intrinsically generates short-term imbalances. Thus, it is currently believed that the introduction of salt into the lumen of the intestine triggers two protective mechanisms: one that slows the absorption of the ingested salt from the lumen (40), and another that accelerates the excretion of systemic salt by the kidney (1,2,3,4,5,6). Together, these mechanisms minimize, or “buffer,” the impact of an incoming salt load. Our working model, shown schematically in Fig. 6, assigns prominent roles in both of these postulated “buffering” mechanisms to proUGn, and, as the most likely possibility, the UGn derived from it. The model highlights new observations presented here and in our companion publication (27), as well as previous findings contributed by a number of other laboratories (reviewed in Refs. 41 and 42). The model ignores potassium, whose reabsorption appears to be regulated by UGn within the kidney (24), but whose postprandial handling is, otherwise, not well understood.
Figure 6.
Model for the postprandial response to salt ingestion in the rat, integrating previously reported information about UGn and proUGn with new results presented in this and a companion publication (27). According to the model, ingesting salt triggers apical and basolateral secretion of proUGn from intestinal EC cells; note that a similar role might be played by ECL cells in the human stomach, where gastric expression of UGn is much higher than in the rat. Apically secreted proUGn is converted to UGn by proteases residing within the intestinal lumen. UGn then acts intraluminally to regulate epithelial electrolyte transport mechanisms. The net effect is to enhance the secretion of sodium and chloride into the lumen, and suppress sodium absorption from the lumen (blue arrows), thus delaying the delivery of salt to the body. In parallel, basolaterally secreted proUGn is delivered to the kidney where it is filtered and converted to smaller peptides and free amino acids by brush border proteases residing within the proximal tubule. Although not shown in the figure, it is also possible that some proUGn may be absorbed from the plasma by tubular epithelial cells, which convert it intracellularly to smaller peptides that are then secreted into the tubular lumen. Free amino acids are returned to the circulation, and one of the luminal peptides (very likely UGn, though possibly an alternate metabolite) acts within the nephron to regulate tubular electrolyte transport. Although the exact site of action is unclear, the net effect on the kidney is to decrease the reabsorption of filtered sodium (blue arrows), thereby accelerating the rate at which sodium is excreted from the body. Thus, this novel endocrine pathway can coordinate the activity of two principal organs involved in whole body electrolyte homeostasis: the intestine, where salt is absorbed, and the kidney, where salt is excreted. Complementary actions at these two sites are thought to buffer the rapid influx of sodium, and perhaps other electrolytes, that would otherwise accompany a salt-rich meal. The proUGn-based signaling pathway may also be activated in disease states that involve volume expansion, such as kidney failure and heart failure.
Central to the model is the identity of the cell that synthesizes proUGn in the alimentary tract. In the rat, proUGn synthesis is most likely performed by a subpopulation of enterochromaffin (EC) cells located within the small intestine (16,17,43), although it should be noted that enterochromaffin-like (ECL) cells in the rat stomach also express a proUGn-like molecule at levels that can be detected immunocytochemically (44). However, when extracts of rat stomach are analyzed by Western blotting (27) or Northern blotting (45), the data suggest that the synthetic capacity of gastric ECL cells is inadequate to support a significant contribution to the high circulating levels of the propeptide that are reported in the companion publication (27), arguing that the gastric actions of proUGn in rats are most likely paracrine (similar to those of histamine and somatostatin), rather than endocrine.b
EC cells were initially identified in the 1950s as the principal site of enteric serotonin synthesis (48) but have subsequently been found to coexpress a variety of peptides, including proUGn (16,17,43), substance P (49), and enkephalin (50). EC cells reside within, and span the width of, the intestinal epithelium (51). Functionally, they have been recognized as enteric sensory receptors that respond to a variety of intraluminal mechanical (tactile) or chemical (acidic, nutrient, osmotic, or ionic) stimuli (52,53,54,55). In the context of our model, it is noteworthy that intraluminal sodium chloride is a prominent stimulus known to activate EC cells (56,57).
EC cells respond to luminal stimuli by releasing serotonin and substance P both apically (into the lumen) and basolaterally (into the interstitium) (51,58,59). Morphological studies show that, like serotonin, immunoreactive proUGn is readily observed both adjacent to basolateral membranes and within the apical extensions of EC cells that project to the lumen (16,17), providing strong indirect evidence that both basolateral and apical secretion of proUGn occur. This presumption is an integral feature of our working model (Fig. 6), which ascribes different functions to apically and basolaterally secreted proUGn. The model proposes that apically secreted propeptide is converted to UGn by proteases residing within the intestinal lumen, a likely candidate protease being chymotrypsin, which rapidly converts proUGn to UGn in vitro (28). UGn, in turn, stimulates chloride channel activity in crypt epithelial cells (which results in a transepithelial electrical potential that drives the paracellular flow of sodium into the lumen) while simultaneously inhibiting sodium/proton exchange in villous epithelial cells (which decreases the movement of sodium out of the lumen) (21,42). These complementary effects strongly retard net intestinal sodium absorption. At the same time, the model proposes that basolaterally secreted proUGn enters the circulation and is transported to the kidney, where it is filtered and metabolized to peptide fragments and free amino acids by brush border proteases located within the proximal tubule. The amino acids are reclaimed from the tubule lumen and returned to the circulation. One of the peptide fragments (very likely UGn, though possibly an alternate metabolite) acts through an as yet unidentified receptor within proximal and/or distal nephron segments to decrease sodium and potassium reabsorption by mechanisms that are under active investigation (60,61,62,63,64,65,66). This accelerates renal salt excretion, which, in conjunction with the delayed intestinal salt absorption, reduces the extent and slows the rate of delivery of ingested salt to the body, thereby minimizing its impact on body fluid compartments.
Such a buffering system would be most appropriately invoked only when the amount of dietary salt exceeds the body’s needs, and might actually be counterproductive during times of salt deficiency. Therefore, it seems likely that these “protective” mechanisms would, themselves, be subject to regulation that considers the body’s overall fluid and electrolyte status. Although little information is currently available to support or refute this conjecture, it has been shown that mRNA expression levels of UGn (along with those of guanylin, a related gut peptide that also regulates intestinal epithelial transport) are influenced by long-term changes in oral sodium intake (18,67).
In a more pathological context, it is well documented that plasma and urinary levels of UGn and proUGn are elevated in human patients with kidney disease, with good correlation between the degree of elevation and severity of the disease (68,69,70). This is consistent with our results showing that plasma clearance of proUGn requires normal kidney function (Fig. 2). However, urinary UGn levels are also significantly elevated in congestive heart failure patients (71), where renal filtration is not typically compromised. The common feature shared by kidney failure and heart failure is volume expansion, not reduced filtration, suggesting that enhanced release of proUGn from the intestine might represent an adaptive mechanism that augments renal salt and fluid excretion in response to pathological salt and fluid retention.
In summary, the results presented here and in our companion publication (27) add to the accumulating evidence that intestinally derived UGn plays a role in renal function and salt homeostasis, and, in particular, focus on proUGn as a relatively neglected player in the signaling pathway. In addition, our results suggest a novel paradigm for the processing of an endocrine peptide, in which an inactive prohormone is converted to active metabolites primarily extracellularly, and only upon reaching its specific target organs. Because processing and, thus, physiological responses are initiated intraluminally within the kidney and gut, this represents a unique situation in which an endocrine agent is constrained, by its unconventional processing mechanisms, to act in the manner of an exocrine agent.
Acknowledgments
We thank Christopher Cazzolla, Randall Rhyne, and Tailun Zhao for technical assistance, James Anderson and Bill Arendshorst for invaluable collegial encouragement, Richard Cheney for generous access to his fluorescence microscope, and Kathleen Dunlap for helpful comments on the manuscript.
Footnotes
This work was supported by awards from the National Institutes of Health (RO1-HL078980), the American Heart Association (Grant-in-Aid NC97GS34 and 0755397U), and the Maren Foundation (to M.F.G.), and by a Pilot/Feasability Award from the University of North Carolina Center for Gastrointestinal Biology and Disease (DK34987) (to N.G.M.).
Present address for X.Q.: University of South Alabama, Department of Medical Genetics, 307 University Boulevard, Mobile, Alabama 36608.
Disclosure Summary: R.C.F. has nothing to declare. N.G.M., X.Q., and M.F.G. are inventors on United States Patent Application No. 11/596,493.
First Published Online May 22, 2008
Abbreviations: AIC, Akaike’s Information Criterion; ANP, atrial natriuretic peptide; EC, enterochromaffin; ECL, enterochromaffin-like; FITC, fluorescein isothiocyanate; proUGn, prouroguanylin; SDS, sodium dodecyl sulfate; UGn, uroguanylin.
As a further consideration, and in contrast to the situation in rats, there is relatively high expression of proUGn mRNA in the stomach of the human (46) and opossum (14,47). Furthermore, even the identity of the UGn-expressing cells may be distinct in these species because UGn appears to be expressed in somatostatin-containing D cells, rather than in EC or ECL cells, in the human intestine and stomach (46). Thus, while the model presented here focuses on EC cells residing in the rat intestine, this cellular detail may not pertain to all species.
References
- Lennane RJ, Carey RM, Goodwin TJ, Peart WS 1975 A comparison of natriuresis after oral and intravenous sodium loading in sodium-depleted man: evidence for a gastrointestinal or portal monitor of sodium intake. Clin Sci Mol Med 49:437–440 [DOI] [PubMed] [Google Scholar]
- Carey RM, Smith JR, Ortt EM 1976 Gastrointestinal control of sodium excretion in sodium-depleted conscious rabbits. Am J Physiol 230:1504–1508 [DOI] [PubMed] [Google Scholar]
- Carey RM 1978 Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res 43:19–23 [DOI] [PubMed] [Google Scholar]
- Singer DR, Markandu ND, Buckley MG, Miller MA, Sagnella GA, MacGregor GA 1998 Contrasting endocrine responses to acute oral compared with intravenous sodium loading in normal humans. Am J Physiol 274(1 Pt 2):F111–F119 [DOI] [PubMed] [Google Scholar]
- Daly JJ, Roe JW, Horrocks P 1967 A comparison of sodium excretion following the infusion of saline into systemic and portal veins in the dog: evidence for a hepatic role in the control of sodium excretion. Clin Sci 33:481–487 [PubMed] [Google Scholar]
- Perlmutt JH, Aziz O, Haberich FJ 1975 A comparison of sodium excretion in response to infusion of isotonic saline into the vena porta and vena cava of conscious rats. Pflugers Arch 357:1–14 [DOI] [PubMed] [Google Scholar]
- Morita H, Ishiki K, Hosomi H 1991 Effects of hepatic NaCl receptor stimulation on renal nerve activity in conscious rabbits. Neurosci Lett 123:1–3 [DOI] [PubMed] [Google Scholar]
- Ishiki K, Morita H, Hosomi H 1991 Reflex control of renal nerve activity originating from the osmoreceptors in the hepato-portal region. J Auton Nerv Syst 36:139–148 [DOI] [PubMed] [Google Scholar]
- Peterson TV, Benjamin BA, Hurst NL, Euler CG 1991 Renal nerves and postprandial renal excretion in the conscious monkey. Am J Physiol 261(5 Pt 2):R1197–R1203 [DOI] [PubMed] [Google Scholar]
- Forte LR 2003 A novel role for uroguanylin in the regulation of sodium balance. J Clin Invest 112:1138–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE 1993 Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci USA 90:10464–10468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R, Kuhn M, Schulz-Knappe P, Raida M, Fuchs M, Klodt J, Adermann K, Kaever V, Cetin Y, Forssmann WG 1995 GCAP-II: isolation and characterization of the circulating form of human uroguanylin. FEBS Lett 374:34–38 [DOI] [PubMed] [Google Scholar]
- Li Z, Perkins AG, Peters MF, Campa MJ, Goy MF 1997 Purification, cDNA sequence, and tissue distribution of rat uroguanylin. Regul Pept 68:45–56 [DOI] [PubMed] [Google Scholar]
- Fan X, Hamra FK, Freeman RH, Eber SL, Krause WJ, Lim RW, Pace VM, Currie MG, Forte LR 1996 Uroguanylin: cloning of preprouroguanylin cDNA, mRNA expression in the intestine and heart and isolation of uroguanylin and prouroguanylin from plasma. Biochem Biophys Res Commun 219:457–462 [DOI] [PubMed] [Google Scholar]
- Miyazato M, Nakazato M, Matsukura S, Kangawa K, Matsuo H 1996 Uroguanylin gene expression in the alimentary tract and extra-gastrointestinal tissues. FEBS Lett 398:170–174 [DOI] [PubMed] [Google Scholar]
- Perkins A, Goy MF, Li Z 1997 Uroguanylin is expressed by enterochromaffin cells in the rat gastrointestinal tract. Gastroenterology 113:1007–1014 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Yamaguchi H, Date Y, Miyazato M, Kangawa K, Goy MF, Chino N, Matsukura S 1998 Tissue distribution, cellular source, and structural analysis of rat immunoreactive uroguanylin. Endocrinology 139:5247–5254 [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Jackson BA, Cai WY, Greenberg RN, Ott CE 2002 Site-specific effects of dietary salt intake on guanylin and uroguanylin mRNA expression in rat intestine. Regul Pept 107:87–95 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Fujimoto S, Nakazato M, Yokota N, Date Y, Yamaguchi H, Hisanaga S, Eto T 1997 Urine and plasma levels of uroguanylin and its molecular forms in renal diseases. Kidney Int 52:1028–1034 [DOI] [PubMed] [Google Scholar]
- Fukae H, Kinoshita H, Fujimoto S, Kita T, Nakazato M, Eto T 2002 Changes in urinary levels and renal expression of uroguanylin on low or high salt diets in rats. Nephron 92:373–378 [DOI] [PubMed] [Google Scholar]
- Forte LR, Fan X, Hamra FK 1996 Salt and water homeostasis: uroguanylin is a circulating peptide hormone with natriuretic activity. Am J Kidney Dis 28:296–304 [DOI] [PubMed] [Google Scholar]
- Greenberg RN, Hill M, Crytzer J, Krause WJ, Eber SL, Hamra FK, Forte LR 1997 Comparison of effects of uroguanylin, guanylin, and Escherichia coli heat-stable enterotoxin STa in mouse intestine and kidney: evidence that uroguanylin is an intestinal natriuretic hormone. J Investig Med 45:276–282 [PubMed] [Google Scholar]
- Carrithers SL, Hill MJ, Johnson BR, O'Hara SM, Jackson BA, Ott CE, Lorenz J, Mann EA, Giannella RA, Forte LR, Greenberg RN 1999 Renal effects of uroguanylin and guanylin in vivo. Braz J Med Biol Res 32:1337–1344 [DOI] [PubMed] [Google Scholar]
- Fonteles MC, Greenberg RN, Monteiro HS, Currie MG, Forte LR 1998 Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol 275(2 Pt 2):F191–F197 [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, Shah RG, Sun C, Mann EA, Fonteles MC, Forte LR, Jackson BA, Giannella RA, Greenberg RN 2004 Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int 65:40–53 [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB 2003 Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest 112:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss NG, Fellner RC, Qian X, Yu SJ, Li Z, Nakazato M, Goy MF 2008 Uroguanylin, an intestinal natriuretic peptide, is delivered to the kidney as an unprocessed propeptide. Endocrinology 149:4486–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Fan X, Krause WJ, Freeman RH, Chin DT, Smith CE, Currie MG, Forte LR 1996 Prouroguanylin and proguanylin: purification from colon, structure, and modulation of bioactivity by proteases. Endocrinology 137:257–265 [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Gruenstein E 1999 A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol 276(1 Pt 2):F172–F177 [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR 2004 Multimodel inference: understanding AIC and BIC in model selection. Soc Methods Res 33:261–304 [Google Scholar]
- Stipanuk MH, Dominy Jr JE, Lee JI, Coloso RM 2006 Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136(Suppl):1652S–1659S [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Varro A, Dimaline R 1996 Gastric endocrine cells: gene expression, processing, and targeting of active products. Physiol Rev 76:767–798 [DOI] [PubMed] [Google Scholar]
- Tanaka S 2003 Comparative aspects of intracellular proteolytic processing of peptide hormone precursors: studies of proopiomelanocortin processing. Zoolog Sci 20:1183–1198 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 1998 The new biology of gastrointestinal hormones. Physiol Rev 78:1087–1108 [DOI] [PubMed] [Google Scholar]
- Sei CA, Hand GL, Murray SF, Glembotski CC 1992 The cosecretional maturation of atrial natriuretic factor by primary atrial myocytes. Mol Endocrinol 6:309–319 [DOI] [PubMed] [Google Scholar]
- Yan W, Wu F, Morser J, Wu Q 2000 Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA 97:8525–8529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Danser AH 2005 Is angiotensin II made inside or outside of the cell? Curr Hypertens Rep 7:124–127 [DOI] [PubMed] [Google Scholar]
- Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR 1994 Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol 266(2 Pt 2):F342–F348 [DOI] [PubMed] [Google Scholar]
- Fan X, Hamra FK, London RM, Eber SL, Krause WJ, Freeman RH, Smith CE, Currie MG, Forte LR 1997 Structure and activity of uroguanylin and guanylin from the intestine and urine of rats. Am J Physiol 273(5 Pt 1):E957–E964 [DOI] [PubMed] [Google Scholar]
- Hosomi H, Morita H 1996 Hepatorenal and hepatointestinal reflexes in sodium homeostasis. News Physiol Sci 11:103–107 [Google Scholar]
- Forte Jr LR 2004 Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104:137–162 [DOI] [PubMed] [Google Scholar]
- Vaandrager AB 2002 Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem 230:73–83 [PubMed] [Google Scholar]
- Cui L, Blanchard RK, Coy LM, Cousins RJ 2000 Prouroguanylin overproduction and localization in the intestine of zinc-deficient rats. J Nutr 130:2726–2732 [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Yamaguchi H, Kangawa K, Kinoshita Y, Chiba T, Ueta Y, Yamashita H, Matsukura S 1999 Enterochromaffin-like cells, a cellular source of uroguanylin in rat stomach. Endocrinology 140:2398–2404 [DOI] [PubMed] [Google Scholar]
- Blanchard RK, Cousins RJ 1997 Upregulation of rat intestinal uroguanylin mRNA by dietary zinc restriction. Am J Physiol 272(5 Pt 1):G972–G978 [DOI] [PubMed] [Google Scholar]
- Magert HJ, Reinecke M, David I, Raab HR, Adermann K, Zucht HD, Hill O, Hess R, Forssmann WG 1998 Uroguanylin: gene structure, expression, processing as a peptide hormone, and co-storage with somatostatin in gastrointestinal D-cells. Regul Pept 73:165–176 [DOI] [PubMed] [Google Scholar]
- London RM, Krause WJ, Fan X, Eber SL, Forte LR 1997 Signal transduction pathways via guanylin and uroguanylin in stomach and intestine. Am J Physiol 273(1 Pt 1):G93–G105 [DOI] [PubMed] [Google Scholar]
- Erspamer V, Asero B 1952 Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169:800–801 [DOI] [PubMed] [Google Scholar]
- Pearse AG, Polak JM 1975 Immunocytochemical localization of substance P in mammalian intestine. Histochemistry 41:373–375 [DOI] [PubMed] [Google Scholar]
- Solcia E, Capella C, Buffa R, Usellini L, Fiocca F, Sessa F 1987 Endocrine cells of the digestive system. In: Johnson LR, ed. Physiology of the gastrointestinal tract. New York: Raven Press; 111–130 [Google Scholar]
- Nilsson O, Ahlman H, Geffard M, Dahlstrom A, Ericson LE 1987 Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res 248:49–54 [DOI] [PubMed] [Google Scholar]
- Bulbring E, Crema A 1959 The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol 146:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke HJ 1998 “Enteric tears:” Chloride secretion and its neural regulation. News Physiol Sci 13:269–274 [PubMed] [Google Scholar]
- Gershon MD 2004 Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20(Suppl 7):3–14 [DOI] [PubMed] [Google Scholar]
- Braun T, Voland P, Kunz L, Prinz C, Gratzl M 2007 Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 132:1890–1901 [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Zhu J, Owyang C 2000 Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 118:1197–1207 [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Zhu JX, Owyang C 2001 Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol 281:G916–G23 [DOI] [PubMed] [Google Scholar]
- Forsberg EJ, Miller RJ 1983 Regulation of serotonin release from rabbit intestinal enterochromaffin cells. J Pharmacol Exp Ther 227:755–766 [PubMed] [Google Scholar]
- Ahlman H, DeMagistris L, Zinner M, Jaffe BM 1981 Release of immunoreactive serotonin into the lumen of the feline gut in response to vagal nerve stimulation. Science 213:1254–1255 [DOI] [PubMed] [Google Scholar]
- Sindice A, Basoglu C, Cerci A, Hirsch JR, Potthast R, Kuhn M, Ghanekar Y, Visweswariah SS, Schlatter E 2002 Guanylin, uroguanylin, and heat-stable euterotoxin activate guanylate cyclase C and/or a pertussis toxin-sensitive G protein in human proximal tubule cells. J Biol Chem 277:17758–17764 [DOI] [PubMed] [Google Scholar]
- Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, Kuhn M, Schlatter E 2005 Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int 68:1008–1017 [DOI] [PubMed] [Google Scholar]
- Sindic A, Hirsch JR, Velic A, Piechota H, Schlatter E 2005 Guanylin and uroguanylin regulate electrolyte transport in isolated human cortical collecting ducts. Kidney Int 67:1420–1427 [DOI] [PubMed] [Google Scholar]
- Schlatter E, Cermak R, Forssmann WG, Hirsch JR, Kleta R, Kuhn M, Sun D, Schafer JA 1996 cGMP-activating peptides do not regulate electrogenic electrolyte transport in principal cells of rat CCD. Am J Physiol 271(6 Pt 2):F1158–F1165 [DOI] [PubMed] [Google Scholar]
- Elitsur N, Lorenz JN, Hawkins JA, Rudolph JA, Witte D, Yang LE, McDonough AA, Cohen MB 2006 The proximal convoluted tubule is a target for the uroguanylin-regulated natriuretic response. J Pediatr Gastroenterol Nutr 43(Suppl 1):S74–S81 [DOI] [PubMed] [Google Scholar]
- Amorim JB, Musa-Aziz R, Lessa LM, Malnic G, Fonteles MC 2006 Effect of uroguanylin on potassium and bicarbonate transport in rat renal tubules. Can J Physiol Pharmacol 84:1003–1010 [DOI] [PubMed] [Google Scholar]
- Sindic A, Schlatter E 2007 Renal electrolyte effects of guanylin and uroguanylin. Curr Opin Nephrol Hypertens 16:10–15 [DOI] [PubMed] [Google Scholar]
- Li Z, Knowles JW, Goyeau D, Prabhakar S, Short DB, Perkins AG, Goy MF 1996 Low salt intake down-regulates the guanylin signaling pathway in rat distal colon. Gastroenterology 111:1714–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Nakazato M, Yamaguchi H, Matsukura S, Fujimoto S, Eto T 1997 Increased plasma guanylin levels in patients with impaired renal function. Clin Nephrol 47:28–32 [PubMed] [Google Scholar]
- Kinoshita H, Fujimoto S, Fukae H, Yokota N, Hisanaga S, Nakazato M, Eto T 1999 Plasma and urine levels of uroguanylin, a new natriuretic peptide, in nephrotic syndrome. Nephron 81:160–164 [DOI] [PubMed] [Google Scholar]
- Fukae H, Kinoshita H, Fujimoto S, Nakazato M, Eto T 2000 Plasma concentration of uroguanylin in patients on maintenance dialysis therapy. Nephron 84:206–210 [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Eber SL, Forte LR, Greenberg RN 2000 Increased urinary excretion of uroguanylin in patients with congestive heart failure. Am J Physiol Heart Circ Physiol 278:H538–H547 [DOI] [PubMed] [Google Scholar]








