Abstract
Ghrelin, a gastric peptide hormone, has been reported to regulate GH secretion and energy homeostasis. Here, we examined the effect of des-acyl ghrelin driven from the fatty acid-binding protein-4 (FABP4) promoter on adiposity and glucose metabolism. A high level of expression of des-acyl ghrelin (692 ± 293 fmol/g fat) in adipose tissue was detected in FABP4-ghrelin transgenic mice, but not in wild-type littermates. Circulating des-acyl ghrelin was significantly higher in FABP4-ghrelin transgenic mice (8409 ± 3390 pm) compared with wild-type mice (513 ± 58 pm). No significant change was observed for plasma acylated ghrelin and obestatin. Epididymal and perirenal fat masses decreased 35 ± 9 and 52 ± 9%, respectively, in FABP4-ghrelin transgenic mice. FABP4-ghrelin transgenic mice are resistant to obesity induced by high-fat diet. Brown fat mass was not affected by overexpression of ghrelin in adipose tissue. Glucose tolerance tests showed glucose levels to be significantly lower in FABP4-ghrelin transgenic mice than in controls after glucose administration. Insulin sensitivity testing showed that FABP4-ghrelin transgenic mice had a 28 ± 5% greater hypoglycemic response to insulin. Our study demonstrates that overexpression of ghrelin from the FABP4 promoter impairs the development of white adipose tissues, and alters glucose tolerance and insulin sensitivity in mice.
GHRELIN IS A 28-amino acid peptide that is secreted by gastric oxyntic glands (1). The ghrelin gene is composed of five exons. Preproghrelin undergoes endoproteolytic processing and posttranslational modification to produce ghrelin and des-acyl ghrelin. Des-acyl ghrelin has the same amino acid sequence as ghrelin, but the third amino acid (serine 3) is not acylated. A third putative proghrelin peptide, termed “obestatin,” has been introduced, but biochemical and functional evidence supporting its existence has not been forthcoming. All these three products of the ghrelin gene are detectable in blood, with des-acyl ghrelin at highest concentration.
Ghrelin is an endogenous ligand of the GH secretagogue receptor (GHSR) 1a (1,2,3), and has stimulated GH release in human and rat, after either peripheral or central administration (1,4,5). Competitive binding experiments show affinity of ghrelin for GHSR1a at subnanomolar concentrations (6). Serum ghrelin levels increase with fasting, suggesting that ghrelin is an orexigenic hormone involved in meal initiation (7,8). In rats, ghrelin has stimulated food intake (4,9), increased gastrointestinal motility (10,11) and acid secretion (12), regulated pancreatic secretion (13), induced adiposity (7), and increased body weight (4,7,9). The catabolic effect of ghrelin is reported to result from the reduction of fat utilization (9).
The concentration of plasma des-acyl ghrelin is higher than that of ghrelin and accounts for more than 90% of total circulating ghrelin (14). Des-acyl ghrelin has been variously reported to either stimulate or reduce food intake in rats (15,16). The inhibitory effect of des-acyl ghrelin is reported to be mediated by corticotropin-releasing factor type 2 receptors in the central nervous system (16), whereas the stimulatory effect of the peptide occurs by the activation of orexin neurons in the hypothalamus via a mechanism independent of the GHSR 1a (15). In vitro, des-acyl ghrelin has promoted adipogenesis (17) and inhibited lipolysis (18). In contrast to these in vitro observations, a mouse model nonspecifically overexpressing des-acyl ghrelin via the cytomegalovirus (CMV) promoter demonstrated a small phenotype (19,20).
Although ghrelin has been reported to affect directly the development of adipocytes in vitro, it is unclear whether ghrelin exercises a direct effect on adipose tissue in vivo. The object of this study was to investigate the effect of des-acyl ghrelin on adiposity in vivo. We created transgenic mice in which the ghrelin gene is overexpressed in adipose tissue via the fatty acid-binding protein-4 (FABP4) promoter. Transgenic mice overexpressing the ghrelin gene in adipose tissue demonstrated significant increases in plasma concentrations of des-acyl ghrelin, whereas ghrelin and obestatin remained unchanged. Overexpression of ghrelin from the FABP4 promoter reduced the weight of white adipose tissues, and changed glucose tolerance and insulin sensitivity.
Materials and Methods
Animals
All studies were approved by the University Committee on Use and Care of Animals, and were overseen by the Unit for Laboratory Animal Medicine (University of Michigan). Transgenic mice that express rat preproghrelin gene (GenBank accession no. NM-021669) under control of the mouse FABP4 promoter were created by the transgenic core facility at the University of Michigan (Fig. 1). Similar expression patterns and phenotypes were observed in three founder lines; the studies reported herein are from a single transgenic line. FABP4-ghrelin founders (C57BL/6 X) F2 were backcrossed to C57BL/6 inbred mice, and male progeny in N2–N4 generations were used for experiments.
Figure 1.
Generation of FABP4-ghrelin transgenic mice. The coding region of the mouse ghrelin was amplified using forward primer 5′-GCG TCG ACA CAT GGT GTC TTC AGC GAC TAT CTG CAG TTT GCT ACT-3′ and reverse primer 5′-AAG GAA AAA AGC GGC CGC CAG TGG TTA CTT GTT AGC TGG CGC CTC-3′ to produce a full-length preproghrelin cDNA. The product was introduced 3′ to the FABP4 promoter sequences in a mammalian expression vector. Orientation and sequence identity were confirmed by sequencing the FABP4-ghrelin construct with primers flanking the insertion site. The plasmid construct was excised from vector sequences, gel purified, and used for pronuclear injection into fertilized oocytes.
Diets.
Where indicated, 3-wk-old mice were assigned to receive standard laboratory chow, or a high-fat diet (45% fat, D12451; Research Diets, New Brunswick, NJ).
Measurement of ghrelin, des-acyl ghrelin, and obestatin
Blood samples were collected transcardially from 8- to 12-wk-old mice and transferred immediately to the prechilled polypropylene tubes containing EDTA (1 mg/ml) and aprotinin. Plasma was separated by centrifugation at 4 C and stored at −80 C until experiments. Epididymal white fat tissues were dissected and homogenized in 1 m acetic acid plus 20 mm HCl. Soluble proteins were separated by centrifugation at 4 C and stored at −80 C until used. Ghrelin and des-acyl ghrelin were measured using commercially available ELISA kits (LINCO Research, Inc., Billerica, MA). The assay used to detect acyl ghrelin has no cross-reaction with des-acyl ghrelin, whereas the one used to measure des-acyl ghrelin has less than 0.1% of cross-reaction with acylated ghrelin. Obestatin was quantified by RIA according to the manufacturer’s instruction (Phoenix Pharmaceuticals, Inc., Burlingame, CA). The assay of insulin, leptin, resistin, and adiponectin was made by ELISA (Pierce Biotechnology, Chicago, IL). Plasma from the same set of animals was used to assay for ghrelin, des-acyl ghrelin, and obestatin, whereas the measurement of insulin, leptin, resistin, and adiponectin was performed using plasma from another group of animals.
Energy balance and body composition.
Food intake of individually housed wild-type (n = 6) and FABP4-ghrelin (n = 6) mice was determined using computer-monitored feeding chambers (Ugo Basile, Comerio, Italy). The measurement of oxygen consumption (VO2) with indirect calorimetry was performed on 8- to 12-wk-old mice over 4 d with the Oxymax System (Columbus Instruments, Columbus, OH). Animals were fed standard laboratory chow and water, and were maintained on 12-h light, 12-h dark cycles beginning at 0600 and 1800 h, respectively. Animals were acclimated in measuring chambers for 1 d before recording. Measurements of VO2 were made every 24 min for each animal over a period of 4 d. Body composition was estimated with dual-energy x-ray absorptiometry (DEXA) as described previously with pDEXA SABRE software (Norland Medical Systems, Fort Atkinson, WI). Differences between genotypes were evaluated with ANOVA analysis.
Glucose and insulin tolerance tests.
For glucose tolerance testing, mice were injected ip with 1.5 mg glucose/g body weight at 0900 h, after a 16-h fast. Blood glucose was determined at the indicated times with samples of tail blood obtained using the OneTouch Ultra Glucometer (LifeScan Canada Ltd., Burnaby, British Columbia, Canada). For insulin sensitivity, insulin (0.5 U/kg body weight) was administered ip, and blood samples were collected at the indicated times after administration of insulin. Blood glucose concentrations were determined as described previously.
RT-PCR.
RT-PCR was performed to analyze the expression of GHSR1a and G protein-coupled receptor 39 (GPR39) in both FABP4-ghrelin transgenic mice and wild-type littermates. Total RNA was isolated from adipose tissues using the RNeasy mini kit (QIAGEN, Inc., Valencia, CA) according to the manufacturer’s directions. Single-strand cDNA synthesis was performed as follows: 30 μl reverse transcriptase (RT) mixture contained 1 μg deoxyribonuclease I pretreated total RNA, 0.75 μg oligo-deoxythymidine primer, 6 μl 5× RT buffer, 10 mm dithiothreitol, 0.5 mm deoxynucleotides, 50 U ribonuclease inhibitor, and 240 U RT (Invitrogen Corp., Carlsbad, CA). The RT reaction was performed at 40 C for 70 min, followed by heat inactivation at 95 C for 3 min. PCR primers used for ghrelin receptor mRNA or GPR39 mRNA detection were deduced from published sequences. The nucleotide sequences of sense and antisense primers with the expected product size are as follows:
Ghrelin receptor: TCC GAT CTG CTC ATC TTC CT (sense, bp 270–289) and CAG CTC TCG CTG ACA AAC TG (antisense, bp 385–366), 116-bp product.
GPR39: GTG AGA GAT GAA GGG CCA GA (sense, bp 2120–2139), GGC TTC TCA CCA CTC TCC TG (antisense, bp 2231–2212), 112-bp product.
β-Actin: AAA TCG TGC GTG ACA TCA AA (sense, bp 700–719) and AAG GAA GGC TGG AAA AGA GC (antisense, bp 858–877), 178-bp product.
Results
High concentrations of plasma des-acyl ghrelin in FABP4-ghrelin mice
Transgenic mice that express ghrelin gene via the mouse FABP-4 promoter were generated. Significant increase in des-acyl ghrelin was observed in visceral fat tissues (Fig. 2A), indicating the successful establishment of transgenic mice overexpressing ghrelin gene in adipose tissue. The adipose tissue-specific expression of ghrelin gene in the transgenic mice was demonstrated by the observation that des-acyl ghrelin was undetectable in other tissues, including muscle, heart, lung, kidney, spleen, and liver. Plasma des-acyl ghrelin concentration in FABP4-ghrelin transgenic mice increased by 16-fold relative to wild-type littermates (Fig. 2B). No significant difference in active ghrelin and obestatin concentrations was observed between FABP4-ghrelin transgenic mice and wild-type mice (Fig. 2, C and D).
Figure 2.
Elevation of des-acyl ghrelin in adipose tissues and serum of FABP4-ghrelin (FABP4-ghr) transgenic mice. FABP4-ghrelin transgenic mice demonstrated a significant increase in des-acyl ghrelin in adipose tissue (A) and in serum (B). Circulatory levels of ghrelin (C) and obestatin (D) were unaltered. *, P < 0.05 vs. wild-type mice.
Effect of FABP4-ghrelin on food intake, body weight, and energy metabolism
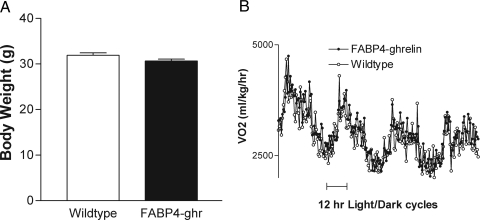
Analysis of food intake showed that there was no difference between FABP4-ghrelin transgenic mice and wild-type mice over a period of observation up to 22 wk (data not shown). FABP4-ghrelin transgenic mice on regular chow demonstrated no significant difference in body weight compared with the wild-type mice (Fig. 3A). No significant difference in body length was observed between FABP4-ghrelin transgenic mice and the wild-type littermates (9.93 ± 0.18 vs. 9.95 ± 0.23 cm). Metabolic analysis revealed that VO2 in FABP4-ghrelin transgenic mice was not different relative to wild-type mice (Fig. 3B).
Figure 3.
Body weight and VO2. A, Twelve- week-old FABP4-ghrelin (FABP4-ghr) transgenic mice showed no significant change in body weight relative to the controls. B, VO2 in FABP4-ghrelin transgenic mice was not different relative to wild-type mice.
Reduction in white adipose tissue in FABP4-ghrelin mice
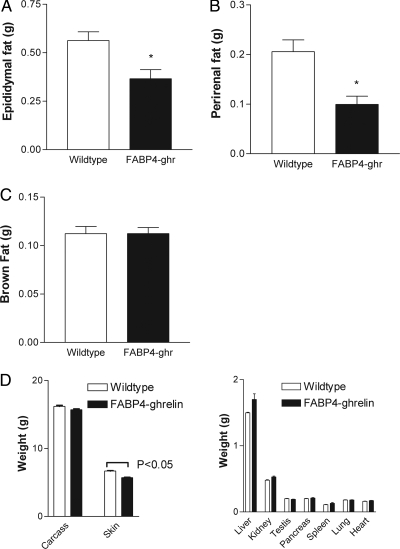
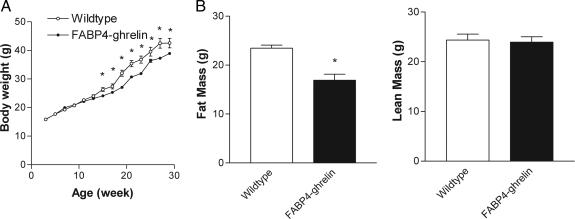
Although no difference in total body weight was observed between transgenic mice and wild-type mice, epididymal and perirenal white adipose tissues weighted 35 ± 9% and 52 ± 9% less, respectively, in FABP4-ghrelin transgenic mice compared with controls (Fig. 4, A and B). The development of brown fat was not affected by overexpression of ghrelin in adipose tissue (Fig. 4C). Skin weight that contains sc adipose tissue showed a significant decrease, whereas no change was demonstrated for other organ weights between FABP4-ghrelin transgenic mice and wild-type controls (Fig. 4D). To evaluate the effect of overexpression of ghrelin gene on the development of high-fat diet-induced obesity, FABP4-ghrelin transgenic mice and wild-type mice were placed on a high-fat diet for 26 wk, and change in body weight was measured. In addition, total body lipid content was determined noninvasively with DEXA. FABP4-ghrelin mice showed a significant decrease in body weight (Fig. 5A). This decrement in body weight is likely due to a decrease in adipose tissue because DEXA examination detected 28 ± 2% less total body fat and no significant change in lean mass when FABP4-ghrelin mice were fed a high-fat diet (Fig. 5B).
Figure 4.
Changes in adipose tissue weight and other organ weights. Mice at 12 wk of age were killed, and visceral fat pad and other organs were harvested and measured. FABP4-ghrelin (FABP4-ghr) transgenic mice showed a significant decrease in epididymal fat pad (A) and perirenal fat pad (B) relative to wild-type animals. C, No change in brown fat pad was detected between FABP4-ghrelin transgenic mice and wild-type controls. D, Skin weights showed a significant decrement in FABP4-ghrelin transgenic mice, whereas other organ weights demonstrated no significant change compared with wild-type controls. *, P < 0.05 vs. wild-type mice.
Figure 5.
Resistance to high-fat diet-induced obesity. A, FABP4-ghrelin transgenic mice were resistant to body weight gain when fed with high-fat diets. ANOVA demonstrated a significant decrement of body weight in FABP4-ghrelin transgenic mice compared with wild-type mice (*, P < 0.05). B, DEXA measurement showed a significant decrease of fat mass in FABP4-ghrelin transgenic mice relative to the control wild-type mice (*, P < 0.05), whereas lean mass was unchanged.
Alterations in glucose tolerance and insulin sensitivity
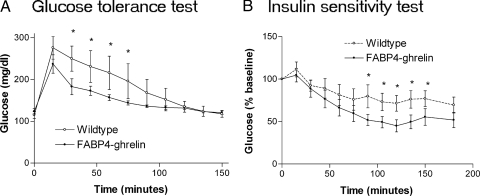
Glucose tolerance and insulin sensitivity were examined in FABP4-ghrelin transgenic mice. Standard glucose tolerance testing showed glucose levels to be significantly lower in FABP4-ghrelin transgenic mice than in controls after glucose administration (Fig. 6A). FABP4-ghrelin transgenic mice had a greater hypoglycemic response to insulin administration than control animals (Fig. 6B).
Figure 6.
Des-acyl ghrelin improves glucose tolerance and insulin sensitivity. Altered glucose tolerance (A) and insulin sensitivity (B) were shown in FABP4-ghrelin transgenic mice. Data shown are mean blood glucose concentration (A) and mean percentage of blood glucose concentration over the basal levels (B). Statistical differences are represented by *, P < 0.05.
Change in plasma levels of leptin and insulin
As shown in Fig. 7, plasma levels of leptin and insulin were significantly higher in FABP4-ghrelin transgenic mice compared with the wild-type littermates. No changes in adiponectin and resistin were observed.
Figure 7.
Elevation in the levels of leptin and insulin. ELISAs were used to measure the levels of plasma leptin, insulin, adiponectin, and resistin. Results are presented as mean ± sem. Statistical differences are indicated by *, P < 0.05.
No expression of GHSR mRNA in fat tissues
To determine whether the previously identified ghrelin receptor GHS-R1a is expressed in adipose tissues, we assayed the expression of ghrelin receptor mRNA by RT-PCR using primers from the published ghrelin receptor sequence. No expression of ghrelin receptor mRNA was detected in white adipose tissues (data not shown). Expression of GPR39, a putative receptor for obestatin, was detected in white adipose tissues by RT-PCR (data not shown).
Discussion
The major finding of the present study is that des-acyl ghrelin alters the mass of white adipose tissues in vivo. This conclusion is supported by two distinct observations: 1) transgenic mice expressing the ghrelin gene driven from the adipocyte-specific FABP4 promoter demonstrated an increase in plasma concentration of des-acyl ghrelin, and 2) FABP4-ghrelin transgenic mice exhibited a reduction in fat pad mass and resistance to high-fat diet-induced obesity.
Although ghrelin was originally reported to stimulate GH release, subsequent studies have provided evidence that ghrelin exercises a wide range of functions, including regulation of food intake and energy metabolism (4,7,9), modulation of cardiovascular function, stimulation of osteoblast proliferation and bone formation, and stimulation of neurogenesis (21,22) and myogenesis (23,24). In the gastrointestinal system, ghrelin affects multiple functions, including secretion of gastric acid (10,12), gastric motility (11,12), and pancreatic protein output (13). Most of these functions have been attributed to the actions of acylated ghrelin.
Although ghrelin has been the focus of numerous studies of neuroendocrine control mechanisms, food intake, and energy metabolism, the physiological role of des-acyl ghrelin is uncertain. In recent reports by Ariyasu (19) and Asakawa (20) et al., des-acyl ghrelin countered the effects of acylated ghrelin, inducing negative energy balance by decreasing food intake and delaying gastric emptying. These observations were supported by a report in which des-acyl ghrelin acted via corticotropin-releasing factor type 2 receptors to alter fasting stomach motility in conscious rats. In contrast, Toshinai et al. (15) reported that des-acyl ghrelin induces food intake by activating orexin neurons in the hypothalamus.
To explore the consequences of long-term expression of the ghrelin gene, we created transgenic mice expressing ghrelin in adipose tissue driven from the FABP4 promoter. FABP4-ghrelin transgenic mice demonstrated a significant increase in plasma des-acyl ghrelin. Meanwhile, FABP4-ghrelin transgenic mice exhibited normal plasma concentrations of ghrelin and obestatin. Ghrelin O-acyltransferase has been recently identified as the only enzyme that octanoylates ghrelin (25). The finding that ghrelin O-acyltransferase mRNA is largely restricted to the stomach and is undetectable in adipose tissue may explain why acyl ghrelin is not elevated in FABP4-ghrelin transgenic mice. Whether the inability of adipose tissue to produce and secrete obestatin into blood accounts for the unchanged plasma level of obestatin remains to be examined.
In contrast to the report by Ariyasu et al. (19), we did not observe any difference in the body length between transgenic mice and wild-type littermates. It is unclear what caused this difference in phenotype of these transgenic mice, despite the common high-circulating des-acyl ghrelin levels in both models. Because the amino acid sequence of ghrelin is identical for rat and mouse, we do not believe that using the rat ghrelin gene accounts for the differences in phenotype of the transgenic mice in our study compared with those reported by Ariyasu et al. (19). It is more likely that the difference comes from the promoter used to drive the expression of ghrelin gene. In the previous study (19), the CMV promoter was used to drive the expression of ghrelin gene. Indeed, overexpression of ghrelin gene has been reported in multiple tissues such as muscle, lung, heart, pituitary, and brain when the CMV promoter is used to drive the expression of the ghrelin gene (26). In contrast to Ariyasu et al. (19), Wei et al. (26) reported no change in body weight of transgenic mice, despite a 14-fold increase in serum levels of acyl ghrelin.
In this study, FABP4-ghrelin transgenic mice demonstrated a decreased amount of white adipose tissues. This decrease may be caused by the impaired development of white adipose tissue because des-acyl ghrelin did not alter food intake and metabolic profiles in transgenic animals. Consistent with our observation, transgenic mice overexpressing the ghrelin gene in a wide variety of tissues driven by CMV promoter demonstrate a significant increase in the plasma concentration of des-acyl ghrelin (19,20). In addition, these transgenic mice have been reported to be shorter and weigh less relative to control littermates. Based on these reports, it appears that des-acyl ghrelin may play a role in the development of adipocytes and the maintenance of energy homeostasis. Because des-acyl ghrelin is unable to stimulate the release of GH (1), the inhibitory effect of des-acyl ghrelin on the development of adipose tissues is unlikely to be mediated by GH.
Although des-acyl ghrelin inhibited the development of white adipose tissues, brown adipose tissue development was unaffected. Consistent with this observation, VO2 remains unchanged between FABP4-ghrelin transgenic mice and control littermates. The differential responses of white and brown adipose tissues to des-acyl ghrelin are interesting. Whether this is because of the absence of a receptor for des-acyl ghrelin in brown adipose tissues is unknown.
The absence of detectable ghrelin receptor GHSR1a mRNA in adipose tissue from both FABP4-ghrelin transgenic mice and wild-type mice suggests that this receptor is not responsible for the reduction in white adipose tissue in these animals. Obestatin is a 23-amino acid peptide derived from proghrelin (27). Original reports demonstrated that this peptide counteracts the effects of ghrelin on food intake, body weight, and gastric motility. The actions of obestatin were reported to be exerted via an orphan receptor, GPR-39 (27). Although mRNA for GPR39 is detected in both FABP4-ghrelin transgenic mice and wild-type mice, we do not believe that GPR39 contributes to the inhibition of adipose development in the FABP4-ghrelin transgenic mice. In the current study, no difference in GPR39 mRNA expression was detected in FABP4-ghrelin transgenic mice relative to wild-type mice. In addition, FABP4-ghrelin transgenic mice did not demonstrate an increase in plasma obestatin levels.
Recent studies have suggested the existence of a novel ghrelin receptor subtype in a variety of cells, including adipocytes (28), skeletal muscle cells (24), cardiomyocytes (29), and bone marrow-derived stromal cells. In bone marrow, Thompson et al. (17) recently reported that acylated ghrelin and des-acyl ghrelin stimulate tibial bone marrow adipogenesis via a receptor other than GHSR1a.
Conflicting results have been reported for the effects of des-acyl ghrelin on glucose uptake by adipocytes. In cultured adipocytes derived from rat retroperitoneal fat pad, des-acyl ghrelin has enhanced glucose uptake by mature adipocytes (30). In contrast to this observation, studies by Patel et al. (31) show that des-acyl ghrelin has no effect on insulin-stimulated glucose uptake by cultured adipocytes derived from perirenal fat pad. In the present study, alterations in glucose tolerance and insulin sensitivity were detected in FABP4-ghrelin transgenic mice, suggesting that des-acyl ghrelin may play a role in the regulation of glucose metabolism. The observation that levels of plasma leptin and insulin are elevated in transgenic mice suggests that interaction among des-acyl ghrelin, leptin, and insulin may exist. Whether des-acyl ghrelin stimulates the release of leptin from adipocytes and insulin from pancreatic islets cells remains to be investigated.
In conclusion, the current study demonstrates that overexpression of ghrelin gene via the FABP4 promoter exhibits high plasma concentration of des-acyl ghrelin, alters the development of the white adipose tissues, and improves glucose tolerance and insulin sensitivity.
Acknowledgments
We thank Dr. Jun Yang at Phoenix Pharmaceuticals, Inc. (Burlingame, CA) for measurement of obestatin. We are grateful to Dr. Ormond Macdougald at the Department of Physiology at the University of Michigan Medical School for providing the fatty acid-binding protein-4 promoter.
Footnotes
This study was supported by National Institutes of Health Grant RO1DK043225.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 5, 2008
Abbreviations: CMV, Cytomegalovirus; DEXA, dual-energy x-ray absorptiometry; FABP4, fatty acid-binding protein-4; GHSR, GH secretagogue receptor; GPR39, G protein-coupled receptor39; RT, reverse transcriptase; VO2, oxygen consumption.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH 1996 A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977 [DOI] [PubMed] [Google Scholar]
- McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, Smith RG, Van der Ploeg LH, Howard AD 1997 Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol 11:415–423 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR 2000 The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW 2003 High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol 17:2201–2210 [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matrukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Kojima M, Kuroiwa T, Matsukura S, Kangawa K, Nakazato M 2001 Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 280:904–907 [DOI] [PubMed] [Google Scholar]
- Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P 2002 Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 282:G948–G952 [DOI] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K 2000 Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276:905–908 [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen M, Chen X, Segura BJ, Mulholland MW 2001 Inhibition of pancreatic protein secretion by ghrelin in rat. J Physiol 537(Pt 1):231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K 2000 Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–913 [DOI] [PubMed] [Google Scholar]
- Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M 2006 Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147: 2306–2314 [DOI] [PubMed] [Google Scholar]
- Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M 2005 Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology 129:8–25 [DOI] [PubMed] [Google Scholar]
- Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T 2004 Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145:234–242 [DOI] [PubMed] [Google Scholar]
- Muccioli G, Pons N, Ghè C, Catapano F, Granata R, Ghigo E 2004 Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol 498:27–35 [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, Kangawa K, Nakao K 2005 Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology 146:355–364 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M 2005 Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, Mulholland MW 2004 Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol 559(Pt 3):729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Hu Y, Lin TR, Fan Y, Mulholland MW 2005 Stimulation of neurogenesis in rat nucleus of the solitary tract by ghrelin. Peptides 26:2280–2288 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Mulholland MW 2007 Ghrelin stimulates myocyte development. Cell Physiol Biochem 20:659–664 [DOI] [PubMed] [Google Scholar]
- Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghe C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, Graziani A 2007 Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell 18:986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL 2008 Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396 [DOI] [PubMed] [Google Scholar]
- Wei W, Qi X, Reed J, Ceci J, Wang HQ, Wang G, Englander EW, Greeley Jr GH 2006 Effect of chronic hyperghrelinemia on ingestive action of ghrelin. Am J Physiol Regul Integr Comp Physiol 290:R803–R808 [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ 2005 Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310:996–999 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Lin TR, Chai B, Fan Y, Gantz I, Mulholland, MW 2004 Inhibition of adipogenesis by ghrelin. Mol Biol Cell 15:2484–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I, Muccioli G, Granata R, Deghenghi R, Ghigo E, Ohlsson C, Isgaard J 2002 Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J Endocrinol 175:201–209 [DOI] [PubMed] [Google Scholar]
- Giovambattista A, Gaillard RC, Spinedi E 2008 Ghrelin gene-related peptides modulate rat white adiposity. Vitam Horm 77:171–205 [DOI] [PubMed] [Google Scholar]
- Patel AD, Stanley SA, Murphy KG, Frost GS, Gardiner JV, Kent AS, White NE, Ghatei MA, Bloom SR 2006 Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regul Pept 134:17–22 [DOI] [PubMed] [Google Scholar]